Carbon-Nanowall Microporous Layers for Proton Exchange Membrane Fuel Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Nanowalls Synthesis
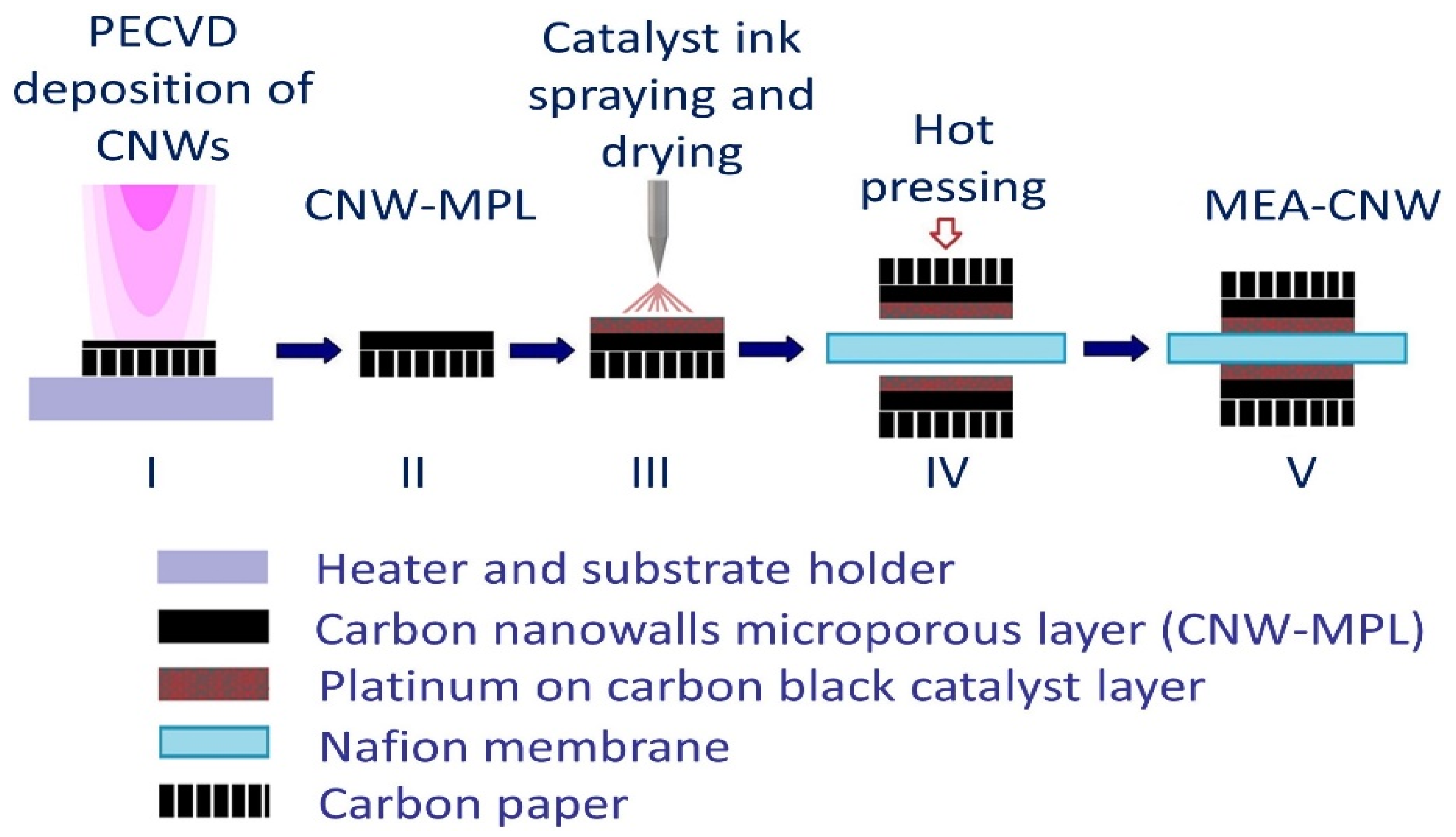
2.2. Membrane–Electrode Assemblies
2.3. Characterization Methods
3. Results and Discussions
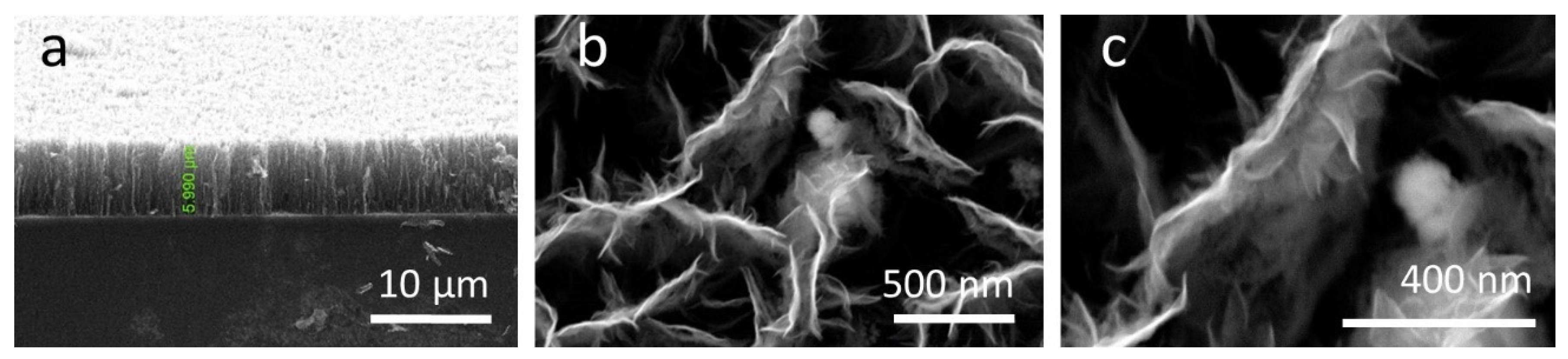
3.1. Morphological Properties
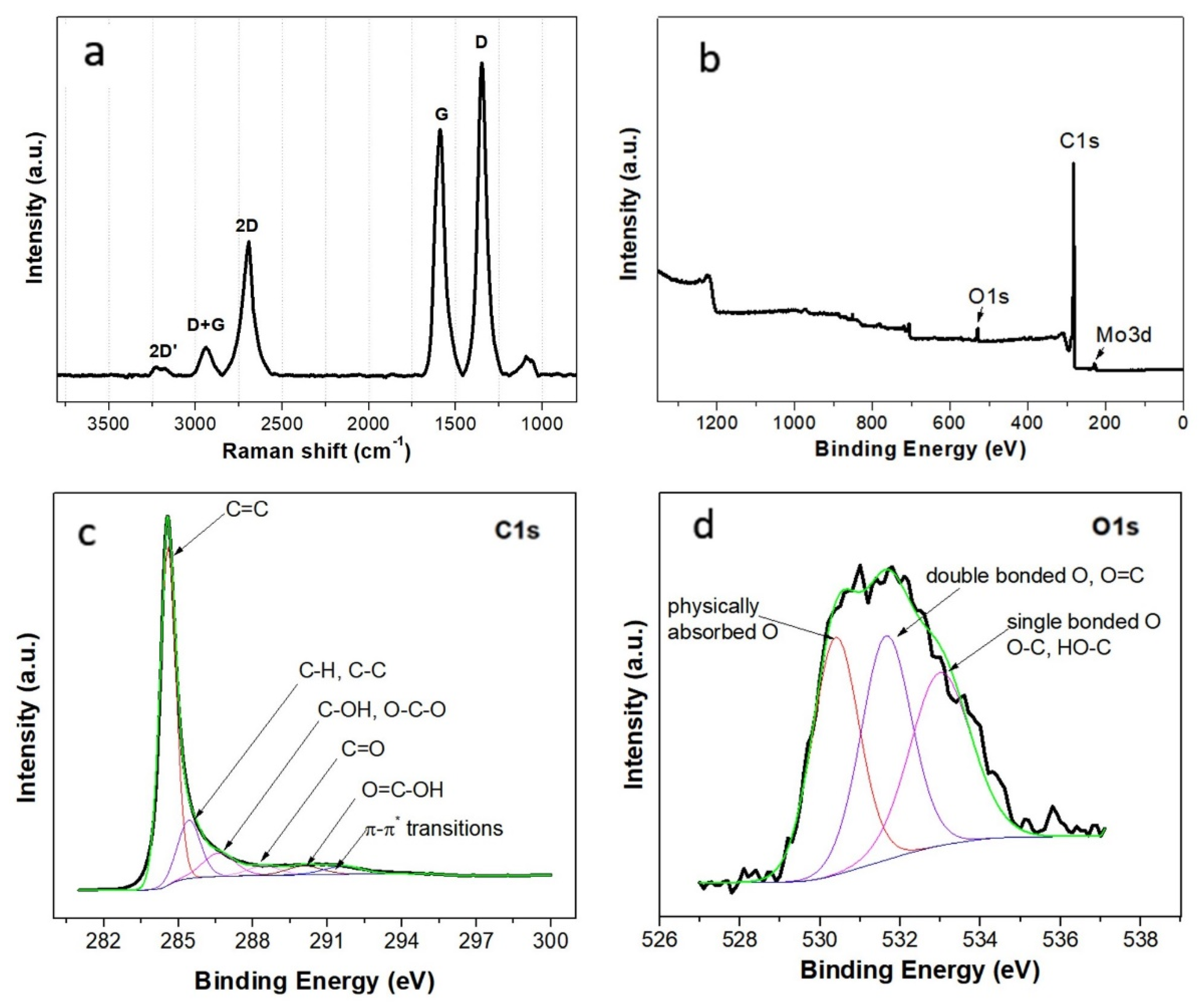
3.2. Structural Characterization
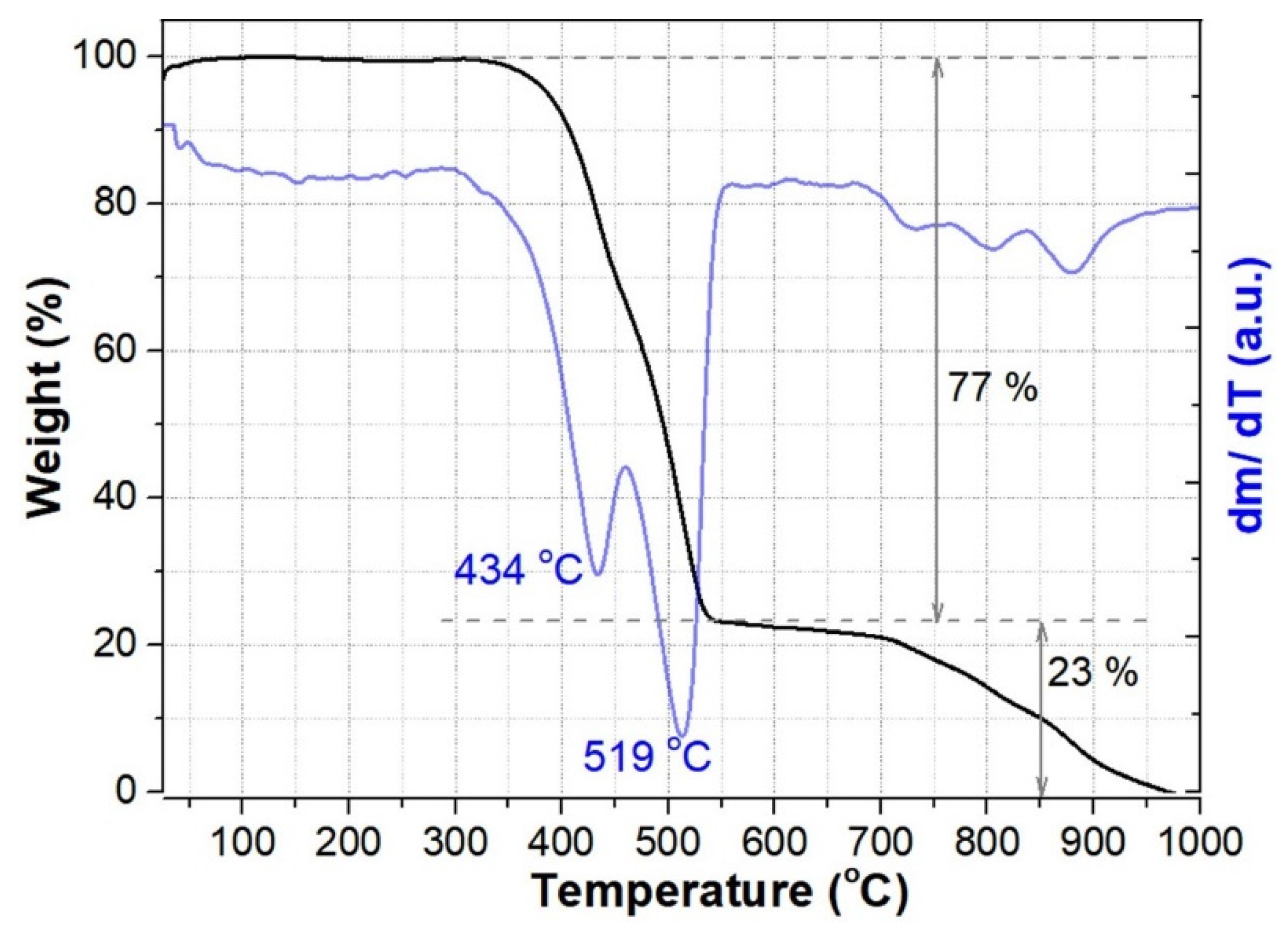
3.3. Thermal Analysis
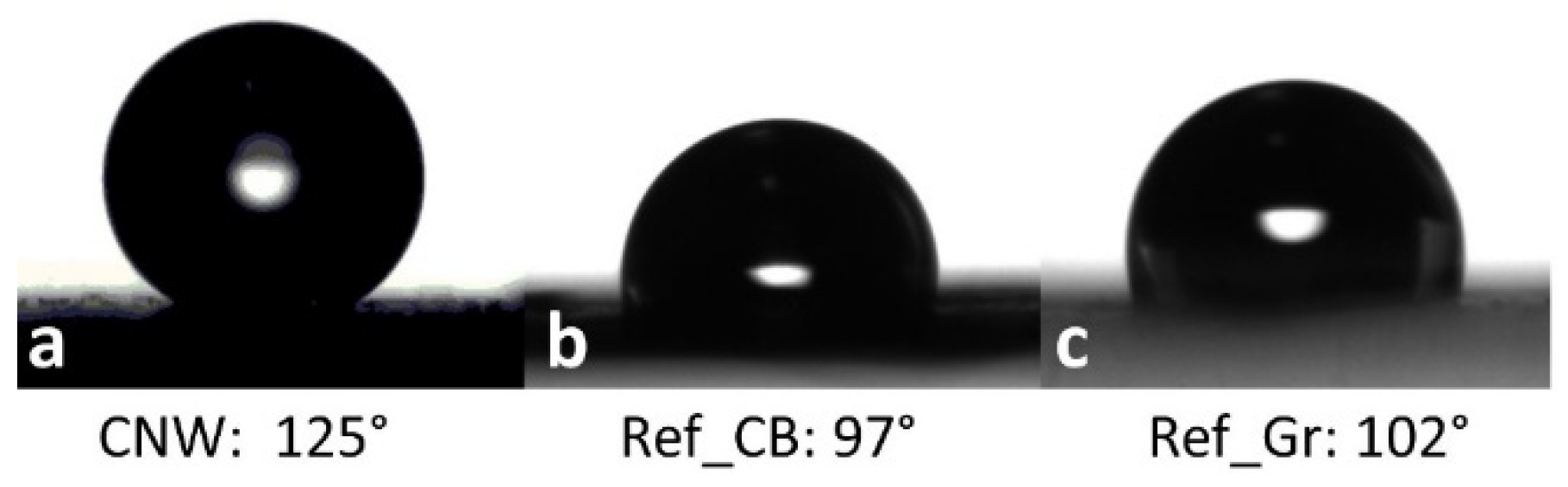
3.4. Physical Properties
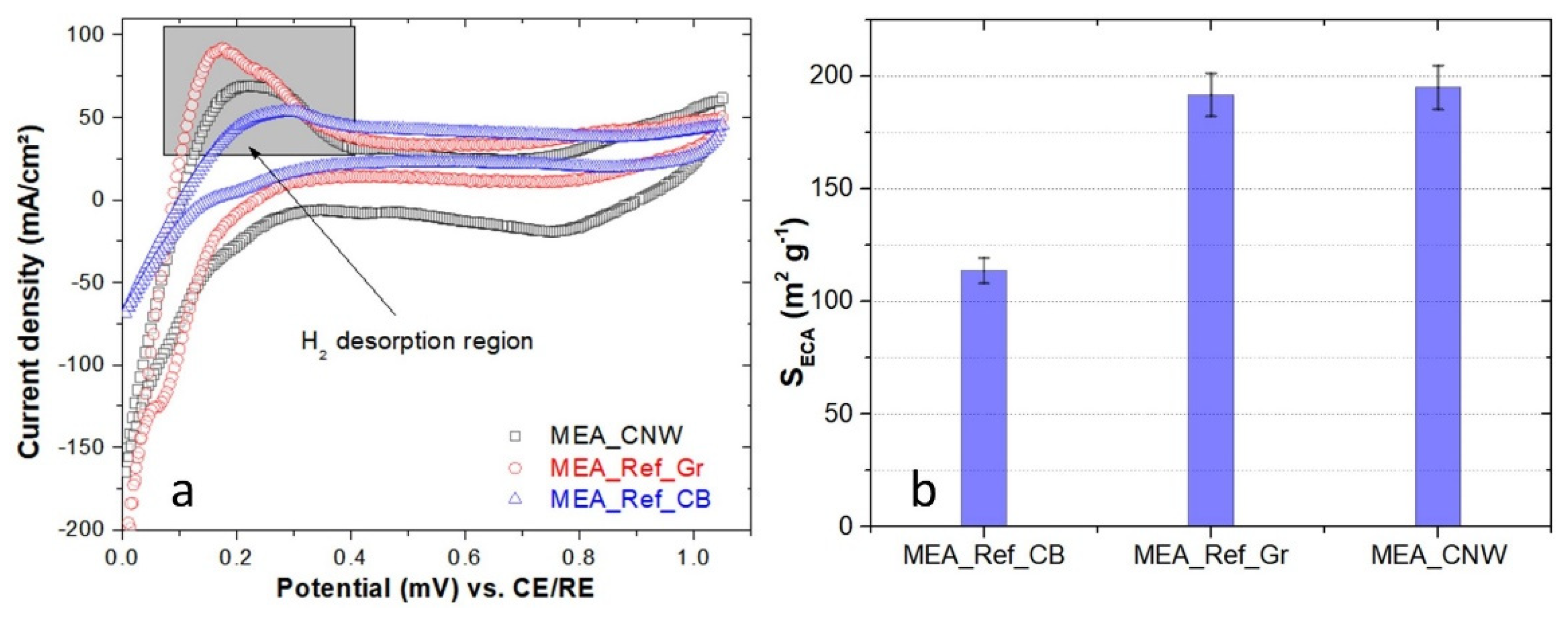
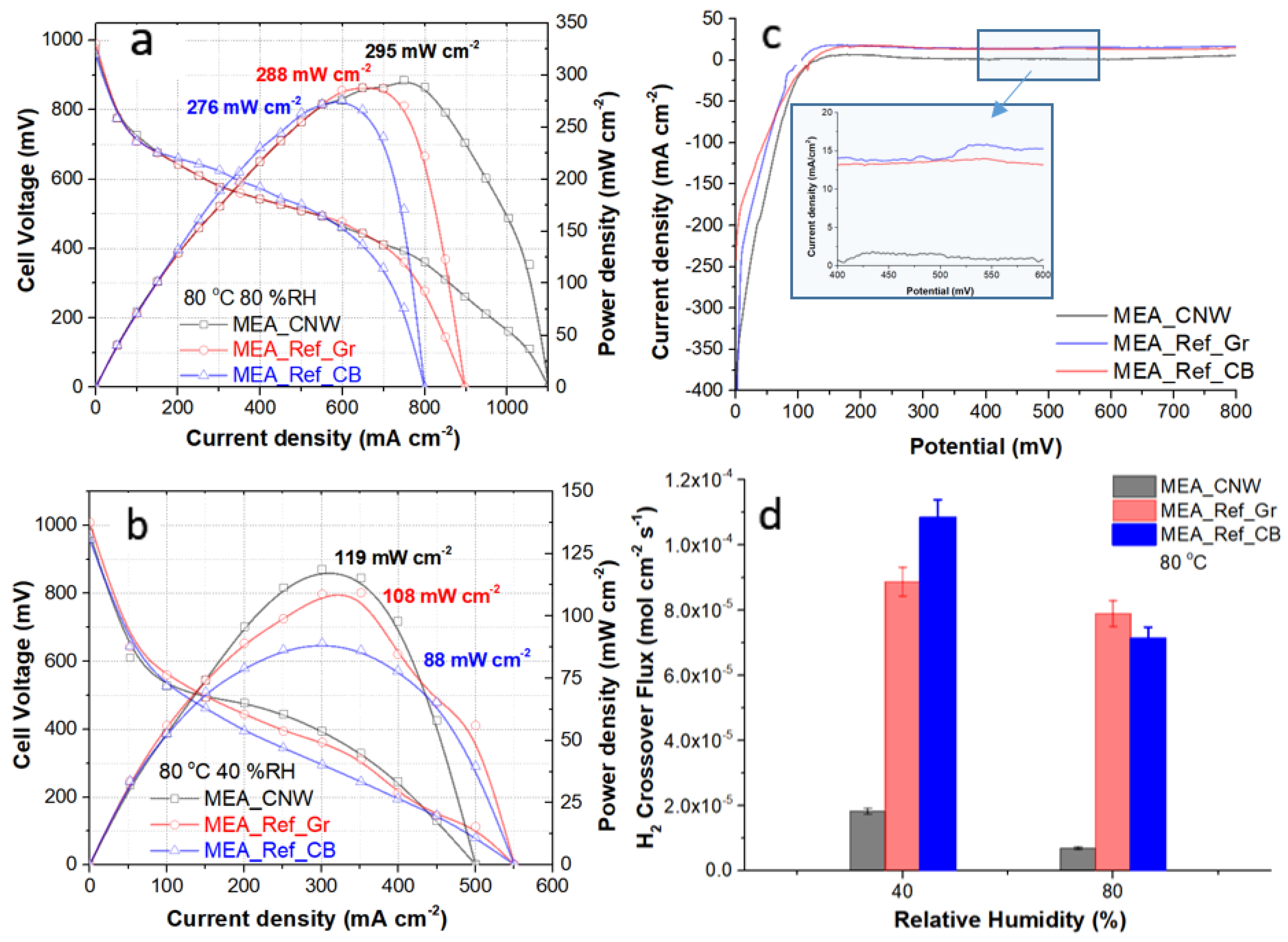
3.5. Fuel Cell Performance Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vielstich, W.; Gasteiger, H.A.; Lamm, A. Handbook of Fuel Cells—Fundamentals, Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Samsun, R.C.; Rex, M.; Antoni, L.; Stolten, D. Deployment of Fuel Cell Vehicles and Hydrogen Refueling Station Infrastructure: A Global Overview and Perspectives. Energies 2022, 15, 4975. [Google Scholar] [CrossRef]
- Yousfi-Steiner, N.; Moçotéguy, P.; Candusso, D.; Hissel, D.; Hernandez, A.; Aslanides, A. A review on PEM voltage degradation associated with water management: Impacts, influent factors and characterization. J. Power Sources 2008, 183, 260–274. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Regnet, F.; Jörissen, L. Graphitized Carbon: A Promising Stable Cathode Catalyst Support Material for Long Term PEMFC Applications. Materials 2018, 11, 907. [Google Scholar] [CrossRef]
