Layer-by-Layer Heterostructure of MnO2@Reduced Graphene Oxide Composites as High-Performance Electrodes for Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Preparation of the Composites
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
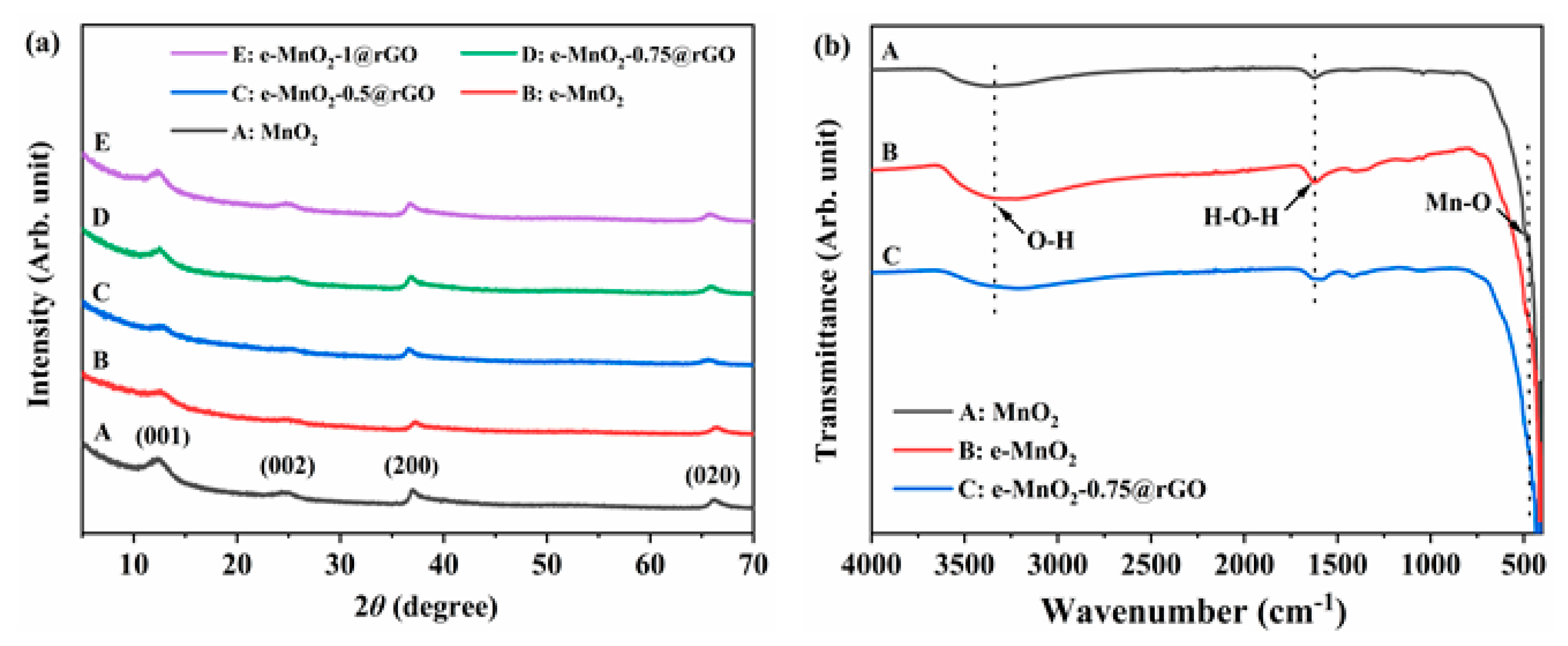
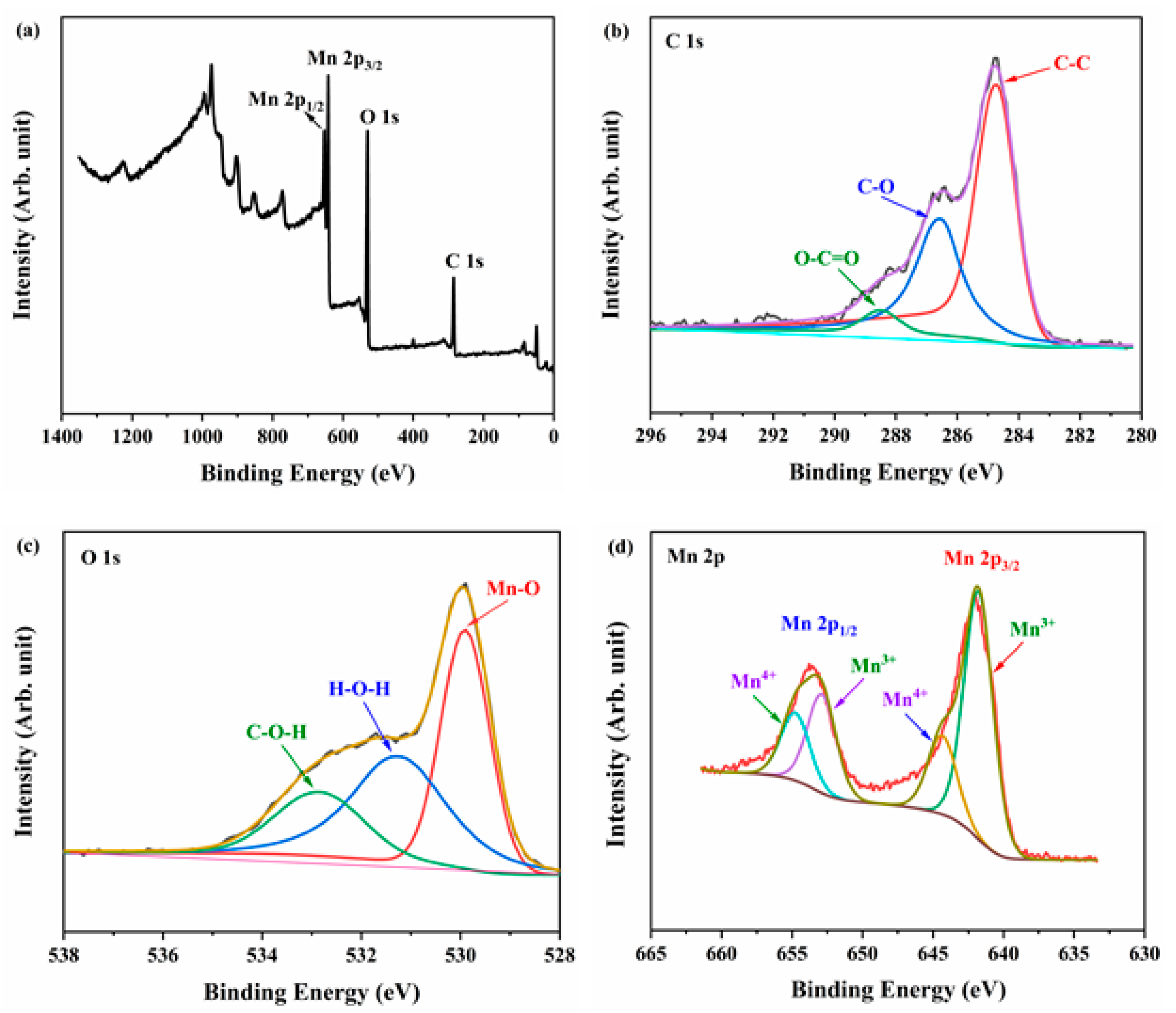
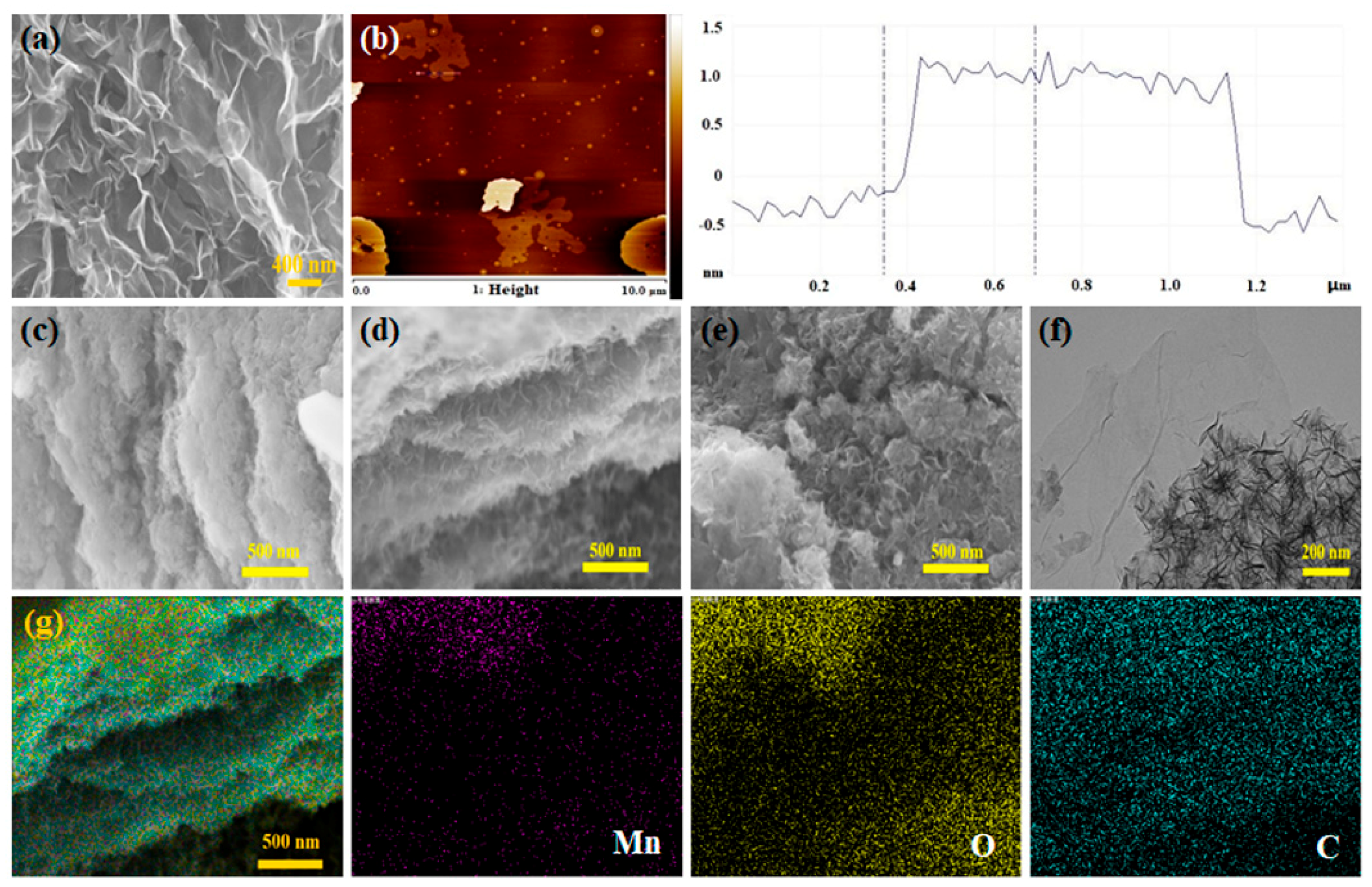
3.1. Structure and Morphology
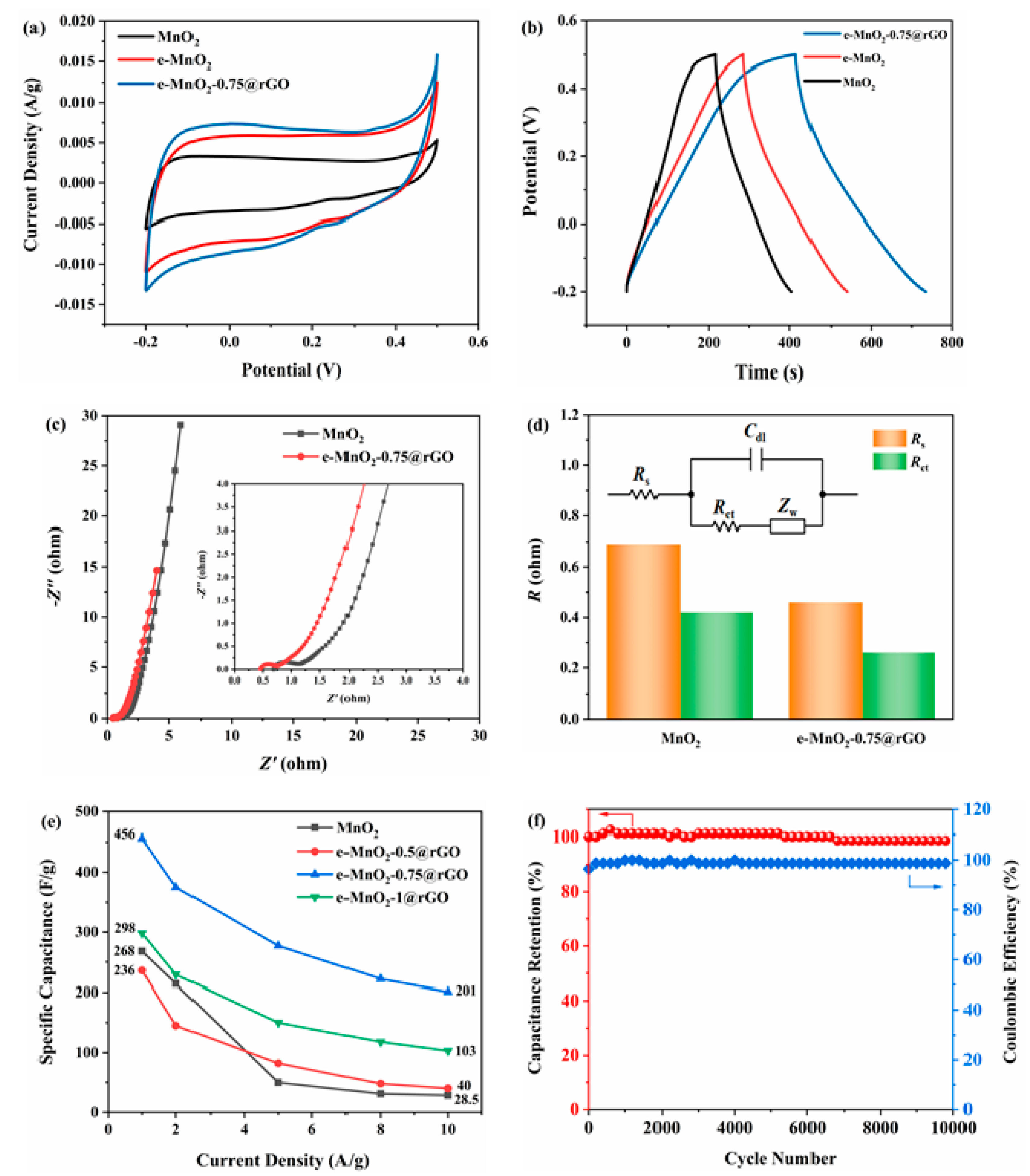
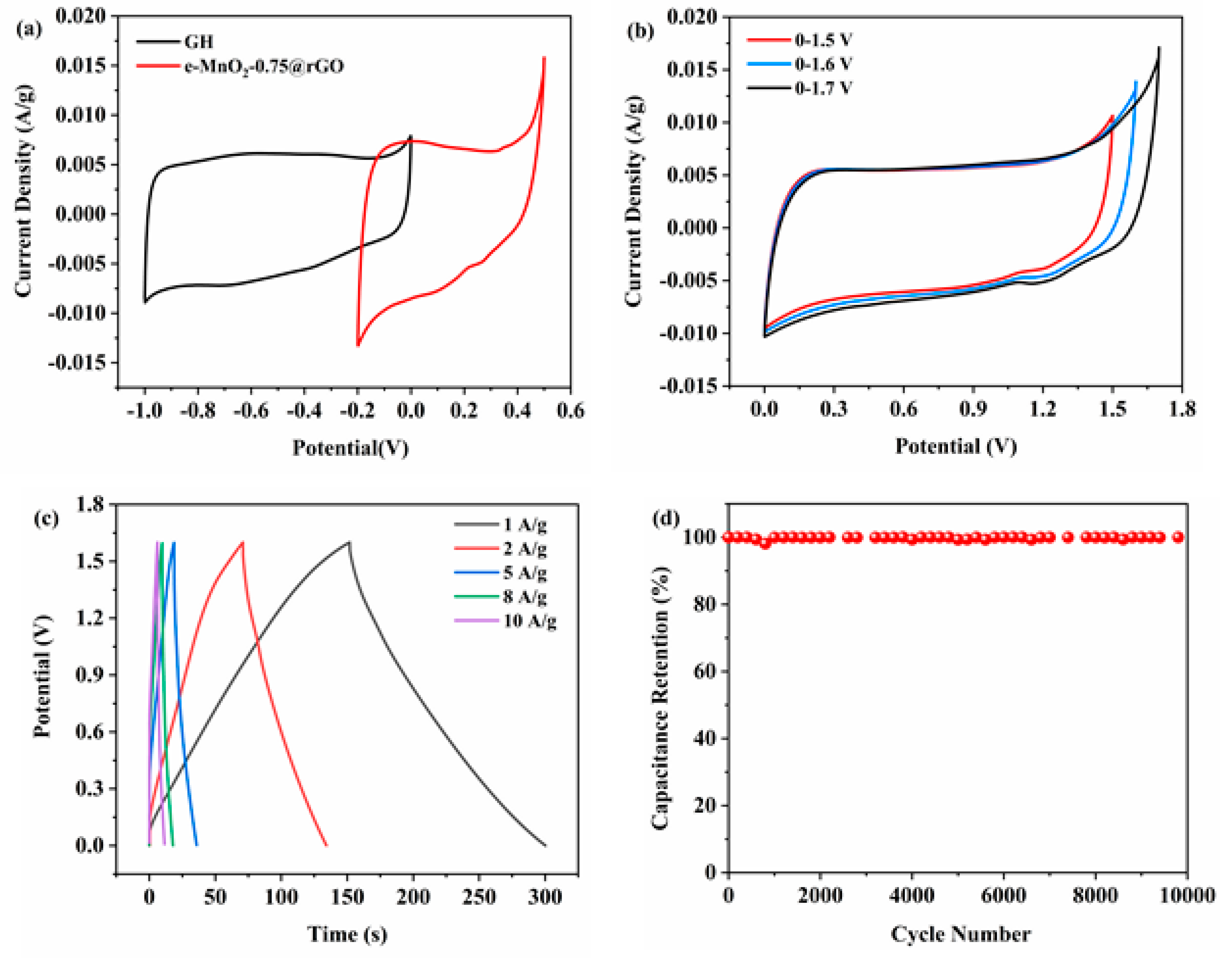
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, J.R.; Simon, P. Materials science: Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, G.Z. Redox electrode materials for supercapatteries. J. Power Sources 2016, 326, 604–612. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal carbonization of abundant renewable natural organic chemicals for high-performance supercapacitor electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Bai, X.; Hu, X.; Zhou, S.; Yan, J.; Sun, C.; Chen, P.; Li, L. In situ polymerization and characterization of grafted poly (3,4-ethylenedioxythiophene)/multiwalled carbon nanotubes composite with high electrochemical performances. Electrochim. Acta 2013, 87, 394–400. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Wang, G.; Liang, R.; Liu, L.; Zhong, B. Improving the specific capacitance of carbon nanotubes-based supercapacitors by combining introducing functional groups on carbon nanotubes with using redox-active electrolyte. Electrochim. Acta 2014, 115, 183–188. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Zhong, X.; Huang, X.; Weiss, N.O.; Huang, Y.; Duan, X. Holey graphene frameworks for highly efficient capacitive energy storage. Nat. Commun. 2014, 5, 4554. [Google Scholar] [CrossRef]
- Yang, W.; Ni, M.; Ren, X.; Tian, Y.; Li, N.; Su, Y.; Zhang, X. Graphene in supercapacitor applications. Curr. Opin. Colloid Interface Sci. 2015, 20, 416–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospective. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, X.; Yang, L.; Wang, T.; Zhang, M.; Duan, G.; Liu, X.; Li, Y. Synthetic melanin facilitates MnO supercapacitors with high specific capacitance and wide operation potential window. Polymer 2021, 235, 124276. [Google Scholar] [CrossRef]
