Recent Developments and Perspectives of Recycled Poly(ethylene terephthalate)-Based Membranes: A Review
Abstract
:1. Introduction
2. Poly(ethylene terephthalate) Recycling Methods
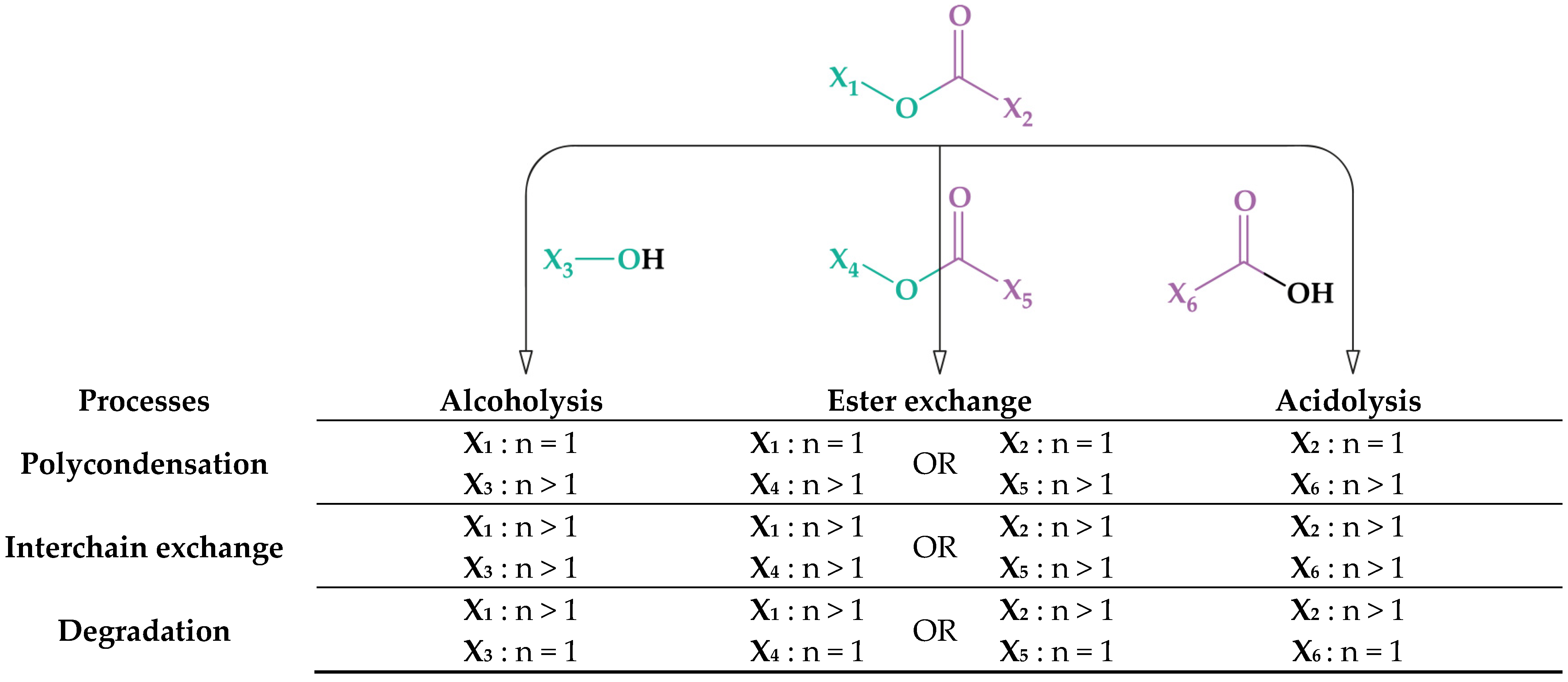
2.1. Chemical Recycling Methods
2.2. Poly(ethylene terephthalate) Preparation Methods
2.3. Copolyester Preparation Methods
3. Membrane Preparation Methods
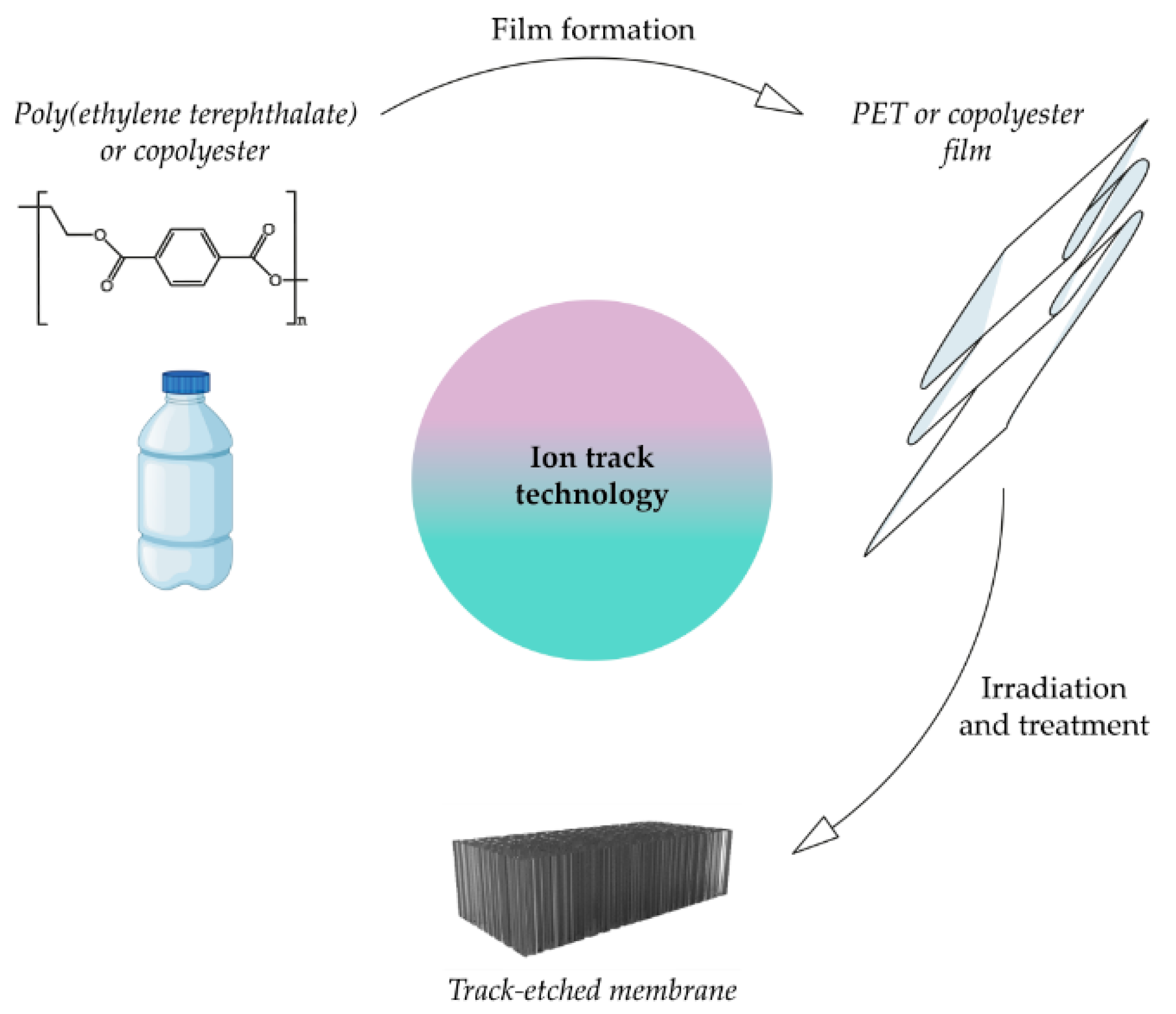
3.1. Ion Tracking Technology
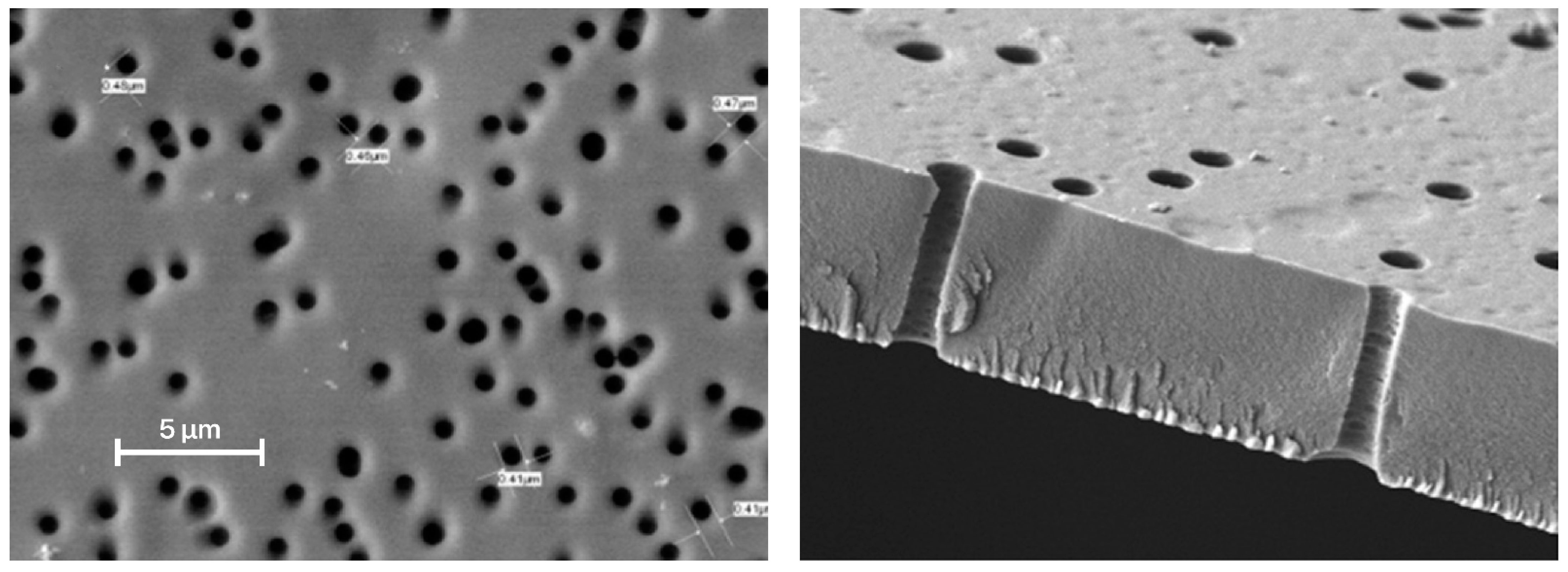
3.1.1. PET-Based Track Membranes
3.1.2. Copolyester-Based Track Membranes
3.2. Electrospinning
| Solvent | Ratio, Weight | References |
|---|---|---|
| Trifluoroacetic acid | — | [139,140] |
| Trifluoroacetic acid, dichloromethane | 7:3 | [140,141,142,143] |
| 1,1,1,3,3,3-Hexafluoro-2-propanol, dichloromethane | 2:8 | [144,145] |
3.2.1. PET-Based Nanofibrous Membranes
3.2.2. Copolyester-Based Nanofibrous Membranes
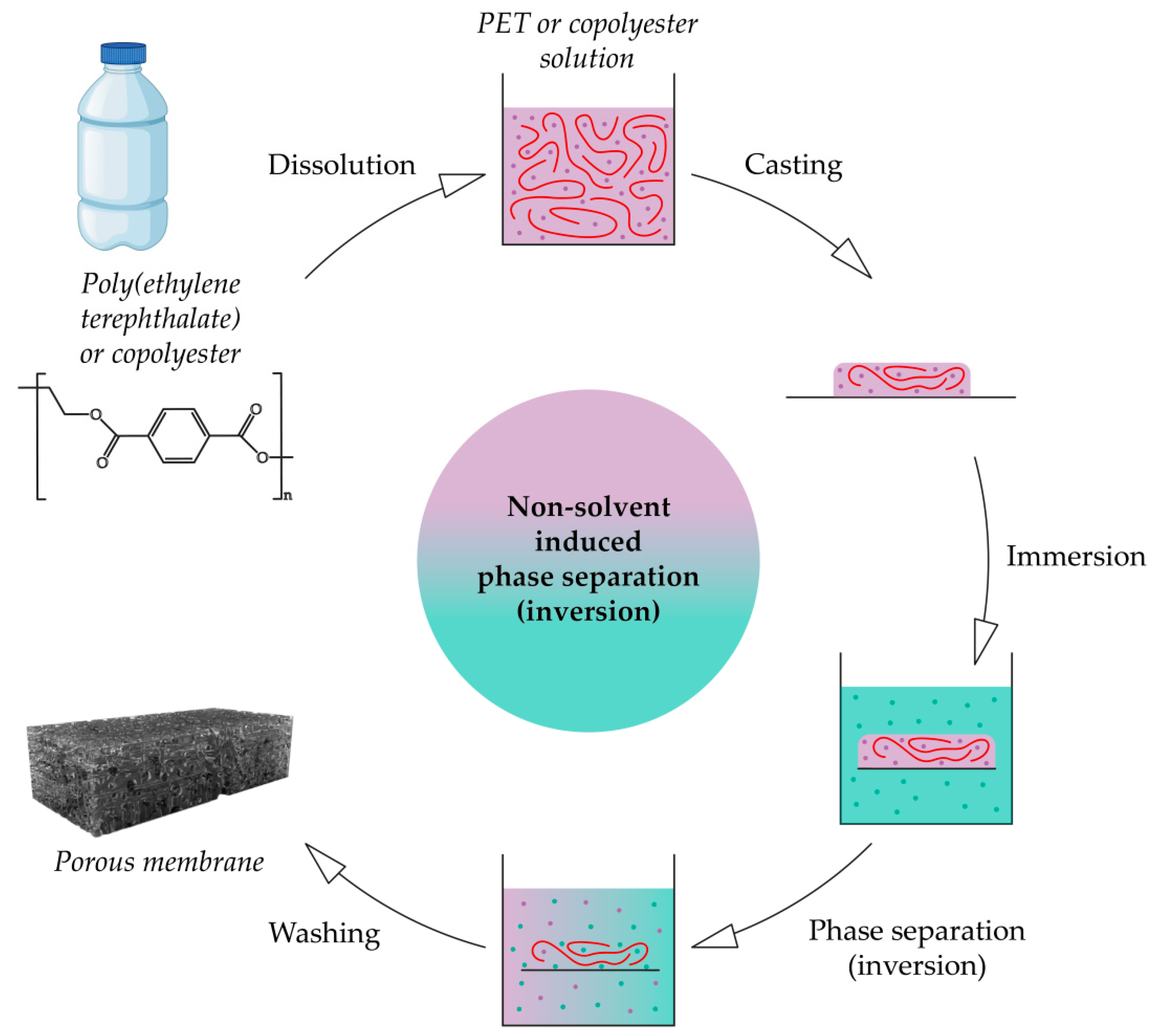
3.3. Phase Inversion or Separation
3.3.1. PET-Based Porous Membranes
3.3.2. Copolyester-Based Porous Membranes
3.4. Other Methods
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watt, E.; Picard, M.; Maldonado, B.; Abdelwahab, M.A.; Mielewski, D.F.; Drzal, L.T.; Misra, M.; Mohanty, A.K. Ocean Plastics: Environmental Implications and Potential Routes for Mitigation—A Perspective. RSC Adv. 2021, 11, 21447–21462. [Google Scholar] [CrossRef] [PubMed]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A Review on Textile Recycling Practices and Challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Investigation of Polyester Tire Cord Glycolysis Accompanied by Rubber Crumb Devulcanization. Polymers 2022, 14, 684. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Toms, R.V.; Gerval’d, A.Y. Prospects of Polyester Tire Cord Waste Utilization. Kauchuk I Rezina 2022, 81, 148–154. [Google Scholar]
- Bogusz, P.; Miedzińska, D.; Wieczorek, M. Experimental Investigation of the Tensile Behavior of Selected Tire Cords Using Novel Testing Equipment. Materials 2022, 15, 4163. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gervald, A.Y.U.; Toms, R.V.; Balashov, M.S. Natural Latex Deproteinization Issues. Kauchuk I Rezina 2020, 79, 310–316. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gervald, A.Y. Elastomeric Compositions in Wound Dressings. Kauchuk I Rezina 2021, 80, 150–154. [Google Scholar] [CrossRef]
- Burrows, S.D.; Ribeiro, F.; O’Brien, S.; Okoffo, E.; Toapanta, T.; Charlton, N.; Kaserzon, S.; Lin, C.-Y.; Tang, C.; Rauert, C.; et al. The Message on the Bottle: Rethinking Plastic Labelling to Better Encourage Sustainable Use. Environ. Sci. Policy 2022, 132, 109–118. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gervald, A.Y.U.; Toms, R.V.; Lobanov, A.N. Obtaining Phthalate Substituted Post-Consumer Polyethylene Terephthalate and Its Isothermal Crystallization. Fine Chem. Technol. 2022, 17, 164–171. [Google Scholar] [CrossRef]
- Hou, D.; Xin, J.; Lu, X.; Guo, X.; Dong, H.; Ren, B.; Zhang, S. Conversion of Bis(2-Hydroxyethylene Terephthalate) into 1,4-Cyclohexanedimethanol by Selective Hydrogenation Using RuPtSn/Al2O3. RSC Adv. 2016, 6, 48737–48744. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Toms, R.V.; Gervald, A.Y. Study of Methods for Obtaining Unsaturated Polyester Resins Based on Recycled Polyethylene Terephthalate. Plast. Massy 2022, 1, 46–47. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Unsaturated Polyester Resin Nanocomposites Based on Post-Consumer Polyethylene Terephthalate. Polymers 2022, 14, 1602. [Google Scholar] [CrossRef] [PubMed]
- Bórquez-Mendivil, A.; Hurtado-Macías, A.; Leal-Pérez, J.E.; Flores-Valenzuela, J.; Vargas-Ortíz, R.Á.; Cabrera-Covarrubias, F.G.; Almaral-Sánchez, J.L. Hybrid Coatings of SiO2–Recycled PET Unsaturated Polyester Resin by Sol-Gel Process. Polymers 2022, 14, 3280. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Huang, Y.-H.; Ju, A.-Q.; Yu, T.-S.; Ge, M.-Q. Synthesis and Characterization of Azo Dyestuff Based on Bis(2-Hydroxyethyl) Terephthalate Derived from Depolymerized Waste Poly(Ethylene Terephthalate) Fibers. Chin. Chem. Lett. 2014, 25, 1550–1554. [Google Scholar] [CrossRef]
- Kamrani, H.; Nosrati, A. Fabrication of Nanofiber Filtration Membranes Using Polyethylene Terephthalate (PET): A Review. J. Membr. Sci. Technol 2018, 8, 1000183. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Al-Juboori, R.A.; al Aani, S.; Ladewig, B.P.; Hilal, N. Natural and Recycled Materials for Sustainable Membrane Modification: Recent Trends and Prospects. Sci. Total Environ. 2022, 838, 156014. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Korolkov, I.V.; Dosmagambetova, S.S.; Zdorovets, M.V.; Güven, O. Recent Progress in the Membrane Distillation and Impact of Track-Etched Membranes. Polymers 2021, 13, 2520. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.H.; Rabie, A.M.; ElMetwally, A.E. Greener Routes for Recycling of Polyethylene Terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Damayanti; Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of Polyethylene Terephthalate Wastes: A Review of Technologies, Routes, and Applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of Poly(Ethylene Terephthalate)—A Review Focusing on Chemical Methods. Express Polym. Lett. 2016, 10, 559–586. [Google Scholar] [CrossRef]
