Clinical Significance of Lipid Transport Function of ABC Transporters in the Innate Immune System
Abstract
:1. Introduction
2. The Innate Immune System
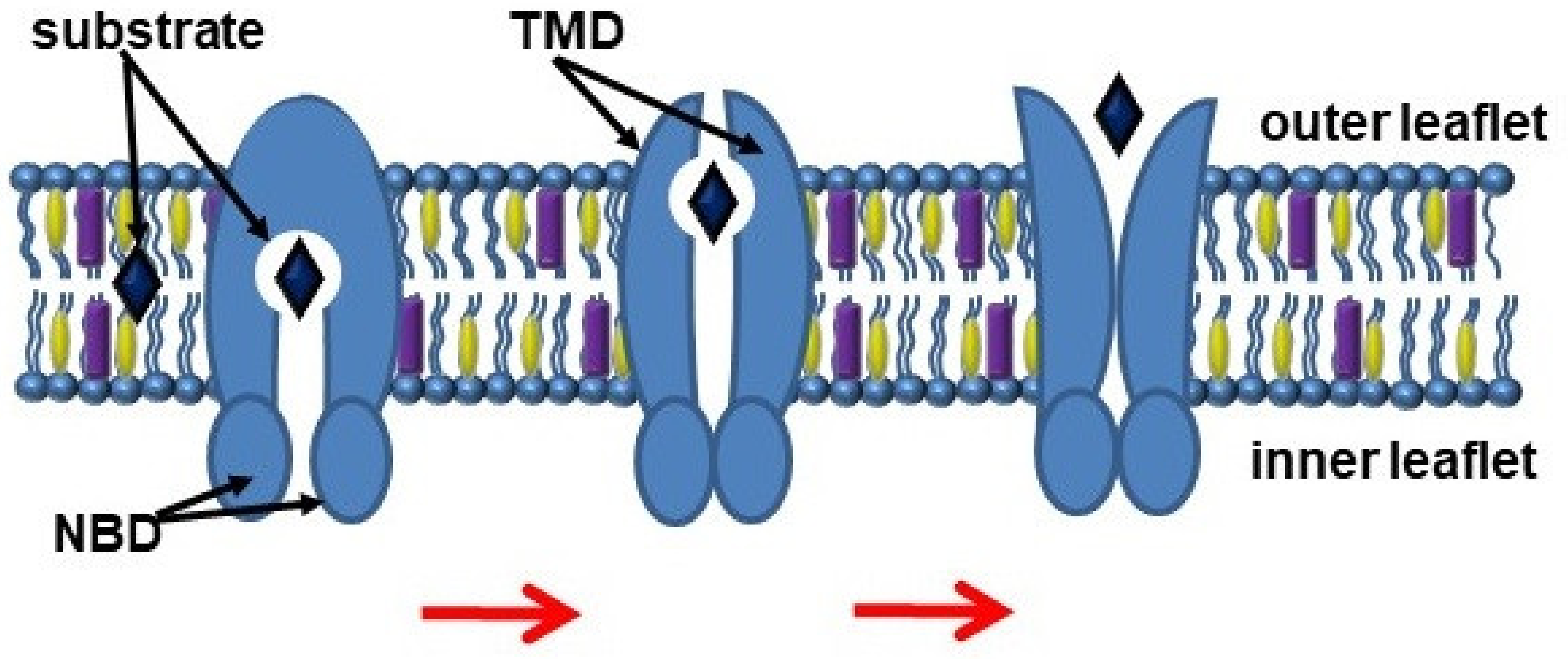
3. ABC Transporters
3.1. The ABCA Subfamily
3.2. The ABCB Subfamily
3.3. The ABCC Subfamily
3.4. The ABCG Subfamily
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Tian, F.; Cai, L.; Zhang, J.; Liu, J.; Zeng, X. Identification of candidate ATP-binding cassette transporter gene family members in Diaphorina citri (Hemiptera: Psyllidae) via adult tissues transcriptome analysis. Sci. Rep. 2019, 9, 15842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, R.J.; Locher, K.P. Structure of a bacterial multidrug ABC transporter. Nature 2006, 443, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Kotlyarova, A. The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 6711. [Google Scholar] [CrossRef] [PubMed]
- Jardetzky, O. Simple allosteric model for membrane pumps. Nature 1966, 211, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Eggensperger, S.; Tampé, R. The transporter associated with antigen processing: A key player in adaptive immunity. Biol. Chem. 2015, 396, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018, 59, 749–763. [Google Scholar] [CrossRef] [Green Version]
- Thélot, F.; Orlando, B.J.; Li, Y.; Liao, M. High-resolution views of lipopolysaccharide translocation driven by ABC transporters MsbA and LptB(2)FGC. Curr. Opin. Struct. Biol. 2020, 63, 26–33. [Google Scholar] [CrossRef]
- Bonifer, C.; Glaubitz, C. MsbA: An ABC transporter paradigm. Biochem. Soc. Trans. 2021, 49, 2917–2927. [Google Scholar] [CrossRef]
- Doerrler, W.T.; Reedy, M.C.; Raetz, C.R. An Escherichia coli mutant defective in lipid export. J. Biol. Chem. 2001, 276, 11461–11464. [Google Scholar] [CrossRef] [Green Version]
- Doerrler, W.T.; Gibbons, H.S.; Raetz, C.R. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J. Biol. Chem. 2004, 279, 45102–45109. [Google Scholar] [CrossRef]
- Zhou, Z.; White, K.A.; Polissi, A.; Georgopoulos, C.; Raetz, C.R. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J. Biol. Chem. 1998, 273, 12466–12475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandeo, P.; Lau, F.K.; Carpentieri, A.; De Castro, C.; Molinaro, A.; Dehò, G.; Silhavy, T.J.; Polissi, A. Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli. J. Bacteriol. 2008, 190, 4460–4469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M.K.; Miu, A.; Oh, A.; Reichelt, M.; Ho, H.; Chalouni, C.; Labadie, S.; Wang, L.; Liang, J.; Nickerson, N.N.; et al. Disrupting Gram-Negative Bacterial Outer Membrane Biosynthesis through Inhibition of the Lipopolysaccharide Transporter MsbA. Antimicrob. Agents Chemother. 2018, 62, e01142-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raetz, C.R.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, W.; Li, Y.; Yoon, S.H.; Ernst, R.K.; Walz, T.; Liao, M. Structural basis of MsbA-mediated lipopolysaccharide transport. Nature 2017, 549, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Buchmann, K. Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, L.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 1983. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26846/ (accessed on 7 October 2022).