- Gao, S.; Sun, Y.; Lei, F.; Liang, L.; Liu, J.; Bi, W.; Pan, B.; Xie, Y. Ultrahigh energy density realized by a single-layer β-Co(OH)2 all-solid-state asymmetric supercapacitor. Angew. Chem. Int. Ed. 2014, 53, 12789–12793. [Google Scholar] [CrossRef]
- Nguyen, T.; Montemor, M. Metal oxide and hydroxide-based aqueous supercapacitors: From charge storage mechanisms and functional electrode engineering to need-tailored devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, Z.; Ding, S.; Chen, J.S.; Lou, X.W. Hierarchical nickel sulfide hollow spheres for high performance supercapacitors. RSC Adv. 2011, 1, 397–400. [Google Scholar] [CrossRef]
- Sugimoto, W.; Iwata, H.; Yokoshima, K.; Murakami, Y.; Takasu, Y. Proton and electron conductivity in hydrous ruthenium oxides evaluated by electrochemical impedance spectroscopy: The origin of large capacitance. J. Phys. Chem. B 2005, 109, 7330–7338. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive materials for electrochemical capacitors: From rational synthesis to capacitance optimization. Natl. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef]
- Jian, T.; Zhu, J.; Ma, W.; Yan, X.; Li, G.; Zhou, J. Interconnected two-dimensional MnO2 nanosheets anchored on threedimensional porous Cu skeleton as a high-performance cathode for energy storage. Appl. Surf. Sci. 2020, 529, 147152. [Google Scholar] [CrossRef]
- Swain, N.; Mitra, A.; Saravanakumar, B.; Balasingam, S.K.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Construction of three-dimensional MnO2/Ni network as an efficient electrode material for high performance supercapacitors. Electrochim. Acta 2020, 342, 136041. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; B’elanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechol. 2011, 6, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Pan, Q.; Yao, S.; Duan, L.; Liu, J. Mesoporous MnO2 nanosphere/graphene sheets as electrodes for supercapacitor synthesized by a simple and inexpensive reflux reaction. Electrochem. Acta 2017, 238, 30–35. [Google Scholar] [CrossRef]