- Nikles, D.E.; Farahat, M.S. New Motivation for the Depolymerization Products Derived from Poly(Ethylene Terephthalate) (PET) Waste: A Review. Macromol. Mater. Eng. 2005, 290, 13–30. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current Developments in Chemical Recycling of Post-Consumer Polyethylene Terephthalate Wastes for New Materials Production: A Review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Ghosal, K.; Nayak, C. Recent Advances in Chemical Recycling of Polyethylene Terephthalate Waste into Value Added Products for Sustainable Coating Solutions—Hope vs. Hype. Mater. Adv. 2022, 3, 1974–1992. [Google Scholar] [CrossRef]
- Aguado, A.; Martínez, L.; Becerra, L.; Arieta-araunabeña, M.; Arnaiz, S.; Asueta, A.; Robertson, I. Chemical Depolymerisation of PET Complex Waste: Hydrolysis vs. Glycolysis. J. Mater. Cycles Waste Manag. 2014, 16, 201–210. [Google Scholar] [CrossRef]
- Raheem, A.B.; Hassan, A.B.; Noor, Z.Z.; Samsudin, S.B.; Hamid, M.A.; Bello, A.; Oladokun, O.; Sabee, A.H.; Shamiri, A. Process Simulation of Bis (2- Hydroxyethyl) Terephthalate and Its Recovery Using Two–Stage Evaporation Systems. Chem. Eng. Trans. 2018, 63, 655–660. [Google Scholar]
- Lu, J.; Li, M.; Li, Y.; Li, X.; Gao, Q.; Ge, M. Synthesis and Sizing Performances of Water-Soluble Polyester Based on Bis(2-Hydroxyethyl) Terephthalate Derived from Depolymerized Waste Poly(Ethylene Terephthalate) Fabrics. Text. Res. J. 2019, 89, 572–579. [Google Scholar] [CrossRef]
- Liu, B.; Lu, X.; Ju, Z.; Sun, P.; Xin, J.; Yao, X.; Zhou, Q.; Zhang, S. Ultrafast Homogeneous Glycolysis of Waste Polyethylene Terephthalate via a Dissolution-Degradation Strategy. Ind. Eng. Chem. Res. 2018, 57, 16239–16245. [Google Scholar] [CrossRef]
- Stoski, A.; Viante, M.F.; Nunes, C.S.; Muniz, E.C.; Felsner, M.L.; Almeida, C.A.P. Oligomer Production through Glycolysis of Poly(Ethylene Terephthalate): Effects of Temperature and Water Content on Reaction Extent. Polym. Int. 2016, 65, 1024–1030. [Google Scholar] [CrossRef]
- Hoang, C.N.; Pham, C.T.; Dang, T.M.; Hoang, D.; Lee, P.-C.; Kang, S.-J.; Kim, J. Novel Oligo-Ester-Ether-Diol Prepared by Waste Poly(Ethylene Terephthalate) Glycolysis and Its Use in Preparing Thermally Stable and Flame Retardant Polyurethane Foam. Polymers 2019, 11, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, C.A.; Gallegos, M.V.; García, J.R.; Sambeth, J.; Peluso, M.A. Catalytic Glycolysis of Poly(Ethylene Terephthalate) Using Zinc and Cobalt Oxides Recycled from Spent Batteries. Waste Biomass Valorization 2020, 11, 4991–5001. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, Q.; Bu, R.; Yang, F.; Li, W. Kinetics of Poly(Ethylene Terephthalate) Fiber Glycolysis in Ethylene Glycol. Fibers Polym. 2015, 16, 1213–1219. [Google Scholar] [CrossRef]
- Scé, F.; Cano, I.; Martin, C.; Beobide, G.; Castillo, Ó.; de Pedro, I. Comparing Conventional and Microwave-Assisted Heating in PET Degradation Mediated by Imidazolium-Based Halometallate Complexes. New J. Chem. 2019, 43, 3476–3485. [Google Scholar] [CrossRef]
- Esquer, R.; García, J.J. Metal-Catalysed Poly(Ethylene) Terephthalate and Polyurethane Degradations by Glycolysis. J. Organomet. Chem. 2019, 902, 120972. [Google Scholar] [CrossRef]
- Fang, P.; Liu, B.; Xu, J.; Zhou, Q.; Zhang, S.; Ma, J.; Lu, X. High-Efficiency Glycolysis of Poly(Ethylene Terephthalate) by Sandwich-Structure Polyoxometalate Catalyst with Two Active Sites. Polym. Degrad. Stab. 2018, 156, 22–31. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Lu, J.; Li, X.; Lu, Y. Decolorization and Reusing of PET Depolymerization Waste Liquid by Electrochemical Method with Magnetic Nanoelectrodes. Environ. Sci. Pollut. Res. 2018, 25, 34531–34539. [Google Scholar] [CrossRef]
- Nabid, M.R.; Bide, Y.; Jafari, M. Boron Nitride Nanosheets Decorated with Fe3O4 Nanoparticles as a Magnetic Bifunctional Catalyst for Post-Consumer PET Wastes Recycling. Polym. Degrad. Stab. 2019, 169, 108962. [Google Scholar] [CrossRef]
- Alzuhairi, M. Bubble Column and CFD Simulation for Chemical Recycling of Polyethylene Terephthalate. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; p. 030041. [Google Scholar]
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; de Pedro, I. Paramagnetic Ionic Liquid-Coated SiO2@Fe3O4 Nanoparticles—The next Generation of Magnetically Recoverable Nanocatalysts Applied in the Glycolysis of PET. Appl. Catal. B 2020, 260, 118110. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.; Zhao, R.; Liu, F.; Ge, X.; Yu, S. Heterogeneous CaO(SrO, BaO)/MCF as Highly Active and Recyclable Catalysts for the Glycolysis of Poly(Ethylene Terephthalate). Res. Chem. Intermed. 2018, 44, 7711–7729. [Google Scholar] [CrossRef]