- Castrillo, A.; Joseph, S.B.; Vaidya, S.A.; Haberland, M.; Fogelman, A.M.; Cheng, G.; Tontonoz, P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 2003, 12, 805–816. [Google Scholar] [CrossRef]
- Hansson, G.r.K.; Libby, P.; Schönbeck, U.; Yan, Z.-Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Cory, T.J.; He, H.; Winchester, L.C.; Kumar, S.; Fletcher, C.V. Alterations in P-Glycoprotein Expression and Function Between Macrophage Subsets. Pharm. Res. 2016, 33, 2713–2721. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Ma, C.; Li, J.; You, S.; Dang, L.; Wu, J.; Hao, Z.; Li, J.; Zhi, Y.; Chen, L.; et al. Proteomic characterization of four subtypes of M2 macrophages derived from human THP-1 cells. J. Zhejiang Univ. Sci. B 2022, 23, 407–422. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Buckley, M.; Britton, B.; Mu, Y.; Warner, K.; Kumar, S.; Cory, T.J. Polarized macrophage subsets differentially express the drug efflux transporters MRP1 and BCRP, resulting in altered HIV production. Antivir. Chem. Chemother. 2018, 26, 2040206617745168. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, M.J.; Teboul, I.; Voloshyna, I.; Reiss, A.B.; Reiss, A.B. Polarization of Human THP-1 Macrophages: Link between Adenosine Receptors, Inflammation and Lipid Accumulation. Int. J. Immunol. Immunother. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, M.E.; Kajani, S.; Ralston, J.C.; Lenighan, Y.M.; Roche, H.M.; McGillicuddy, F.C. Nutritionally Derived Metabolic Cues Typical of the Obese Microenvironment Increase Cholesterol Efflux Capacity of Adipose Tissue Macrophages. Mol. Nutr. Food Res. 2019, 63, 1800713. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Tarling, E.J.; McMillen, T.S.; Tang, C.; LeBoeuf, R.C. ABCG1 regulates mouse adipose tissue macrophage cholesterol levels and ratio of M1 to M2 cells in obesity and caloric restriction. J. Lipid Res. 2015, 56, 2337–2347. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Tang, X.; Zhang, Z.; Dong, C. Structural insight into lipopolysaccharide transport from the Gram-negative bacterial inner membrane to the outer membrane. Biochim. Biophys. Acta Mol Cell Biol. Lipids 2017, 1862, 1461–1467. [Google Scholar] [CrossRef]
- Li, H.; Sun, B. Toll-like receptor 4 in atherosclerosis. J. Cell. Mol. Med. 2007, 11, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, E.; Ding, J.L.; Byrne, B. SARM modulates MyD88-mediated TLR activation through BB-loop dependent TIR-TIR interactions. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 244–253. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A. MyD88 Is an Adaptor Protein in the hToll/IL-1 Receptor Family Signaling Pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Mori, K.; Hoshino, K.; Takeuchi, O.; Takeda, K.; Akira, S. Cutting Edge: A Novel Toll/IL-1 Receptor Domain-Containing Adapter That Preferentially Activates the IFN-β Promoter in the Toll-Like Receptor Signaling. J. Immunol. 2002, 169, 6668–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Uematsu, S.; Hoshino, K.; Kaisho, T.; Takeuchi, O.; Takeda, K.; Akira, S. TRAM is specifically involved in the Toll-like receptor 4–mediated MyD88-independent signaling pathway. Nat. Immunol. 2003, 4, 1144–1150. [Google Scholar] [CrossRef]
- Imler, J.L.; Hoffmann, J.A. Toll receptors in innate immunity. Trends Cell Biol. 2001, 11, 304–311. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Welch, C.; Pagler, T.A.; Ranalletta, M.; Lamkanfi, M.; Han, S.; Ishibashi, M.; Li, R.; Wang, N.; Tall, A.R. Increased inflammatory gene expression in ABC transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008, 118, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerterp, M.; Gautier, E.L.; Ganda, A.; Molusky, M.M.; Wang, W.; Fotakis, P.; Wang, N.; Randolph, G.J.; D’Agati, V.D.; Yvan-Charvet, L.; et al. Cholesterol Accumulation in Dendritic Cells Links the Inflammasome to Acquired Immunity. Cell Metab. 2017, 25, 1294–1304.e1296. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, M.; Fotakis, P.; Ouimet, M.; Bochem, A.E.; Zhang, H.; Molusky, M.M.; Wang, W.; Abramowicz, S.; la Bastide-van Gemert, S.; Wang, N.; et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation 2018, 138, 898–912. [Google Scholar] [CrossRef] [Green Version]
- Quazi, F.; Molday, R.S. Lipid transport by mammalian ABC proteins. Essays Biochem. 2011, 50, 265–290. [Google Scholar] [CrossRef] [Green Version]
- Francone, O.L.; Royer, L.; Boucher, G.; Haghpassand, M.; Freeman, A.; Brees, D.; Aiello, R.J. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1198–1205. [Google Scholar] [CrossRef]