- Cho, J.; Oh, H.; Park, J.; Min, K.; Lee, E.; Jyoung, J.Y. Effect of the microporous layer design on the dynamic performance of a proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2014, 39, 459–468. [Google Scholar] [CrossRef]
- Kreitmeier, S.; Wokaun, A.; Büchi, F.N. Local Catalyst Support Degradation during Polymer Electrolyte Fuel Cell Start-Up and Shutdown. J. Electrochem. Soc. 2012, 159, F787–F793. [Google Scholar] [CrossRef]
- Roen, L.M.; Paik, C.H.; Jarvi, T.D. Electrocatalytic Corrosion of Carbon Support in PEMFC Cathodes. Electrochem. Solid-State Lett. 2004, 7, A19–A22. [Google Scholar] [CrossRef]
- Tiliakos, A.; Trefilov, A.M.I.; Tanasă, E.; Balan, A.; Stamatin, I. Laser-induced graphene as the microporous layer in proton exchange membrane fuel cells. Appl. Surf. Sci. 2020, 504, 144096. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, G.; Yu, X.; Hou, M.; Shao, Z.; Hong, S.; Mu, C. Carbon nanotubes grown in situ on carbon paper as a microporous layer for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 8958–8965. [Google Scholar] [CrossRef]
- Leeuwner, M.J.; Wilkinson, D.P.; Gyenge, E.L. Novel graphene foam microporous layers for PEM fuel cells: Interfacial characteristics and comparative performance. Fuel Cells 2015, 15, 790–801. [Google Scholar] [CrossRef]