- Park, G.; Bartolome, L.; Lee, K.G.; Lee, S.J.; Kim, D.H.; Park, T.J. One-Step Sonochemical Synthesis of a Graphene Oxide–Manganese Oxide Nanocomposite for Catalytic Glycolysis of Poly(Ethylene Terephthalate). Nanoscale 2012, 4, 3879. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lindqvist, K.; de la Motte, H. An Efficient Recycling Process of Glycolysis of PET in the Presence of a Sustainable Nanocatalyst. J. Appl. Polym. Sci. 2018, 135, 46285. [Google Scholar] [CrossRef]
- Veregue, F.R.; Pereira da Silva, C.T.; Moisés, M.P.; Meneguin, J.G.; Guilherme, M.R.; Arroyo, P.A.; Favaro, S.L.; Radovanovic, E.; Girotto, E.M.; Rinaldi, A.W. Ultrasmall Cobalt Nanoparticles as a Catalyst for PET Glycolysis: A Green Protocol for Pure Hydroxyethyl Terephthalate Precipitation without Water. ACS Sustain. Chem. Eng. 2018, 6, 12017–12024. [Google Scholar] [CrossRef]
- Lima, G.R.; Monteiro, W.F.; Ligabue, R.; Santana, R.M.C. Titanate Nanotubes as New Nanostrutured Catalyst for Depolymerization of PET by Glycolysis Reaction. Mater. Res. 2017, 20, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Lima, G.R.; Monteiro, W.F.; Toledo, B.O.; Ligabue, R.A.; Santana, R.M.C. Titanate Nanotubes Modified with Zinc and Its Application in Post-Consumer PET Depolymerization. Macromol. Symp. 2019, 383, 1800008. [Google Scholar] [CrossRef] [Green Version]
- Al-Sabagh, A.M.; Yehia, F.Z.; Harding, D.R.K.; Eshaq, G.H.; ElMetwally, A.E. Fe3O4-Boosted MWCNT as an Efficient Sustainable Catalyst for PET Glycolysis. Green Chem. 2016, 18, 3997–4003. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.H.; ElMetwally, A.E. Ionic Liquid-Coordinated Ferrous Acetate Complex Immobilized on Bentonite as a Novel Separable Catalyst for PET Glycolysis. Ind. Eng. Chem. Res. 2015, 54, 12474–12481. [Google Scholar] [CrossRef]
- Sert, E.; Yılmaz, E.; Atalay, F.S. Chemical Recycling of Polyethlylene Terephthalate by Glycolysis Using Deep Eutectic Solvents. J. Polym. Environ. 2019, 27, 2956–2962. [Google Scholar] [CrossRef]
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis Acid–Base Synergistic Catalysis for Polyethylene Terephthalate Degradation by 1,3-Dimethylurea/Zn(OAc)2 Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 3292–3300. [Google Scholar] [CrossRef]
- Yunita, I.; Putisompon, S.; Chumkaeo, P.; Poonsawat, T.; Somsook, E. Effective Catalysts Derived from Waste Ostrich Eggshells for Glycolysis of Post-Consumer PET Bottles. Chem. Pap. 2019, 73, 1547–1560. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, X.; Tang, S.; Lu, X.; Zhang, X.; Zhang, S. Urea as an Efficient and Reusable Catalyst for the Glycolysis of Poly(Ethylene Terephthalate) Wastes and the Role of Hydrogen Bond in This Process. Green Chem. 2012, 14, 2559. [Google Scholar] [CrossRef]
- De Castro, A.M.; Carniel, A. A Novel Process for Poly(Ethylene Terephthalate) Depolymerization via Enzyme-Catalyzed Glycolysis. Biochem. Eng. J. 2017, 124, 64–68. [Google Scholar] [CrossRef]
- Jamdar, V.; Kathalewar, M.; Jagtap, R.N.; Dubey, K.A.; Sabnis, A. Effect of γ-Irradiation on Glycolysis of PET Waste and Preparation of Ecofriendly Coatings Using Bio-Based and Recycled Materials. Polym. Eng. Sci. 2015, 55, 2653–2660. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gerval’d, A.Y.; Toms, R.V. Obtaining Oligoesters by Directed Glycolytic Destruction of Polyethylene Terephthalate Waste. Plast. Massy 2020, 1, 51–53. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Toms, R.V. Study of Polyethylene Terephthalate Glycolysis with a Mixture of Bis(2-Hydroxyethyl) Terephthalate and Its Oligomers. Plast. Massy 2021, 1, 50–52. [Google Scholar] [CrossRef]
- El Mejjatti, A.; Harit, T.; Riahi, A.; Khiari, R.; Bouabdallah, I.; Malek, F. Chemical Recycling of Poly(Ethylene Terephthalate). Application to the Synthesis of Multiblock Copolyesters. Express Polym. Lett. 2014, 8, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Nagahata, R.; Sugiyama, J.; Velmathi, S.; Nakao, Y.; Goto, M.; Takeuchi, K. Synthesis of Poly(Ethylene Terephthalate-Co-Isophthalate) by Copolymerization of Ethylene Isophthalate Cyclic Dimer and Bis(2-Hydroxyethyl) Terephthalate. Polym. J. 2004, 36, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, Y.; Wang, J.; Liu, F.; Jia, Z.; Liu, X.; Zhu, J. 2,5-Furandicarboxylic Acid as a Sustainable Alternative to Isophthalic Acid for Synthesis of Amorphous Poly(Ethylene Terephthalate) Copolyester with Enhanced Performance. J. Appl. Polym. Sci. 2019, 136, 47186. [Google Scholar] [CrossRef]
- Gan, Z.; Qu, S.; Li, S.; Tan, T.; Yang, J. Facile Synthesis of PET-Based Poly(Ether Ester)s with Striking Physical and Mechanical Properties. React. Funct. Polym. 2021, 164, 104936. [Google Scholar] [CrossRef]