- Lawn, R.M.; Wade, D.P.; Garvin, M.R.; Wang, X.; Schwartz, K.; Porter, J.G.; Seilhamer, J.J.; Vaughan, A.M.; Oram, J.F. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Investig. 1999, 104, R25–R31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld, E.B.; Remaley, A.T.; Demosky, S.J.; Stonik, J.A.; Cooney, A.M.; Comly, M.; Dwyer, N.K.; Zhang, M.; Blanchette-Mackie, J.; Santamarina-Fojo, S.; et al. Cellular localization and trafficking of the human ABCA1 transporter. J. Biol. Chem. 2001, 276, 27584–27590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singaraja, R.R.; Kang, M.H.; Vaid, K.; Sanders, S.S.; Vilas, G.L.; Arstikaitis, P.; Coutinho, J.; Drisdel, R.C.; El-Husseini Ael, D.; Green, W.N.; et al. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ. Res. 2009, 105, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Landry, Y.D.; Denis, M.; Nandi, S.; Bell, S.; Vaughan, A.M.; Zha, X. ATP-binding Cassette Transporter A1 Expression Disrupts Raft Membrane Microdomains through Its ATPase-related Functions. J. Biol. Chem. 2006, 281, 36091–36101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, O.; Ito, S.; Kato, R.; Shimizu, Y.; Kobayashi, A.; Kimura, Y.; Kioka, N.; Hanada, K.; Ueda, K.; Matsuo, M. ABCA1, ABCG1, and ABCG4 are distributed to distinct membrane meso-domains and disturb detergent-resistant domains on the plasma membrane. PLoS ONE 2014, 9, e109886. [Google Scholar] [CrossRef] [Green Version]
- Mendez, A.J.; Lin, G.; Wade, D.P.; Lawn, R.M.; Oram, J.F. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J. Biol. Chem. 2001, 276, 3158–3166. [Google Scholar] [CrossRef] [Green Version]
- Iatan, I.; Bailey, D.; Ruel, I.; Hafiane, A.; Campbell, S.; Krimbou, L.; Genest, J. Membrane microdomains modulate oligomeric ABCA1 function: Impact on apoAI-mediated lipid removal and phosphatidylcholine biosynthesis. J. Lipid Res. 2011, 52, 2043–2055. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, Y.; Yokoyama, S.; Chang, T.Y. ABCA1-dependent sterol release: Sterol molecule specificity and potential membrane domain for HDL biogenesis. J. Lipid Res. 2016, 57, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Witting, S.R.; Maiorano, J.N.; Davidson, W.S. Ceramide enhances cholesterol efflux to apolipoprotein A-I by increasing the cell surface presence of ATP-binding cassette transporter A1. J. Biol. Chem. 2003, 278, 40121–40127. [Google Scholar] [CrossRef]
- Fantini, J.; Epand, R.M.; Barrantes, F.J. Cholesterol-recognition motifs in membrane proteins. Direct Mech. Cholest. Modul. Protein Funct. 2019, 3–25. [Google Scholar]
- Ruysschaert, J.-M.; Lonez, C. Role of lipid microdomains in TLR-mediated signalling. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Kusnadi, A.; Park, S.H.; Yuan, R.; Pannellini, T.; Giannopoulou, E.; Oliver, D.; Lu, T.; Park-Min, K.H.; Ivashkiv, L.B. The Cytokine TNF Promotes Transcription Factor SREBP Activity and Binding to Inflammatory Genes to Activate Macrophages and Limit Tissue Repair. Immunity 2019, 51, 241–257.e249. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.J.; Rao, G.; Jones, P.M.; Glancey, E.; Aleidi, S.M.; George, A.M.; Brown, A.J.; Gelissen, I.C. Cholesterol sensing by the ABCG1 lipid transporter: Requirement of a CRAC motif in the final transmembrane domain. Biochim. Biophys. Acta. 2015, 1851, 956–964. [Google Scholar] [CrossRef]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef]
- Zhu, X.; Owen, J.S.; Wilson, M.D.; Li, H.; Griffiths, G.L.; Thomas, M.J.; Hiltbold, E.M.; Fessler, M.B.; Parks, J.S. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010, 51, 3196–3206. [Google Scholar] [CrossRef] [Green Version]
- Frisdal, E.; Lesnik, P.; Olivier, M.; Robillard, P.; Chapman, M.J.; Huby, T.; Guerin, M.; Le Goff, W. Interleukin-6 protects human macrophages from cellular cholesterol accumulation and attenuates the proinflammatory response. J. Biol. Chem. 2011, 286, 30926–30936. [Google Scholar] [CrossRef] [Green Version]
- Azzam, K.M.; Fessler, M.B. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol. Metab. 2012, 23, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.A.; Gauthier, K.C.; Varley, A.W.; Kitchens, R.L. ABCA1 promotes the efflux of bacterial LPS from macrophages and accelerates recovery from LPS-induced tolerance. J. Lipid Res. 2010, 51, 2672–2685. [Google Scholar] [CrossRef] [Green Version]
- Burnett, J.R.; Hooper, A.J.; McCormick, S.P.A.; Hegele, R.A. Tangier Disease. In GeneReviews(®); Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Liu, H.-F.; Cui, K.-F.; Wang, J.-P.; Zhang, M.; Guo, Y.-P.; Li, X.-Y.; Jiang, C. Significance of ABCA1 in human carotid atherosclerotic plaques. Exp. Ther. Med. 2012, 4, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Soumian, S.; Amey, J.S.; Sardini, A.; Higgins, C.F.; Davies, A.H.; Gibbs, R.G.J. ABCA1 Expression in Carotid Atherosclerotic Plaques. Stroke 2004, 35, 2801–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eck, M.; Bos, I.S.; Kaminski, W.E.; Orsó, E.; Rothe, G.; Twisk, J.; Böttcher, A.; Van Amersfoort, E.S.; Christiansen-Weber, T.A.; Fung-Leung, W.P.; et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 6298–6303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.; Gautier, T.; Masson, D. Non-lipogenic ABCA1 inducers: The holy grail in cardio-metabolic diseases? eBioMedicine 2021, 66, 103324. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Ren, Y.; Holm, M.L.; Asmann, Y.W.; Alam, A.; Fitzgerald, M.L.; Bu, G.; Kanekiyo, T. ABCA7 Regulates Brain Fatty Acid Metabolism During LPS-Induced Acute Inflammation. Front. Neurosci. 2021, 15, 647974. [Google Scholar] [CrossRef]