- Imai, S.; Kondo, H.; Hyungjun, C.; Ishikawa, K.; Tsutsumi, T.; Sekine, M.; Hiramatsu, M.; Hori, M. Pt nanoparticle-supported carbon nanowalls electrode with improved durability for fuel cell applications using C2F6/H2 plasma-enhanced chemical vapor deposition. Appl. Phys. Express 2018, 12, 015001. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, P.; Chong, T.; Shen, Z. Carbon Nanowalls Grown by Microwave Plasma Enhanced Chemical Vapor Deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Santhosh, N.M.; Filipič, G.; Tatarova, E.; Baranov, O.; Kondo, H.; Sekine, M.; Hori, M.; Ostrikov, K.; Cvelbar, U. Oriented Carbon Nanostructures by Plasma Processing: Recent Advances and Future Challenges. Micromachines 2018, 9, 565. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D. Scalable production of graphene via wet chemistry: Progress and challenges. Mater. Today 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Hori, M. Carbon Nanowalls: Synthesis and Emerging Applications; Springer: Wien, Germany, 2010; ISBN 9783211997178. [Google Scholar]
- Yerlanuly, Y.; Zhumadilov, R.; Nemkayeva, R.; Uzakbaiuly, B.; Beisenbayev, A.R.; Bakenov, Z.; Ramazanov, T.; Gabdullin, M.; Ng, A.; Brus, V.V.; et al. Physical properties of carbon nanowalls synthesized by the ICP-PECVD method vs. the growth time. Sci. Rep. 2021, 11, 19287. [Google Scholar] [CrossRef]