- Shirali, H.; Rafizadeh, M.; Taromi, F.A. Synthesis and Characterization of Amorphous and Impermeable Poly(Ethylene-Co-1,4-Cyclohexylenedimethylene Terephthalate)/Organoclay Nanocomposite via in Situ Polymerization. J. Compos. Mater. 2014, 48, 301–315. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.Y.; Park, J.H.; Lyoo, W.S.; Noh, S.K. Kinetics of Polycondensation and Copolycondensation of Bis(3-Hydroxypropyl Terephthalate) and Bis(2-Hydroxyethyl Terephthalate). J. Appl. Polym. Sci. 2000, 77, 693–698. [Google Scholar] [CrossRef]
- Lewis, C.L.; Spruiell, J.E. Crystallization of 2-Methyl-1,3-Propanediol Substituted Poly(Ethylene Terephthalate). I. Thermal Behavior and Isothermal Crystallization. J. Appl. Polym. Sci. 2006, 100, 2592–2603. [Google Scholar] [CrossRef]
- Tsai, Y.; Fan, C.-H.; Wu, J.-H. Synthesis, Microstructures and Properties of Amorphous Poly(Ethylene Terephthalate-Co- Tricyclodecanedimethylene Terephthalate). J. Polym. Res. 2016, 23, 42. [Google Scholar] [CrossRef]
- Heidarzadeh, N.; Rafizadeh, M.; Taromi, F.A.; del Valle, L.J.; Franco, L.; Puiggalí, J. Biodegradability and Biocompatibility of Copoly(Butylene Sebacate-Co-Terephthalate)s. Polym. Degrad. Stab. 2017, 135, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.; Peace, S.K.; Richards, R.W.; MacDonald, W.A.; Mills, P.; King, S.M. Transesterification in Poly(Ethylene Terephthalate). Molecular Weight and End Group Effects. Macromolecules 2000, 33, 2981–2988. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Korolkov, I.V.; Gorin, Y.G.; Dosmagambetova, S.S.; Zdorovets, M.V. Membrane Distillation of Pesticide Solutions Using Hydrophobic Track-Etched Membranes. Chem. Pap. 2020, 74, 3445–3453. [Google Scholar] [CrossRef]
- Kozhina, E.; Kulesh, E.; Bedin, S.; Doludenko, I.; Piryazev, A.; Korolkov, I.; Kozlovskiy, A.; Zdorovets, M.; Rogachev, A.; Shumskaya, A. One-Dimensional Magneto-Optical Nanostructures: Template Synthesis, Structure, Properties, and Application in Spectroscopy Based on Plasmon Resonance. IEEE Magn. Lett. 2022, 13, 6101905. [Google Scholar] [CrossRef]
- Kovalets, N.P.; Panov, D.V.; Filippova, Y.U.A.; Razumovskaya, I.V. Point Agglomeration of Nickel and Iron Nanowires Synthesized in the Pores of Track Membranes. Bull. Russ. Acad. Sci. Phys. 2021, 85, 1400–1403. [Google Scholar] [CrossRef]
- Doludenko, I.M.; Volchkov, I.S.; Turenko, B.A.; Koshelev, I.O.; Podkur, P.L.; Zagorskiy, D.L.; Kanevskii, V.M. Electrical Properties Arrays of Intersecting of Nanowires Obtained in the Pores of Track Membranes. Mater. Chem. Phys. 2022, 287, 126285. [Google Scholar] [CrossRef]
- Doludenko, I.M. Aspects of Pore Filling in Synthesis of FeNi Alloy Nanowires Using Track-Etched Membranes. Inorg. Mater. Appl. Res. 2022, 13, 531–535. [Google Scholar] [CrossRef]
- Lee, P.L.J.; Thangavel, V.; Guery, C.; Trautmann, C.; Toimil-Molares, M.E.; Morcrette, M. Etched Ion-Track Membranes as Tailored Separators in Li–S Batteries. Nanotechnology 2021, 32, 365401. [Google Scholar] [CrossRef] [PubMed]
- Temnov, D.; Rossouw, A.; Vinogradov, I.; Shabanova, N.; Mamonova, T.; Lizunov, N.; Perold, W.; Nechaev, A. Thermo-Activation Spectroscopy of Track-Etched Membranes Based on Polyethylene Terephthalate Films Irradiated by Swift Xe Ions. Radiat. Phys. Chem. 2022, 191, 109868. [Google Scholar] [CrossRef]
- Sokhoreva, V.V.; Kanaev, V.G.; Kashkarov, E.B.; Kulyukina, E.S.; Kuznetsov, S.I. Formation of a Track Template during PETP Irradiation with High-Energy Helium Ions for the Template Synthesis of Regular Microstructures. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2018, 12, 598–602. [Google Scholar] [CrossRef]
- Apel, P.Y.U.; Blonskaya, I.V.; Ivanov, O.M.; Kristavchuk, O.V.; Lizunov, N.E.; Nechaev, A.N.; Orelovich, O.L.; Polezhaeva, O.A.; Dmitriev, S.N. Creation of Ion-Selective Membranes from Polyethylene Terephthalate Films Irradiated with Heavy Ions: Critical Parameters of the Process. Membr. Membr. Technol. 2020, 2, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Negi, S. Photo Driven Ion Transport and Pumping through Synthetic Nanochannels. Mater. Today Commun. 2021, 26, 102127. [Google Scholar] [CrossRef]
- Apel, P.Y.; Blonskaya, I.V.; Ivanov, O.M.; Kristavchuk, O.V.; Nechaev, A.N.; Olejniczak, K.; Orelovich, O.L.; Polezhaeva, O.A.; Dmitriev, S.N. Do the Soft-Etched and UV-Track Membranes Actually Have Uniform Cylindrical Subnanometer Channels? Radiat. Phys. Chem. 2022, 198, 110266. [Google Scholar] [CrossRef]