- Tanaka, N.; Abe-Dohmae, S.; Iwamoto, N.; Yokoyama, S. Roles of ATP-binding cassette transporter A7 in cholesterol homeostasis and host defense system. J. Atheroscler. Thromb. 2011, 18, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Jehle, A.W.; Gardai, S.J.; Li, S.; Linsel-Nitschke, P.; Morimoto, K.; Janssen, W.J.; Vandivier, R.W.; Wang, N.; Greenberg, S.; Dale, B.M.; et al. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J. Cell Biol. 2006, 174, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Abe-Dohmae, S.; Iwamoto, N.; Fitzgerald, M.L.; Yokoyama, S. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J. Lipid Res. 2010, 51, 2591–2599. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Lan, D.; Gerbod-Giannone, M.; Linsel-Nitschke, P.; Jehle, A.W.; Chen, W.; Martinez, L.O.; Tall, A.R. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 2003, 278, 42906–42912. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Abe-Dohmae, S.; Munehira, Y.; Aoki, R.; Kawamoto, S.; Furuya, A.; Shitara, K.; Amachi, T.; Kioka, N.; Matsuo, M.; et al. Posttranscriptional regulation of human ABCA7 and its function for the apoA-I-dependent lipid release. Biochem. Biophys. Res. Commun. 2003, 311, 313–318. [Google Scholar] [CrossRef]
- Sasaki, M.; Shoji, A.; Kubo, Y.; Nada, S.; Yamaguchi, A. Cloning of rat ABCA7 and its preferential expression in platelets. Biochem. Biophys. Res. Commun. 2003, 304, 777–782. [Google Scholar] [CrossRef]
- Abe-Dohmae, S.; Ikeda, Y.; Matsuo, M.; Hayashi, M.; Okuhira, K.; Ueda, K.; Yokoyama, S. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 2004, 279, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, N.; Abe-Dohmae, S.; Sato, R.; Yokoyama, S. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J. Lipid Res. 2006, 47, 1915–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petry, F.; Ritz, V.; Meineke, C.; Middel, P.; Kietzmann, T.; Schmitz-Salue, C.; Hirsch-Ernst, K.I. Subcellular localization of rat Abca5, a rat ATP-binding-cassette transporter expressed in Leydig cells, and characterization of its splice variant apparently encoding a half-transporter. Biochem. J. 2006, 393, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, T.; Benndorf, R.A. ABC Transport Proteins in Cardiovascular Disease-A Brief Summary. Molecules 2017, 22, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotlyarov, S.; Kotlyarova, A. Bioinformatic Analysis of ABCA1 Gene Expression in Smoking and Chronic Obstructive Pulmonary Disease. Membranes 2021, 11, 674. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Bulgakov, A. Lipid Metabolism Disorders in the Comorbid Course of Nonalcoholic Fatty Liver Disease and Chronic Obstructive Pulmonary Disease. Cells 2021, 10, 2978. [Google Scholar] [CrossRef]
- Bossennec, M.; Di Roio, A.; Caux, C.; Ménétrier-Caux, C. MDR1 in immunity: Friend or foe? OncoImmunology 2018, 7, e1499388. [Google Scholar] [CrossRef] [Green Version]
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Barreto-Ojeda, E.; Corradi, V.; Gu, R.X.; Tieleman, D.P. Coarse-grained molecular dynamics simulations reveal lipid access pathways in P-glycoprotein. J. Gen. Physiol. 2018, 150, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Sharom, F.J. The P-glycoprotein efflux pump: How does it transport drugs? J. Membr. Biol. 1997, 160, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.; Condic-Jurkic, K.; O’Mara, M.L. Structural and dynamic perspectives on the promiscuous transport activity of P-glycoprotein. Neurochem. Int. 2016, 98, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siarheyeva, A.; Lopez, J.J.; Glaubitz, C. Localization of Multidrug Transporter Substrates within Model Membranes. Biochemistry 2006, 45, 6203–6211. [Google Scholar] [CrossRef]
- Higgins, C.F.; Gottesman, M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992, 17, 18–21. [Google Scholar] [CrossRef]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. The role of size and charge for blood-brain barrier permeation of drugs and fatty acids. J. Mol. Neurosci. 2007, 33, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Gerebtzoff, G.; Seelig, A. In silico prediction of blood-brain barrier permeation using the calculated molecular cross-sectional area as main parameter. J. Chem. Inf. Model. 2006, 46, 2638–2650. [Google Scholar] [CrossRef]
- Neumann, J.; Rose-Sperling, D.; Hellmich, U.A. Diverse relations between ABC transporters and lipids: An overview. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 605–618. [Google Scholar] [CrossRef]
- Batetta, B.; Dessì, S.; Putzolu, M.; Sanna, F.; Spano, O.; Mulas, M.F.; Petruzzo, P.; Cappai, A.; Brotzu, G. MDR1 gene expression in normal and atherosclerotic human arteries(1). J. Vasc. Res. 1999, 36, 261–271. [Google Scholar] [CrossRef]