- Jadhav, S.; Kalubarme, R.S.; Terashima, C.; Kale, B.B.; Godbole, V.; Fujishima, A.; Gosavi, S.W. Manganese dioxide/reduced graphene oxide composite an electrode material for high-performance solid state supercapacitor. Electrochim. Acta 2019, 299, 34–44. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.; Wang, G.; Zhang, J.; Zhang, S. Sandwich-like NiO/rGO nanoarchitectures for 4 V solid-state asymmetric-supercapacitors with high energy density. Electrochim. Acta 2018, 283, 1401–1410. [Google Scholar] [CrossRef]
- Kumar, A.; Sarkar, D.; Mukherjee, S.; Patil, S.; Sarma, D.D.; Shukla, A. Realizing an asymmetric supercapacitor employing carbon nanotubes anchored to Mn3O4 cathode and Fe3O4 anode. ACS Appl. Mater. Interfaces 2018, 10, 42484–42493. [Google Scholar] [CrossRef]
- Shi, Z.; Xing, L.; Liu, Y.; Gao, Y.; Liu, J. A porous biomass-based sandwich-structured Co3O4@carbon fiber@Co3O4 composite for high-performance supercapacitors. Carbon 2018, 129, 819–825. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Tian, Z.; Guo, H.; Cai, C.; Wu, Q.; Du, H.; Liu, K.; Hao, Z.; He, S.; et al. Wood-derived high-mass-loading MnO2 composite carbon electrode enabling high energy density and high-rate supercapacitor. Small 2022, 18, 2201307. [Google Scholar] [CrossRef]
- Xu, C.; Li, Z.; Yang, C.; Zou, P.; Xie, B.; Lin, Z.; Zhang, Z.; Li, B.; Kang, F.; Wong, C.P. An ultralong, highly oriented nickel-nanowire-array electrode scaffold for high-performance compressible pseudocapacitors. Adv. Mater. 2016, 28, 4105–4110. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Wang, Z.; Yu, J.; Huang, H.; Qiu, J. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives. Nano Energy 2019, 57, 459–472. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Samanta, P.; Murmu, N.C.; Kim, N.H.; Kuila, T.; Lee, J.H. Growth of Ni-Co binary hydroxide on reduced graphene oxide surface by a successive ionic layer adsorption and reaction (SILAR) method for high performance asymmetric supercapacitor electrode. J. Mater. Chem. A 2016, 4, 2188–2197. [Google Scholar] [CrossRef]
- Govindasamy, M.; Shanthi, S.; Elaiyappillai, E.; Wang, S.F.; Johnson, P.M.; Ikeda, H.; Hayakawa, Y.; Ponnusamy, S.; Muthamizhchelvan, C. Fabrication of hierarchical NiCo2S4@CoS2 nanostructures on highly conductive flexible carbon cloth substrate as a hybrid electrode material for supercapacitors with enhanced electrochemical performance. Electrochim. Acta 2019, 293, 328–337. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Das, H.T.; Chen, S.M.; Govindasamy, M.; Alshgari, R.A.; Fan, C.H.; Huang, C.H. CoAl2O4 nanoparticles modified carbon nanofibers as high-efficiency bifunctional electrocatalyst: An efficient electrochemical aqueous asymmetric supercapacitors and non-enzymatic electrochemical sensors. J. Alloy. Compd. 2023, 931, 167553. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Wang, J.; Ma, J.; Zhang, M.; Liu, L. Solvothermal one-step synthesis of Ni-Al layered double hydroxide/carbon nanotube/reduced graphene oxide sheet ternary nanocomposite with ultrahigh capacitance for supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 5443–5454. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Shao, G.; Fan, Y.; Wang, G.; Song, J.; Shen, D. Construction of hierarchical α-MnO2 nanowires@ultrathin δ-MnO2 nanosheets core-shell nanostructure with excellent cycling stability for high-power asymmetric supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 9050–9058. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Zong, Q.; Zhang, Q.; Mei, X.; Li, Q.; Zhou, Z.; Li, D.; Chen, M.; Shi, F.; Sun, J.; Yao, Y.; et al. Facile synthesis of Na-doped MnO2 nanosheets on carbon nanotube fibers for ultrahigh-energy-density all-solid-state wearable asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 37233–37241. [Google Scholar] [CrossRef]
- Chen, X.P.; Wen, J.; Zhao, C.X.; Li, Y.T.; Wang, N. Synthesis of core-shell structured MnO2 petal nanosheet@carbon sphere composites and their application as supercapacitor electrodes. Chem. Sel. 2018, 3, 9301–9307. [Google Scholar] [CrossRef]
- Ren, K.; Liu, Z.; Wei, T.; Fan, Z. Recent developments of transition metal compounds-carbon hybrid electrodesfor high energy/power supercapacitor. Nano-Micro Lett. 2021, 13, 129. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Samanta, P.; Murmu, N.C.; Kim, N.H.; Kuila, T.; Lee, J.H. A successive ionic layer adsorption and reaction (SILAR) method to fabricate a layer-by-layer (LbL) MnO2-reduced graphene oxide assembly for supercapacitor application. J. Power Sources 2017, 340, 380–392. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Lutkenhaus, J.L.; Siqueira, J.R., Jr. Building up nanostructured layer-by-layer films combining reduced graphene oxide-manganese dioxide nanocomposite in supercapacitor electrodes. Thin Solid Film. 2021, 718, 138483. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, N.; Zhang, J.; Wang, X.; Jin, J.; Zheng, X.; Fang, Y. The additive-free electrode based on the layered MnO2 nanoflowers/reduced, graphene oxide film for high performance supercapacitor. Ceram. Int. 2017, 43, 5374–5381. [Google Scholar] [CrossRef]
- Amir, F.Z.; Pham, V.H.; Schultheis, E.M.; Dickerson, J.H. Flexible, all-solid-state, high-cell potential supercapacitors based on holey reduced graphene oxide/manganese dioxide nanosheets. Electrochim. Acta 2018, 260, 944–951. [Google Scholar] [CrossRef]
- Yuan, T.; Cui, X.; Liu, X.; Qu, X.; Sun, J. Highly tough, stretchable, self-healing, and recyclable hydrogels reinforced by in situ-formed polyelectrolyte complex nanoparticles. Macromolecules 2019, 52, 3141–3149. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, K.; Sun, H.; Yin, S. One-step synthesis of single-layer MnO2 nanosheets with multi-role sodium dodecyl sulfate for high-performance pseudocapacitors. Small 2015, 11, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M. PE-Based Lithium Ion Battery Separator Was Prepared Based on Layer-by-layer Self-Assembly Technology. Master’s Thesis, Tianjin University of Science & Technology, Tianjin, China, 2019. [Google Scholar]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Hao, Q. Preparation and supercapacitive performances of layer by layer assembled graphene/polyaniline composite electrode. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2017. [Google Scholar]
- Huang, M.; Wang, Y.; Chen, J.; He, D.; He, J.; Wang, Y. Biomimetic design of Ni Co LDH composites linked by carbon nanotubes with plant conduction tissues characteristic for hybrid supercapacitors. Electrochim. Acta 2021, 381, 138289. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, W.; Li, C.; Sun, X.; Wang, K.; Ma, Y. Microwave-assisted rapid synthesis of birnessite-type MnO2 nanoparticles for high performance supercapacitor applications. Mater. Res. Bull. 2015, 71, 111–115. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, Y.; Hu, C.; Zhao, Y.; Shao, H.; Chen, N.; Qu, L. MnO2-modified hierarchical graphene fiber electrochemical supercapacitor. J. Power Sources 2014, 247, 32–39. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene-MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Yang, X.; Makita, Y.; Liu, Z.H.; Sakane, K.; Ooi, K. Structural characterization of self-assembled MnO2 nanosheets from birnessite manganese oxide single crystals. Chem. Mater. 2004, 16, 5581–5588. [Google Scholar] [CrossRef]
- Huang, S.Y.; Le, P.A.; Yen, P.J.; Lu, Y.C.; Sahoo, S.K.; Cheng, H.W.; Chiu, P.W.; Tseng, T.Y.; Wei, K.H. Cathodic plasma-induced syntheses of graphene nanosheet/MnO2/WO3 architectures and their use in supercapacitors. Electrochim. Acta 2020, 342, 136043. [Google Scholar] [CrossRef]
- Niu, Z.; Yue, T.; Hu, W.; Sun, W.; Hu, Y.; Xu, Z. Covalent bonding of MnO2 onto graphene aerogel forwards: Efficiently catalytic degradation of organic wastewater. Appl. Surf. Sci. 2019, 496, 143585. [Google Scholar] [CrossRef]
- Liu, B.; Cao, Z.; Yang, Z.; Qi, W.; He, J.; Pan, P.; Li, H.; Zhang, P. Flexible micro-supercapacitors fabricated from MnO2 nanosheet/graphene composites with black phosphorus additive. Prog. Nat. Sci. Mater. Int. 2022, 32, 10–19. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, T.; Liu, Z.; Qi, W.; He, J.; Pan, P.; Li, H.; Zhang, P. Facile fabrication of binder-free reduced graphene oxide/MnO2/Ni foam hybrid electrode for high-performance supercapacitors. J. Alloy. Compd. 2019, 812, 152124. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently enhancing electrocatalytic activity of α-MnO2 nanorods/N-doped ketjenblack carbon for oxygen reduction reaction and oxygen evolution reaction using facile regulated hydrothermal treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef]
- Hastuti, E.; Subhan, A.; Amonpattaratkit, P.; Zainuri, M.; Suasmoro, S. The effects of Fe-doping on MnO2: Phase transitions, defect structures and its influence on electrical properties. RSC Adv. 2021, 11, 7808–7823. [Google Scholar] [CrossRef]