- Zhao, J.; Du, G.; Yao, H.; Guo, J.; Mao, G.; Liu, W.; Wu, R.; Shen, C.; Mou, H.; Zhao, C.; et al. Fabrication of Double Conical PET Nanochannel for Molecular Detection. Vacuum 2022, 202, 111198. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Ling, Y.; Wang, M.; Ding, S.; She, W.; Wang, Z.; Wang, Y.; Liu, F. Ultrafast Selective Ionic Transport through Heat-Treated Polyethylene Terephthalate Track Membranes with Sub-Nanometer Pores. Radiat. Meas. 2018, 119, 80–84. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Yeszhanov, A.B.; Gorin, Y.G.; Zdorovets, M.V.; Khlebnikov, N.A.; Serkov, K.V. Hydrophobization of PET Track-Etched Membranes for Direct Contact Membrane Distillation. Mater. Res. Express 2018, 5, 065317. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Narmukhamedova, A.R.; Melnikova, G.B.; Muslimova, I.B.; Yeszhanov, A.B.; Zhatkanbayeva, Z.K.; Chizhik, S.A.; Zdorovets, M.V. Preparation of Hydrophobic PET Track-Etched Membranes for Separation of Oil–Water Emulsion. Membranes 2021, 11, 637. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Gorin, Y.G.; Yeszhanov, A.B.; Kozlovskiy, A.L.; Zdorovets, M.V. Preparation of PET Track-Etched Membranes for Membrane Distillation by Photo-Induced Graft Polymerization. Mater. Chem. Phys. 2018, 205, 55–63. [Google Scholar] [CrossRef]
- Shumskaya, A.; Kaniukov, E.; Yakimchuk, D.; Plisko, T.; Burts, K.; Bildyukevich, A.; Nikolaevich, L.; Kozlovskiy, A.; Zdorovets, M. Modified Ion-Track Membranes for Separation of Biological Objects. Mater. Res. Express 2019, 6, 0850h3. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Zdorovets, M.V. Protein Fouling of Modified Microporous PET Track-Etched Membranes. Radiat. Phys. Chem. 2018, 151, 141–148. [Google Scholar] [CrossRef]
- Mashentseva, A.A. Effect of the Oxidative Modification and Activation of Templates Based on Poly(Ethylene Terephthalate) Track-Etched Membranes on the Electroless Deposition of Copper and the Catalytic Properties of Composite Membranes. Pet. Chem. 2019, 59, 1337–1344. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C. Surface Characterization of Polyimide and Polyethylene Terephthalate Membranes toward Plasma and UV Treatments. Prog. Nat. Sci. Mater. Int. 2022, 32, 314–327. [Google Scholar] [CrossRef]
- Filippova, E.O.; Karpov, D.A.; Pichugin, V.F.; Ulbricht, M. The Investigation of the Influence of Low-Temperature Plasma and Steam Sterilization on the Properties of Track Membranes Made of Polyethylene Terephthalate. Inorg. Mater. Appl. Res. 2020, 11, 1116–1123. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Yeszhanov, A.B.; Shakayeva, A.K.; Shlimas, D.I.; Zhumazhanova, A.; Zdorovets, M.V. Photo-Induced Graft (Co)Polymerization of Glycidyl Methacrylate and Acrylonitrile on PET Ion-Track Membranes for Electrochemical Detection of Uranyl Ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129086. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Korolkov, I.V.; Yeszhanov, A.B.; Gorin, Y.G. Functionalization of PET Track-Etched Membranes by UV-Induced Graft (Co)Polymerization for Detection of Heavy Metal Ions in Water. Polymers 2019, 11, 1876. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.-E.; Ahn, S.; Kang, J.H. Robust Chemical Bonding of PMMA Microfluidic Devices to Porous PETE Membranes for Reliable Cytotoxicity Testing of Drugs. Lab. Chip. 2019, 19, 3706–3713. [Google Scholar] [CrossRef]
- Parmanbek, N.; Sütekin, D.S.; Barsbay, M.; Mashentseva, A.A.; Zheltov, D.A.; Aimanova, N.A.; Jakupova, Z.Y.; Zdorovets, M.V. Hybrid PET Track-Etched Membranes Grafted by Well-Defined Poly(2-(Dimethylamino)Ethyl Methacrylate) Brushes and Loaded with Silver Nanoparticles for the Removal of As(III). Polymers 2022, 14, 4026. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, Y.; Li, P.; Lai, J.; Kong, X.-Y.; Liu, W.; Xiao, K.; Xie, G.; Tian, Y.; Wen, L.; et al. DNAzyme Tunable Lead (ii) Gating Based on Ion-Track Etched Conical Nanochannels. Chem. Commun. 2015, 51, 5979–5981. [Google Scholar] [CrossRef] [PubMed]
- Muench, F. Direct Surface Functionalization with Metal and Metal Oxide Nanostructures. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Muench, F. Electroless Plating of Metal Nanomaterials. ChemElectroChem 2021, 8, 2993–3012. [Google Scholar] [CrossRef]
- Do Nascimento, K.T.O.; Ratkovski, G.P.; Pedro, G.D.C.; Gorza, F.D.S.; da Silva, R.J.; de Melo, C.P. Intrinsically Conductive Polymers Hybrid Bilayer Films for the Fluorescence Molecular Diagnosis of the Zika Virus. Colloids Surf. B Biointerfaces 2021, 208, 112120. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-L.; Hua, Y.; Pan, Z.-Q.; Qian, J.-H.; Yu, X.-Y.; Bao, N.; Huo, X.-L.; Wu, Z.-Q.; Xia, X.-H. PNP Nanofluidic Transistor with Actively Tunable Current Response and Ionic Signal Amplification. Nano Lett. 2022, 22, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Wiedenhöft, L.; Elleithy, M.M.A.; Ulbricht, M.; Schacher, F.H. Polyelectrolyte Functionalisation of Track Etched Membranes: Towards Charge-Tuneable Adsorber Materials. Membranes 2021, 11, 509. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Zhumanazar, N.; Gorin, Y.G.; Yeszhanov, A.B.; Zdorovets, M.V. Enhancement of Electrochemical Detection of Pb2+ by Sensor Based on Track-Etched Membranes Modified with Interpolyelectrolyte Complexes. J. Mater. Sci. Mater. Electron. 2020, 31, 20368–20377. [Google Scholar] [CrossRef]