- van Helvoort, A.; Smith, A.J.; Sprong, H.; Fritzsche, I.; Schinkel, A.H.; Borst, P.; van Meer, G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 1996, 87, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Kioka, N.; Kato, H.; Matsuo, M.; Ueda, K. Modulation of drug-stimulated ATPase activity of human MDR1/P-glycoprotein by cholesterol. Biochem. J. 2007, 401, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, A.; Escargueil, A.E.; Orlowski, S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc. Natl. Acad. Sci. USA 2002, 99, 10347–10352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clay, A.T.; Lu, P.; Sharom, F.J. Interaction of the P-glycoprotein multidrug transporter with sterols. Biochemistry 2015, 54, 6586–6597. [Google Scholar] [CrossRef] [PubMed]
- Bosch, I.; Dunussi-Joannopoulos, K.; Wu, R.-L.; Furlong, S.T.; Croop, J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry 1997, 36, 5685–5694. [Google Scholar] [CrossRef] [PubMed]
- Grybauskas, A.; Koga, T.; Kuprys, P.V.; Nolan, M.; McCarty, R.; Walker, L.; Green, K.A.; Norkett, W.M.; Yue, B.Y.J.T.; Knepper, P.A. ABCB1 transporter and Toll-like receptor 4 in trabecular meshwork cells. Mol. Vis. 2015, 21, 201–212. [Google Scholar] [PubMed]
- Barancík, M.; Bohácová, V.; Kvackajová, J.; Hudecová, S.; Krizanová, O.; Breier, A. SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur. J. Pharm. Sci. 2001, 14, 29–36. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Saegusa, K.; Noguchi, T.; Sadamitsu, C.; Nishitoh, H.; Nagai, S.; Koyasu, S.; Matsumoto, K.; Takeda, K.; Ichijo, H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 2005, 6, 587–592. [Google Scholar] [CrossRef]
- Frank, M.; Hennenberg, E.M.; Eyking, A.; Rünzi, M.; Gerken, G.; Scott, P.; Parkhill, J.; Walker, A.W.; Cario, E. TLR signaling modulates side effects of anticancer therapy in the small intestine. J. Immunol. 2015, 194, 1983–1995. [Google Scholar] [CrossRef] [Green Version]
- Mottaz, H.; Schönenberger, R.; Fischer, S.; Eggen, R.I.L.; Schirmer, K.; Groh, K.J. Dose-dependent effects of morphine on lipopolysaccharide (LPS)-induced inflammation, and involvement of multixenobiotic resistance (MXR) transporters in LPS efflux in teleost fish. Environ. Pollut. 2017, 221, 105–115. [Google Scholar] [CrossRef]
- Kooij, G.; Backer, R.; Koning, J.J.; Reijerkerk, A.; van Horssen, J.; van der Pol, S.M.; Drexhage, J.; Schinkel, A.; Dijkstra, C.D.; den Haan, J.M.; et al. P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS ONE 2009, 4, e8212. [Google Scholar] [CrossRef]
- Sigal, N.; Kaplan Zeevi, M.; Weinstein, S.; Peer, D.; Herskovits, A.A. The human P-glycoprotein transporter enhances the type I interferon response to Listeria monocytogenes infection. Infect. Immun. 2015, 83, 2358–2368. [Google Scholar] [CrossRef]
- Wyska, E. Pretreatment with R(+)-verapamil significantly reduces mortality and cytokine expression in murine model of septic shock. Int. Immunopharmacol. 2009, 9, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Pendse, S.S.; Behjati, S.; Schatton, T.; Izawa, A.; Sayegh, M.H.; Frank, M.H. P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation. Am. J. Transpl. 2006, 6, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.H.; Denton, M.D.; Alexander, S.I.; Khoury, S.J.; Sayegh, M.H.; Briscoe, D.M. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J. Immunol. 2001, 166, 2451–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloberas, N.; Rama, I.; Llaudó, I.; Torras, J.; Cerezo, G.; Cassis, L.; Franquesa, M.; Merino, A.; Benitez-Ribas, D.; Cruzado, J.M.; et al. Dendritic cells phenotype fitting under hypoxia or lipopolysaccharide; adenosine 5’-triphosphate-binding cassette transporters far beyond an efflux pump. Clin. Exp. Immunol. 2013, 172, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J.; Beaulieu, S.; Pope, M.; Sugawara, I.; Hoffman, L.; Steinman, R.M.; Muller, W.A. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc. Natl. Acad. Sci. USA 1998, 95, 6924–6929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggers, R.J.; Vogels, I.; van Meer, G. Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem. J. 2001, 357, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Masuda, M.; Nakai, E.; Furumiya, K.; Togawa, H.; Nakamura, Y.; Kawai, Y.; Nakahira, K.; Shinkai, S.; Takahashi, K. Genuine functions of P-glycoprotein (ABCB1). Curr. Drug Metab. 2008, 9, 167–174. [Google Scholar] [CrossRef]
- Panwala, C.M.; Jones, J.C.; Viney, J.L. A novel model of inflammatory bowel disease: Mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J. Immunol. 1998, 161, 5733–5744. [Google Scholar]
- Foley, S.E.; Tuohy, C.; Dunford, M.; Grey, M.J.; De Luca, H.; Cawley, C.; Szabady, R.L.; Maldonado-Contreras, A.; Houghton, J.M.; Ward, D.V.; et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome 2021, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.E.; Dente, M.J.; Lei, X.; Sallis, B.F.; Loew, E.B.; Meza-Segura, M.; Fitzgerald, K.A.; McCormick, B.A. Microbial Metabolites Orchestrate a Distinct Multi-Tiered Regulatory Network in the Intestinal Epithelium That Directs P-Glycoprotein Expression. mBio 2022, 13, e0199322. [Google Scholar] [CrossRef] [PubMed]