- Kai, K.; Yoshida, Y.; Kageyama, H.; Saito, G.; Ishigaki, T.; Furukawa, Y.; Kawamata, J. Room-temperature synthesis of manganese oxide monosheets. J. Am. Chem. Soc. 2008, 130, 15938–15943. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, S.; Yang, S.; Chen, W.; Cheng, Y.; Guo, Y.; Peng, S.; Ramakrishna, S.; Zhu, M. Hierarchical MnO2 nanowire/graphene hybrid fibers with excellent electrochemical performance for flexible solid-state supercapacitors. J. Power Sources 2016, 306, 481–488. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Zhang, S.; Chen, X.; Li, Y.; Liu, J.; Lu, F.; Tang, Y. Compounding δ-MnO2 with modified graphene nanosheets for highly stable asymmetric supercapacitors. Colloids Surf. A 2019, 573, 57–66. [Google Scholar] [CrossRef]
- Vedpathak, A.S.; Desai, M.A.; Bhagwat, S.; Sartale, S.D. Green strategy for the synthesis of K+ pre-inserted MnO2/rGO and its electrochemical conversion to Na-MnO2/rGO for high-performance supercapacitors. Energy Fuels 2022, 36, 4596–4608. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, L.; Wu, B.; Hu, N. Vertical carbon skeleton introduced three-dimensional MnO2 nanostructured composite electrodes for high-performance asymmetric supercapacitors. J. Power Sources 2020, 476, 228527. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Zhang, L.; Cui, X.; Zhu, X.; Zhao, J.; Jin, D.; Yang, D.; Li, J. Reduced graphene oxide coated manganese dioxide electrode prepared by polyvinylpyrrolidone assisted electrodeposition. Vacuum 2022, 199, 110925. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Boore-Talari, O. Sonochemical assisted synthesis MnO2/RGO nanohybrid as effective electrode material for supercapacitor. Ultrason. Sonochemistry 2017, 40, 675–685. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, X.; Fang, J.; Zhang, X.; Chen, S.; Li, W.; Feng, W.; Chen, Y.; Wang, W.; Zhang, Y. Layered-MnO2 nanosheet grown on nitrogen-doped graphene template as a composite cathode for flexible solid-state asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 5251–5260. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, X.; Zhang, Q.; Yang, F.; Dong, H.; Sui, J.; Dong, L. One-step hydrothermal synthesis of MnO2/graphene composite for electrochemical energy storage. J. Electroanal. Chem. 2019, 837, 108–115. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Liu, X.; Liu, S.; Zhang, L.; Xu, W.; Yang, H.; Hou, H.; He, S.; Zhao, Y.; et al. Nitrogen, sulfur co-doped hierarchical carbon encapsulated in graphenewith ‘‘sphere-in-layer” interconnection for high-performance supercapacitor. J. Colloid Interface Sci. 2021, 599, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Sui, L.; Jiang, S.; Hou, H. Self-adhesive polyimide (PI)@reduced graphene oxide (RGO)/PI@carbon nanotube (CNT) hierarchically porous electrodes: Maximizing the utilization of electroactive materials for organic Li-ion batteries. Energy Technol. 2020, 8, 2000397. [Google Scholar] [CrossRef]



| Materials | Preparation Methods | Specific Capacitance | References |
|---|---|---|---|
| MnO2 NF/RGO@Ni foam | layer-by-layer (LBL) self-assembly | 246 F/g (0.5 A/g) | [47] |
| δ-MnO2/modified graphene | Hydrothermal method | 270 F/g (0.5 A/g) | [63] |
| Na-MnO2/rGO | Hydrothermal method | 451 F/g (0.5 A/g) | [64] |
| rGO/C/MnO2 | Carbonization + Hydrothermal treatment | 215.2 F/g (0.15 A/g) | [65] |
| MnO2 and polyvinylpyrrolidone (PVP)@rGO | Electrodeposition | 358 F/g (1 A/g) | [66] |
| MnO2/rGO | Sonochemical assisted synthesis | 375 F/g (1 A/g) | [67] |
| MnO2/nitrogen-doped graphene (NG) | Hydrothermal method | 305 F/g (5 mV/s) | [68] |
| MnO2/graphene | Hydrothermal method | 255 F/g (0.5 A/g) | [69] |
| MnO2/rGO | Electrostatic self-assembly + Hydrothermal method | 456 F/g (1 A/g) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Chen, L.; Chen, L.; Tian, G.; Ji, M.; Zhou, S. Layer-by-Layer Heterostructure of MnO2@Reduced Graphene Oxide Composites as High-Performance Electrodes for Supercapacitors. Membranes 2022, 12, 1044. https://doi.org/10.3390/membranes12111044
Liu T, Chen L, Chen L, Tian G, Ji M, Zhou S. Layer-by-Layer Heterostructure of MnO2@Reduced Graphene Oxide Composites as High-Performance Electrodes for Supercapacitors. Membranes. 2022; 12(11):1044. https://doi.org/10.3390/membranes12111044
Chicago/Turabian StyleLiu, Tingting, Lei Chen, Ling Chen, Guoxing Tian, Mingtong Ji, and Shuai Zhou. 2022. "Layer-by-Layer Heterostructure of MnO2@Reduced Graphene Oxide Composites as High-Performance Electrodes for Supercapacitors" Membranes 12, no. 11: 1044. https://doi.org/10.3390/membranes12111044
APA StyleLiu, T., Chen, L., Chen, L., Tian, G., Ji, M., & Zhou, S. (2022). Layer-by-Layer Heterostructure of MnO2@Reduced Graphene Oxide Composites as High-Performance Electrodes for Supercapacitors. Membranes, 12(11), 1044. https://doi.org/10.3390/membranes12111044