- Rossouw, A.; Olejniczak, A.; Olejniczak, K.; Gorberg, B.; Vinogradov, I.; Kristavchuk, O.; Nechaev, A.; Petrik, L.; Perold, W.; Dmitriev, S. Ti and TiO2 Magnetron Sputtering in Roll-to-Roll Fabrication of Hybrid Membranes. Surf. Interfaces 2022, 31, 101975. [Google Scholar] [CrossRef]
- Rossouw, A.; Kristavchuk, O.; Olejniczak, A.; Bode-Aluko, C.; Gorberg, B.; Nechaev, A.; Petrik, L.; Perold, W.; Apel, P. Modification of Polyethylene Terephthalate Track Etched Membranes by Planar Magnetron Sputtered Ti/TiO2 Thin Films. Thin Solid Films 2021, 725, 138641. [Google Scholar] [CrossRef]
- Pereao, O.; Uche, C.; Bublikov, P.S.; Bode-Aluko, C.; Rossouw, A.; Vinogradov, I.I.; Nechaev, A.N.; Opeolu, B.; Petrik, L. Chitosan/PEO Nanofibers Electrospun on Metallized Track-Etched Membranes: Fabrication and Characterization. Mater. Today Chem. 2021, 20, 100416. [Google Scholar] [CrossRef]
- Kutuzau, M.; Shumskaya, A.; Kaniukov, E.; Alisienok, O.; Shidlouskaya, V.; Melnikova, G.; Shemukhin, A.; Nazarov, A.; Kozlovskiy, A.; Zdorovets, M. Photocatalytically Active Filtration Systems Based on Modified with Titanium Dioxide PET-Membranes. Nucl. Instrum. Methods Phys. Res. B 2019, 460, 212–215. [Google Scholar] [CrossRef]
- Altynbaeva, L.S.H.; Barsbay, M.; Aimanova, N.A.; Jakupova, Z.Y.; Nurpeisova, D.T.; Zdorovets, M.V.; Mashentseva, A.A. A Novel Cu2O/ZnO@PET Composite Membrane for the Photocatalytic Degradation of Carbendazim. Nanomaterials 2022, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Khashij, M.; Salmani, M.H.; Dalvand, A.; Fallahzadeh, H.; Haghirosadat, F.; Mokhtari, M. Fabrication of ZnO/y-FeOOH Nanoparticles Embedded on the Polyethylene Terephthalate Membrane: Evaluation of Antifouling Behavior and COD Removal. Environ. Sci. Pollut. Res. 2022, 29, 67014–67025. [Google Scholar] [CrossRef] [PubMed]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.V.; Zhidkov, I.S.; Cholach, S.O. Electron/Gamma Radiation-Induced Synthesis and Catalytic Activity of Gold Nanoparticles Supported on Track-Etched Poly(Ethylene Terephthalate) Membranes. Mater. Chem. Phys. 2018, 217, 31–39. [Google Scholar] [CrossRef]
- Ndilowe, G.M.; Bode-Aluko, C.A.; Chimponda, D.; Kristavchuk, O.; Kochnev, I.; Nechaev, A.; Petrik, L. Fabrication of Silver-Coated PET Track-Etched Membrane as SERS Platform for Detection of Acetaminophen. Colloid Polym. Sci. 2021, 299, 1729–1741. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Shaliutina-Kolešová, A.; Bouša, D.; Sofer, Z.; Friess, K. Co0·5Ni0·5FeCrO4 Spinel Nanoparticles Decorated with UiO-66-Based Metal-Organic Frameworks Grafted onto GO and O-SWCNT for Gas Adsorption and Water Purification. Chemosphere 2020, 255, 126966. [Google Scholar] [CrossRef]
- Ashtiani, S.; Sofer, Z.; Průša, F.; Friess, K. Molecular-Level Fabrication of Highly Selective Composite ZIF-8-CNT-PDMS Membranes for Effective CO2/N2, CO2/H2 and Olefin/Paraffin Separations. Sep. Purif. Technol. 2021, 274, 119003. [Google Scholar] [CrossRef]
- Usman, M.; Ali, M.; Al-Maythalony, B.A.; Ghanem, A.S.; Saadi, O.W.; Ali, M.; Jafar Mazumder, M.A.; Abdel-Azeim, S.; Habib, M.A.; Yamani, Z.H.; et al. Highly Efficient Permeation and Separation of Gases with Metal–Organic Frameworks Confined in Polymeric Nanochannels. ACS Appl. Mater. Interfaces 2020, 12, 49992–50001. [Google Scholar] [CrossRef]
- Awasthi, K.; Choudhury, S.; Komber, H.; Simon, F.; Formanek, P.; Sharma, A.; Stamm, M. Functionalization of Track-Etched Poly (Ethylene Terephthalate) Membranes as a Selective Filter for Hydrogen Purification. Int. J. Hydrogen Energy 2014, 39, 9356–9365. [Google Scholar] [CrossRef]
- Kamakshi, K.R.; Saraswat, V.K.; Kumar, M.; Awasthi, K. Palladium Nanoparticle Binding in Functionalized Track Etched PET Membrane for Hydrogen Gas Separation. Int. J. Hydrogen Energy 2017, 42, 16186–16194. [Google Scholar] [CrossRef]
- Kozhina, E.P.; Bedin, S.A.; Nechaeva, N.L.; Podoynitsyn, S.N.; Tarakanov, V.P.; Andreev, S.N.; Grigoriev, Y.V.; Naumov, A.V. Ag-Nanowire Bundles with Gap Hot Spots Synthesized in Track-Etched Membranes as Effective SERS-Substrates. Appl. Sci. 2021, 11, 1375. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Shumskaya, A.; Kozlovskiy, A.L.; Kaliyekperov, M.E.; Lissovskaya, L.I.; Zdorovets, M.V. Magnetic-Plasmonic Ni Nanotubes Covered with Gold for Improvement of SERS Analysis. J. Alloys Compd. 2022, 901, 163661. [Google Scholar] [CrossRef]
- Shumskaya, A.; Kozhina, E.; Bedin, S.; Andreev, S.; Kulesh, E.; Rogachev, A.; Yarmolenko, M.; Korolkov, I.; Kozlovskiy, A.; Zdorovets, M.; et al. Detection of Polynitro Compounds at Low Concentrations by SERS Using Ni@Au Nanotubes. Chemosensors 2022, 10, 306. [Google Scholar] [CrossRef]