- Mickley, L.A.; Bates, S.E.; Richert, N.D.; Currier, S.; Tanaka, S.; Foss, F.; Rosen, N.; Fojo, A.T. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J. Biol. Chem. 1989, 264, 18031–18040. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e655. [Google Scholar] [CrossRef]
- Szabady, R.L.; Louissaint, C.; Lubben, A.; Xie, B.; Reeksting, S.; Tuohy, C.; Demma, Z.; Foley, S.E.; Faherty, C.S.; Llanos-Chea, A.; et al. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J. Clin. Investig. 2018, 128, 4044–4056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, V.; Svenningsen, K.; Knudsen, L.A.; Hansen, A.K.; Holmskov, U.; Stensballe, A.; Vogel, U. Novel understanding of ABC transporters ABCB1/MDR/P-glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology. World J. Gastroenterol. 2015, 21, 11862–11876. [Google Scholar] [CrossRef] [Green Version]
- Paik, J.; Fierce, Y.; Treuting, P.M.; Brabb, T.; Maggio-Price, L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a−/− male mice. J. Nutr. 2013, 143, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Englund, G.; Jacobson, A.; Rorsman, F.; Artursson, P.; Kindmark, A.; Rönnblom, A. Efflux transporters in ulcerative colitis: Decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm. Bowel Dis. 2007, 13, 291–297. [Google Scholar] [CrossRef]
- Langmann, T.; Moehle, C.; Mauerer, R.; Scharl, M.; Liebisch, G.; Zahn, A.; Stremmel, W.; Schmitz, G. Loss of detoxification in inflammatory bowel disease: Dysregulation of pregnane X receptor target genes. Gastroenterology 2004, 127, 26–40. [Google Scholar] [CrossRef]
- Andersen, V.; Vogel, U.; Godiksen, S.; Frenzel, F.B.; Sæbø, M.; Hamfjord, J.; Kure, E.; Vogel, L.K. Low ABCB1 gene expression is an early event in colorectal carcinogenesis. PLoS ONE 2013, 8, e72119. [Google Scholar] [CrossRef]
- Pazos, M.; Siccardi, D.; Mumy, K.L.; Bien, J.D.; Louie, S.; Shi, H.N.; Gronert, K.; Mrsny, R.J.; McCormick, B.A. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J. Immunol. 2008, 181, 8044–8052. [Google Scholar] [CrossRef] [PubMed]
- Neudeck, B.L.; Loeb, J.M.; Faith, N.G.; Czuprynski, C.J. Intestinal P glycoprotein acts as a natural defense mechanism against Listeria monocytogenes. Infect. Immun. 2004, 72, 3849–3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercado-Lubo, R.; McCormick, B.A. The interaction of gut microbes with host ABC transporters. Gut Microbes 2010, 1, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Siccardi, D.; Mumy, K.L.; Wall, D.M.; Bien, J.D.; McCormick, B.A. Salmonella enterica serovar Typhimurium modulates P-glycoprotein in the intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1392–G1400. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Lubo, R.; Zhang, Y.; Zhao, L.; Rossi, K.; Wu, X.; Zou, Y.; Castillo, A.; Leonard, J.; Bortell, R.; Greiner, D.L.; et al. A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours. Nat. Commun. 2016, 7, 12225. [Google Scholar] [CrossRef] [Green Version]
- Becerra-Báez, E.I.; Meza-Toledo, S.E.; Muñoz-López, P.; Flores-Martínez, L.F.; Fraga-Pérez, K.; Magaño-Bocanegra, K.J.; Juárez-Hernández, U.; Mateos-Chávez, A.A.; Luria-Pérez, R. Recombinant Attenuated Salmonella enterica as a Delivery System of Heterologous Molecules in Cancer Therapy. Cancers 2022, 14, 4224. [Google Scholar] [CrossRef]
- Ye, S.; MacEachran, D.P.; Hamilton, J.W.; O’Toole, G.A.; Stanton, B.A. Chemotoxicity of doxorubicin and surface expression of P-glycoprotein (MDR1) is regulated by the Pseudomonas aeruginosa toxin Cif. Am. J. Physiol. Cell Physiol. 2008, 295, C807–C818. [Google Scholar] [CrossRef] [Green Version]
- Wirths, S.; Lanzavecchia, A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur. J. Immunol. 2005, 35, 3433–3441. [Google Scholar] [CrossRef]
- Pierce, S.K. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2002, 2, 96–105. [Google Scholar] [CrossRef]
- Weng, H.J.; Tsai, T.F. ABCB1 in dermatology: Roles in skin diseases and their treatment. J. Mol. Med. 2021, 99, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Nakamichi, N.; Nanmo, H.; Kimura, K.-i.; Masuo, Y.; Sakai, Y.; Schinkel, A.H.; Sato, S.; Soga, T.; Kato, Y. Metabolome Analysis Reveals Dermal Histamine Accumulation in Murine Dermatitis Provoked by Genetic Deletion of P-Glycoprotein and Breast Cancer Resistance Protein. Pharm. Res. 2019, 36, 158. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Akimoto, N.; Kawamura, M.; Nakase, K.; Noguchi, N.; Sato, T. Involvement of adenosine triphosphate-binding cassette subfamily B member 1 in the augmentation of triacylglycerol excretion by Propionibacterium acnes in differentiated hamster sebocytes. J. Dermatol. 2017, 44, 1404–1407. [Google Scholar] [CrossRef]