- Woehrl, N.; Ochedowski, O.; Gottlieb, S.; Shibasaki, K.; Schulz, S. Plasma-enhanced chemical vapor deposition of graphene on copper substrates. AIP Adv. 2014, 4, 047128. [Google Scholar] [CrossRef]
- Vizireanu, S.; Dinescu, G.; Nistor, L.C.; Baibarac, M.; Ruxanda, G.; Stancu, M.; Ciuparu, D. Stability of carbon nanowalls against chemical attack with acid solutions. Phys. E Low Dimens. Syst. Nanostruct. 2013, 47, 59–65. [Google Scholar] [CrossRef]
- González, Z.; Vizireanu, S.; Dinescu, G.; Blanco, C.; Santamaría, R. Carbon nanowalls thin films as nanostructured electrode materials in vanadium redox flow batteries. Nano Energy 2012, 1, 833–839. [Google Scholar] [CrossRef]
- Dinh, T.M.; Achour, A.; Vizireanu, S.; Dinescu, G.; Nistor, L.; Armstrong, K.; Guay, D.; Pech, D. Hydrous RuO2/carbon nanowalls hierarchical structures for all-solid-state ultrahigh-energy-density micro-supercapacitors. Nano Energy 2014, 10, 288–294. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, G.; Jeong, S.M.; Rout, C.S. A review on supercapacitors based on plasma enhanced chemical vapor deposited vertical graphene arrays. J. Energy Storage 2022, 53, 105212. [Google Scholar] [CrossRef]
- Wu, W.; Ma, H.; Zhang, Z.; Zhang, Z.; Gu, Y.; Gao, W.; Zhou, W.; Zhang, R. In-situ synthesis of a unique 0D/2D porous carbon integrated architecture for high-performance flexible micro-supercapacitors. J. Power Sources 2022, 541, 231687. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, H.; Lee, S.; Lee, G.; Kim, Y.; Choi, W.; Kang, H. Room Temperature Gas Sensor Application of Carbon Nanowalls using Electrical Resistance Change by Surface Adsorption of Toxic Gases. Mater. Res. Bull. 2021, 141, 111377. [Google Scholar] [CrossRef]
- Ohta, T.; Iwata, H.; Hiramatsu, M.; Kondo, H.; Hori, M. Power Generation Characteristics of Polymer Electrolyte Fuel Cells Using Carbon Nanowalls as Catalyst Support Material. C 2022, 8, 44. [Google Scholar] [CrossRef]
- Shin, S.-C.; Yoshimura, A.; Matsuo, T.; Mori, M.; Tanimura, M.; Ishihara, A.; Ota, K.; Tachibana, M. Carbon nanowalls as platinum support for fuel cells. J. Appl. Phys. 2011, 110, 104308. [Google Scholar] [CrossRef]
- Imai, S.; Kondo, H.; Hyungjun, C.; Ishikawa, K.; Tsutsumi, T.; Sekine, M.; Hiramatsu, M.; Hori, M. Effects of three-dimensional structure on electrochemical oxygen reduction characteristics of Pt-nanoparticle-supported carbon nanowalls. J. Phys. D 2019, 52, 105503. [Google Scholar] [CrossRef]
- Vizireanu, S.; Stoica, S.D.; Mitu, B.; Husanu, M.A.; Galca, A.; Nistor, L.; Dinescu, G. Carbon Nanowalls Growth by Radiofrequency Plasma-Beam-Enhanced Chemical Vapor Deposition. Plasma Process. Polym. 2008, 5, 263–268. [Google Scholar] [CrossRef]