- Mashentseva, A.; Borgekov, D.; Kislitsin, S.; Zdorovets, M.; Migunova, A. Comparative Catalytic Activity of PET Track-Etched Membranes with Embedded Silver and Gold Nanotubes. Nucl. Instrum. Methods Phys. Res. B 2015, 365, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Mashentseva, A.; Borgekov, D.; Zdorovets, M.; Russakova, A. Synthesis, Structure, and Catalytic Activity of Au/Poly(Ethylene Terephthalate) Composites. Acta Phys. Pol. A 2014, 125, 1263–1267. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Korolkov, I.V.; Yeszhanov, A.B.; Zdorovets, M.V.; Russakova, A.V. The Application of Composite Ion Track Membranes with Embedded Gold Nanotubes in the Reaction of Aminomethylation of Acetophenone. Mater. Res. Express 2019, 6, 115022. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Barsbay, M.; Aimanova, N.A.; Zdorovets, M.V. Application of Silver-Loaded Composite Track-Etched Membranes for Photocatalytic Decomposition of Methylene Blue under Visible Light. Membranes 2021, 11, 60. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Zdorovets, M.V. Composites Based on Polyethylene Terephthalate Track-Etched Membranes and Silver as Hydrogen Peroxide Decomposition Catalysts. Pet. Chem. 2017, 57, 954–960. [Google Scholar] [CrossRef]
- Muench, F.; Rauber, M.; Stegmann, C.; Lauterbach, S.; Kunz, U.; Kleebe, H.-J.; Ensinger, W. Ligand-Optimized Electroless Synthesis of Silver Nanotubes and Their Activity in the Reduction of 4-Nitrophenol. Nanotechnology 2011, 22, 415602. [Google Scholar] [CrossRef]
- Borgekov, D.; Mashentseva, A.; Kislitsin, S.; Kozlovskiy, A.; Russakova, A.; Zdorovets, M. Temperature Dependent Catalytic Activity of Ag/PET Ion-Track Membranes Composites. Acta Phys. Pol. A 2015, 128, 871–875. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Barsbay, M.; Zdorovets, M.V.; Zheltov, D.A.; Güven, O. Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III). Nanomaterials 2020, 10, 1552. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Zdorovets, M.V. Catalytic Activity of Composite Track-Etched Membranes Based on Copper Nanotubes in Flow and Static Modes. Pet. Chem. 2019, 59, 552–557. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Kozlovskiy, A.L.; Zdorovets, M.V. Influence of Deposition Temperature on the Structure and Catalytic Properties of the Copper Nanotubes Composite Membranes. Mater. Res. Express 2018, 5, 065041. [Google Scholar] [CrossRef]
- Russakova, A.V.; Altynbaeva, L.S.; Barsbay, M.; Zheltov, D.A.; Zdorovets, M.V.; Mashentseva, A.A. Kinetic and Isotherm Study of As(III) Removal from Aqueous Solution by PET Track-Etched Membranes Loaded with Copper Microtubes. Membranes 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ning, W.; Peng, Q.; Yang, M.; Lei, D.; Guo, S.; Liu, P.; Jiang, K.; He, X.; Li, Y. Superbroad-Band Actively Tunable Acoustic Metamaterials Driven from Poly (Ethylene Terephthalate)/Carbon Nanotube Nanocomposite Membranes. Nano Res. 2021, 14, 100–107. [Google Scholar] [CrossRef]
- Kaniukov, E.; Shumskaya, A.; Yakimchuk, D.; Kozlovskiy, A.; Korolkov, I.; Ibragimova, M.; Zdorovets, M.; Kadyrzhanov, K.; Rusakov, V.; Fadeev, M.; et al. FeNi Nanotubes: Perspective Tool for Targeted Delivery. Appl. Nanosci. 2019, 9, 835–844. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Korolkov, I.; Rusakov, V.; Nikolaevich, L.; Fesenko, O.; Budnyk, O.; Yakimchuk, D.; Shumskaya, A.; et al. FeCo Nanotubes: Possible Tool for Targeted Delivery of Drugs and Proteins. Appl. Nanosci. 2019, 9, 1091–1099. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Zdorovets, M.V.; Shumskaya, A.E.; Kadyrzhanov, K.K. Study of the Applicability of FE Nanotubes as an Anode Material of Lithium-ion Batteries. Prog. Electromagn. Res. M 2019, 82, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Starosta, W.; Wawszczak, D.; Sartowska, B.; Buczkowski, M. Investigations of Heavy Ion Tracks in Polyethylene Naphthalate Films. Radiat. Meas. 1999, 31, 149–152. [Google Scholar] [CrossRef]
- Molokanova, L.G.; Kochnev, Y.U.K.; Nechaev, A.N.; Chukova, S.N.; Apel, P.Y.U. Effect of Ultraviolet Radiation on Polyethylene Naphthalate Films Irradiated with High-Energy Heavy Ions. High Energy Chem. 2017, 51, 182–188. [Google Scholar] [CrossRef]
- Ivanova, N.M.; Filippova, E.O.; Tverdokhlebov, S.I.; Levkovich, N.V.; Apel, P.Y.U. Preparation, Structure, and Properties of Track-Etched Membranes Based on Polylactic Acid. Membr. Membr. Technol. 2021, 3, 282–290. [Google Scholar] [CrossRef]
- Kang, H.J.; Youm, J.S.; Kim, J.H. Characteristics of PET-PEN Copolymer as a Material for Flexible Substrate. Polym. Korea 2011, 35, 599–604. [Google Scholar] [CrossRef]
- Krentsel’, L.B.; Makarova, V.V.; Kudryavtsev, Y.A.V.; Govorun, E.N.; Litmanovich, A.D.; Markova, G.D.; Vasnev, V.A.; Kulichikhin, V.G. Interchain Exchange and Interdiffusion in Blends of Poly(Ethylene Terephthalate) and Poly(Ethylene Naphthalate). Polym. Sci. Ser. A 2009, 51, 1241–1248. [Google Scholar] [CrossRef]