- Landreville, S.; Agapova, O.A.; Kneass, Z.T.; Salesse, C.; Harbour, J.W. ABCB1 identifies a subpopulation of uveal melanoma cells with high metastatic propensity. Pigment Cell Melanoma Res. 2011, 24, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, F.C.; Liu, R.; Lu, P.; Shapiro, A.B.; Renoir, J.M.; Sharom, F.J.; Reiner, P.B. beta-Amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001, 76, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Vogelgesang, S.; Cascorbi, I.; Schroeder, E.; Pahnke, J.; Kroemer, H.K.; Siegmund, W.; Kunert-Keil, C.; Walker, L.C.; Warzok, R.W. Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 2002, 12, 535–541. [Google Scholar] [CrossRef]
- Vogelgesang, S.; Warzok, R.W.; Cascorbi, I.; Kunert-Keil, C.; Schroeder, E.; Kroemer, H.K.; Siegmund, W.; Walker, L.C.; Pahnke, J. The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 121–125. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Deane, R.; Fagan, A.M.; Spinner, M.L.; Parsadanian, M.; Finn, M.B.; Jiang, H.; Prior, J.L.; Sagare, A.; Bales, K.R.; et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Investig. 2005, 115, 3285–3290. [Google Scholar] [CrossRef] [Green Version]
- Kuhnke, D.; Jedlitschky, G.; Grube, M.; Krohn, M.; Jucker, M.; Mosyagin, I.; Cascorbi, I.; Walker, L.C.; Kroemer, H.K.; Warzok, R.W.; et al. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer’s amyloid-beta peptides—Implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007, 17, 347–353. [Google Scholar] [CrossRef]
- Silverberg, G.D.; Messier, A.A.; Miller, M.C.; Machan, J.T.; Majmudar, S.S.; Stopa, E.G.; Donahue, J.E.; Johanson, C.E. Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J. Neuropathol. Exp. Neurol. 2010, 69, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Vogelgesang, S.; Glatzel, M.; Walker, L.C.; Kroemer, H.K.; Aguzzi, A.; Warzok, R.W. Cerebrovascular P-glycoprotein expression is decreased in Creutzfeldt-Jakob disease. Acta Neuropathol. 2006, 111, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L. Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr. Pharm. Des. 2011, 17, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- van Assema, D.M.; Lubberink, M.; Boellaard, R.; Schuit, R.C.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. P-glycoprotein function at the blood-brain barrier: Effects of age and gender. Mol. Imaging Biol. 2012, 14, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Q.; Zhang, J.; Chan, E.; Duan, W.; Zhou, S. Multidrug resistance proteins (MRPs) and implication in drug development. Drug Dev. Res. 2005, 64, 1–18. [Google Scholar] [CrossRef]
- Leier, I.; Jedlitschky, G.; Buchholz, U.; Cole, S.P.; Deeley, R.G.; Keppler, D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994, 269, 27807–27810. [Google Scholar] [CrossRef]
- Cui, Y.; König, J.; Buchholz, U.; Spring, H.; Leier, I.; Keppler, D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 1999, 55, 929–937. [Google Scholar]
- Zeng, H.; Liu, G.; Rea, P.A.; Kruh, G.D. Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res. 2000, 60, 4779–4784. [Google Scholar]
- Rius, M.; Hummel-Eisenbeiss, J.; Keppler, D. ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4). J. Pharm. Exp. 2008, 324, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Belinsky, M.G.; Chen, Z.S.; Shchaveleva, I.; Zeng, H.; Kruh, G.D. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res. 2002, 62, 6172–6177. [Google Scholar]
- Chen, Z.S.; Hopper-Borge, E.; Belinsky, M.G.; Shchaveleva, I.; Kotova, E.; Kruh, G.D. Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10). Mol. Pharm. 2003, 63, 351–358. [Google Scholar] [CrossRef]
- Chen, Z.S.; Guo, Y.; Belinsky, M.G.; Kotova, E.; Kruh, G.D. Transport of bile acids, sulfated steroids, estradiol 17-beta-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol. Pharm. 2005, 67, 545–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Ven, R.; Oerlemans, R.; van der Heijden, J.W.; Scheffer, G.L.; de Gruijl, T.D.; Jansen, G.; Scheper, R.J. ABC drug transporters and immunity: Novel therapeutic targets in autoimmunity and cancer. J. Leukoc. Biol. 2009, 86, 1075–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijnholds, J.; Evers, R.; van Leusden, M.R.; Mol, C.A.; Zaman, G.J.; Mayer, U.; Beijnen, J.H.; van der Valk, M.; Krimpenfort, P.; Borst, P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat. Med. 1997, 3, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, M.J.; Wijnholds, J.; Peppelenbosch, M.P.; Vervoordeldonk, M.J.; Speelman, P.; van Deventer, S.J.; Borst, P.; van der Poll, T. Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. J. Immunol. 2001, 166, 4059–4064. [Google Scholar] [CrossRef] [PubMed]
- Luft, T.; Jefford, M.; Luetjens, P.; Toy, T.; Hochrein, H.; Masterman, K.A.; Maliszewski, C.; Shortman, K.; Cebon, J.; Maraskovsky, E. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: Prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood 2002, 100, 1362–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scandella, E.; Men, Y.; Legler, D.F.; Gillessen, S.; Prikler, L.; Ludewig, B.; Groettrup, M. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood 2004, 103, 1595–1601. [Google Scholar] [CrossRef] [Green Version]