- Trefilov, A.M.; Tiliakos, A.; Serban, E.C.; Ceaus, C.; Iordache, S.M.; Voinea, S.; Balan, A. Carbon xerogel as gas diffusion layer in PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 10448–10454. [Google Scholar] [CrossRef]
- Trefilov, A.M.I.; Balan, A.; Stamatin, I. Hybrid Proton-Exchange Membrane Based on Perfluorosulfonated Polymers and Resorcinol–Formaldehyde Hydrogel. Polymers 2021, 13, 4123. [Google Scholar] [CrossRef]
- Holton, O.T.; Stevenson, J.W. The Role of Platinum in Proton Exchange Membrane Fuel Cells. Platin. Met. Rev. 2013, 57, 259. [Google Scholar] [CrossRef]
- Bita, B.; Vizireanu, S.; Stoica, D.; Ion, V.; Yehia, S.; Radu, A.; Iftimie, S.; Dinescu, G. On the Structural, Morphological, and Electrical Properties of Carbon Nanowalls Obtained by Plasma-Enhanced Chemical Vapor Deposition. J. Nanomater. 2020, 2020, 814459. [Google Scholar] [CrossRef]
- Azizighannad, S.; Mitra, S. Stepwise Reduction of Graphene Oxide (GO) and Its Effects on Chemical and Colloidal Properties. Sci. Rep. 2018, 8, 10083. [Google Scholar] [CrossRef] [PubMed]
- Paillard, V. On the origin of the 1100 cm−1 Raman band in amorphous and nanocrystalline sp3 carbon. Europhys. Lett. 2001, 54, 194–198. [Google Scholar] [CrossRef]
- Liu, R.; Chi, Y.; Fang, L.; Tang, Z.; Yi, X. Synthesis of carbon nanowall by plasma-enhanced chemical vapor deposition method. J. Nanosci. Nanotechnol. 2014, 14, 1647–1657. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, S.; Kang, H.; Kim, J.H.; Choi, W. Adhesion-increased carbon nanowalls for the electrodes of energy storage systems. Energies 2019, 12, 4759. [Google Scholar] [CrossRef]
- Vizireanu, S.; Ionita, M.D.; Ionita, R.E.; Stoica, S.D.; Teodorescu, C.M.; Husanu, M.A.; Apostol, N.G.; Baibarac, M.; Panaitescu, D.; Dinescu, G. Aging phenomena and wettability control of plasma deposited carbon nanowall layers. Plasma Process. Polym. 2017, 14, 1700023. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, B.; Itkis, M.E.; Haddon, R.C. Nitric acid purification of single-walled carbon nanotubes. J. Phys. Chem. B. 2003, 107, 13838–13842. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y. A simple purification for single-walled carbon nanotubes. Phys. E Low-Dimens. Syst. Nanostruct. 2005, 28, 309–312. [Google Scholar] [CrossRef]
- Luxembourg, D.; Flamant, G.; Laplaze, D. Solar synthesis of single-walled carbon nanotubes at medium scale. Carbon 2005, 43, 2302–2310. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Kurmashov, P.B. Thermal analysis of carbon nanomaterials: Advantages and problems of interpretation. J. Therm. Anal. Calorim. 2020, 142, 349–370. [Google Scholar] [CrossRef]
- Chen, D.; Tao, Q.; Liao, L.W.; Liu, S.X.; Chen, Y.X.; Ye, S. Determining the active surface area for various platinum electrodes. Electrocatalysis 2011, 2, 207–219. [Google Scholar] [CrossRef]