- Legler, D.F.; Krause, P.; Scandella, E.; Singer, E.; Groettrup, M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 2006, 176, 966–973. [Google Scholar] [CrossRef] [Green Version]
- van de Ven, R.; Scheffer, G.L.; Reurs, A.W.; Lindenberg, J.J.; Oerlemans, R.; Jansen, G.; Gillet, J.-P.; Glasgow, J.N.; Pereboev, A.; Curiel, D.T.; et al. A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood 2008, 112, 2353–2359. [Google Scholar] [CrossRef] [Green Version]
- van de Ven, R.; de Groot, J.; Reurs, A.W.; Wijnands, P.G.; van de Wetering, K.; Schuetz, J.D.; de Gruijl, T.D.; Scheper, R.J.; Scheffer, G.L. Unimpaired immune functions in the absence of Mrp4 (Abcc4). Immunol. Lett. 2009, 124, 81–87. [Google Scholar] [CrossRef]
- Kryczka, J.; Boncela, J. Cell Migration Related to MDR-Another Impediment to Effective Chemotherapy? Molecules 2018, 23, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, C.; Arora, K.; Naren, A.P. Methods to Study Mrp4-containing Macromolecular Complexes in the Regulation of Fibroblast Migration. J. Vis. Exp. 2016, 53973. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.F.; Finch, R.A.; Jäger, D.; Muller, W.A.; Sartorelli, A.C.; Randolph, G.J. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 2000, 103, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Pace-Asciak, C.R.; Reynaud, D.; Demin, P.; Nigam, S. The hepoxilins. A review. Adv. Exp. Med. Biol. 1999, 447, 123–132. [Google Scholar]
- Nigam, S.; Zafiriou, M.P.; Deva, R.; Ciccoli, R.; Roux-Van der Merwe, R. Structure, biochemistry and biology of hepoxilins: An update. FEBS J. 2007, 274, 3503–3512. [Google Scholar] [CrossRef]
- Nigam, S.; Patabhiraman, S.; Ciccoli, R.; Ishdorj, G.; Schwarz, K.; Petrucev, B.; Kühn, H.; Haeggström, J.Z. The rat leukocyte-type 12-lipoxygenase exhibits an intrinsic hepoxilin A3 synthase activity. J. Biol. Chem. 2004, 279, 29023–29030. [Google Scholar] [CrossRef]
- Yu, Z.; Schneider, C.; Boeglin, W.E.; Marnett, L.J.; Brash, A.R. The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc. Natl. Acad. Sci. USA 2003, 100, 9162–9167. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Takanezawa, Y.; Hirata, T.; Shimizu, Y.; Misasa, K.; Kioka, N.; Arai, H.; Ueda, K.; Matsuo, M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006, 47, 1791–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cserepes, J.; Szentpétery, Z.; Seres, L.; Özvegy-Laczka, C.; Langmann, T.; Schmitz, G.; Glavinas, H.; Klein, I.; Homolya, L.; Váradi, A. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: Indications for heterodimerization. Biochem. Biophys. Res. Commun. 2004, 320, 860–867. [Google Scholar] [CrossRef]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef] [Green Version]
- Terasaka, N.; Wang, N.; Yvan-Charvet, L.; Tall, A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA 2007, 104, 15093–15098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Out, R.; Hoekstra, M.; Habets, K.; Meurs, I.; de Waard, V.; Hildebrand, R.B.; Wang, Y.; Chimini, G.; Kuiper, J.; Van Berkel, T.J. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006, 47, 2433–2443. [Google Scholar] [CrossRef] [Green Version]
- Gelissen, I.C.; Harris, M.; Rye, K.A.; Quinn, C.; Brown, A.J.; Kockx, M.; Cartland, S.; Packianathan, M.; Kritharides, L.; Jessup, W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 534–540. [Google Scholar] [CrossRef]
- Jessup, W.; Gelissen, I.C.; Gaus, K.; Kritharides, L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 2006, 17, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Vaughan, A.M. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef]
- Seres, L.; Cserepes, J.; Elkind, N.B.; Törocsik, D.; Nagy, L.; Sarkadi, B.; Homolya, L. Functional ABCG1 expression induces apoptosis in macrophages and other cell types. Biochim. Biophys. Acta 2008, 1778, 2378–2387. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, A.J.; Skaflen, M.D.; Srinivasan, S.; Hedrick, C.C. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J. Immunol. 2008, 180, 4273–4282. [Google Scholar] [CrossRef] [Green Version]
- Sag, D.; Purcu, D.U.; Altunay, M. The cholesterol transporter ABCG1 modulates macrophage polarization in human monocyte-derived macrophages. J. Immunol. 2019, 202, 187-22. [Google Scholar]
- Sag, D.; Cekic, C.; Wu, R.; Linden, J.; Hedrick, C.C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 2015, 6, 6354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlyarov, S.; Kotlyarova, A. Clinical Significance of Lipid Transport Function of ABC Transporters in the Innate Immune System. Membranes 2022, 12, 1083. https://doi.org/10.3390/membranes12111083
Kotlyarov S, Kotlyarova A. Clinical Significance of Lipid Transport Function of ABC Transporters in the Innate Immune System. Membranes. 2022; 12(11):1083. https://doi.org/10.3390/membranes12111083
Chicago/Turabian StyleKotlyarov, Stanislav, and Anna Kotlyarova. 2022. "Clinical Significance of Lipid Transport Function of ABC Transporters in the Innate Immune System" Membranes 12, no. 11: 1083. https://doi.org/10.3390/membranes12111083