- Shao, M.; Odell, J.H.; Choi, S.I.; Xia, Y. Electrochemical surface area measurements of platinum-and palladium-based nanoparticles. Electrochem. Commun. 2013, 31, 46–48. [Google Scholar] [CrossRef]
- Sim, J.; Kang, M.; Min, K.; Lee, E.; Jyoung, J.I. Effect of capillary pressure gradient in microporous layer on proton exchange membrane fuel cell performance. Int. J. Hydrogen Energy 2022, 47, 17762–17777. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, A.E.; Bita, B.I.; Vizireanu, S.; Dinescu, G.; Stamatin, I.; Trefilov, A.M.I. Carbon-Nanowall Microporous Layers for Proton Exchange Membrane Fuel Cell. Membranes 2022, 12, 1064. https://doi.org/10.3390/membranes12111064
Balan AE, Bita BI, Vizireanu S, Dinescu G, Stamatin I, Trefilov AMI. Carbon-Nanowall Microporous Layers for Proton Exchange Membrane Fuel Cell. Membranes. 2022; 12(11):1064. https://doi.org/10.3390/membranes12111064
Chicago/Turabian StyleBalan, Adriana Elena, Bogdan Ionut Bita, Sorin Vizireanu, Gheorghe Dinescu, Ioan Stamatin, and Alexandra Maria Isabel Trefilov. 2022. "Carbon-Nanowall Microporous Layers for Proton Exchange Membrane Fuel Cell" Membranes 12, no. 11: 1064. https://doi.org/10.3390/membranes12111064
APA StyleBalan, A. E., Bita, B. I., Vizireanu, S., Dinescu, G., Stamatin, I., & Trefilov, A. M. I. (2022). Carbon-Nanowall Microporous Layers for Proton Exchange Membrane Fuel Cell. Membranes, 12(11), 1064. https://doi.org/10.3390/membranes12111064