- Gunes, K.; Isayev, A.I.; Li, X.; Wesdemiotis, C. Fast in Situ Copolymerization of PET/PEN Blends by Ultrasonically-Aided Extrusion. Polymer 2010, 51, 1071–1081. [Google Scholar] [CrossRef]
- Buasri, A.; Ongmali, D.; Sriboonpeng, P.; Prompanut, S.; Loryuenyong, V. Synthesis of PET-PLA Copolymer from Recycle Plastic Bottle and Study of Its Applications in the Electrochromic Devices with Graphene Conductive Ink. Mater. Today Proc. 2018, 5, 11060–11067. [Google Scholar] [CrossRef]
- Flores, I.; Etxeberria, A.; Irusta, L.; Calafel, I.; Vega, J.F.; Martínez-Salazar, J.; Sardon, H.; Müller, A.J. PET-PLA Partially Degradable Random Copolymers Prepared by Organocatalysis: Effect of Poly( Lactic Acid) Incorporation on Crystallization and Morphology. ACS Sustain. Chem. Eng. 2019, 7, 8647–8659. [Google Scholar] [CrossRef]
- Yasin, S.; Bakr, Z.H.; Ali, G.A.M.; Saeed, I. Recycling Nanofibers from Polyethylene Terephthalate Waste Using Electrospinning Technique. In Waste Recycling Technologies for Nanomaterials Manufacturing; Springer: Cham, Switzerland, 2021; pp. 805–821. [Google Scholar]
- Xu, G.-R.; An, X.-C.; Das, R.; Xu, K.; Xing, Y.-L.; Hu, Y.-X. Application of Electrospun Nanofibrous Amphiphobic Membrane Using Low-Cost Poly (Ethylene Terephthalate) for Robust Membrane Distillation. J. Water Process Eng. 2020, 36, 101351. [Google Scholar] [CrossRef]
- Topuz, F.; Oldal, D.G.; Szekely, G. Valorization of Polyethylene Terephthalate (PET) Plastic Wastes as Nanofibrous Membranes for Oil Removal: Sustainable Solution for Plastic Waste and Oil Pollution. Ind. Eng. Chem. Res. 2022, 61, 9077–9086. [Google Scholar] [CrossRef]
- Doan, H.N.; Phong Vo, P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Recycled PET as a PDMS-Functionalized Electrospun Fibrous Membrane for Oil-Water Separation. J. Environ. Chem. Eng. 2020, 8, 103921. [Google Scholar] [CrossRef]
- Strain, I.N.; Wu, Q.; Pourrahimi, A.M.; Hedenqvist, M.S.; Olsson, R.T.; Andersson, R.L. Electrospinning of Recycled PET to Generate Tough Mesomorphic Fibre Membranes for Smoke Filtration. J. Mater. Chem. A Mater. 2015, 3, 1632–1640. [Google Scholar] [CrossRef] [Green Version]
- Bonfim, D.P.F.; Cruz, F.G.S.; Bretas, R.E.S.; Guerra, V.G.; Aguiar, M.L. A Sustainable Recycling Alternative: Electrospun PET-Membranes for Air Nanofiltration. Polymers 2021, 13, 1166. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Frajová, J.; Nosko, M. Recycling of Poly(Ethylene Terephthalate) by Electrospinning to Enhanced the Filtration Efficiency. Mater. Lett. 2020, 278, 128426. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Mosnáčková, K.; Hrůza, J.; Frajová, J.; Opálek, A.; Bučková, M.; Kozics, K.; Peer, P.; Eckstein Andicsová, A. Electrospun Poly(Ethylene Terephthalate)/Silk Fibroin Composite for Filtration Application. Polymers 2021, 13, 2499. [Google Scholar] [CrossRef]
- Song, J.; Zhao, Q.; Meng, C.; Meng, J.; Chen, Z.; Li, J. Hierarchical Porous Recycled PET Nanofibers for High-Efficiency Aerosols and Virus Capturing. ACS Appl. Mater. Interfaces 2021, 13, 49380–49389. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, H.; Lin, Y.; Liu, X.; Yao, H.; Yu, L.; Wang, H.; Wang, X. Fabrication of Polysulfone Membrane with Sponge-like Structure by Using Different Non-Woven Fabrics. Sep. Purif. Technol. 2022, 297, 121553. [Google Scholar] [CrossRef]
- Tas, M.; Musa, U.G.; Ahmed, I.; Xu, F.; Smartt, C.; Hou, X. Functionalised SiO2 Modified Icephobic Nanocomposite Electrospun Membranes for Outdoor Electromagnetic Shielding Applications. Polymer 2022, 240, 124499. [Google Scholar] [CrossRef]
- Liu, R.; Qu, M.; Qiu, X.; Wang, H.; Fan, M.; Zhang, A.; Chen, Q.; Bin, Y. Poly (Ethylene Terephthalate) Nonwoven Fabrics-based Membranes Modified by Electrospinning of Thermoplastic Polyurethane, Nano SiO 2 and Ag Particles as Medical Packing Materials. Packag. Technol. Sci. 2022, 35, 557–567. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Stoica, A.E.; Dima-Bălcescu, M.-Ș.; Chircov, C.; Gharbia, S.; Baltă, C.; Roșu, M.; Herman, H.; Holban, A.M.; Ficai, A.; et al. Electrospun Polyethylene Terephthalate Nanofibers Loaded with Silver Nanoparticles: Novel Approach in Anti-Infective Therapy. J. Clin. Med. 2019, 8, 1039. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Santos, R.P.; Ramos, L.A.; Frollini, E. Bio-Based Electrospun Mats Composed of Aligned and Nonaligned Fibers from Cellulose Nanocrystals, Castor Oil, and Recycled PET. Int. J. Biol. Macromol. 2020, 163, 878–887. [Google Scholar] [CrossRef]
- Essa, W.K.; Yasin, S.A.; Abdullah, A.H.; Thalji, M.R.; Saeed, I.A.; Assiri, M.A.; Chong, K.F.; Ali, G.A.M. Taguchi L25 (54) Approach for Methylene Blue Removal by Polyethylene Terephthalate Nanofiber-Multi-Walled Carbon Nanotube Composite. Water 2022, 14, 1242. [Google Scholar] [CrossRef]
- Gün Gök, Z.; İnal, M.; Bozkaya, O.; Yiğitoğlu, M.; Vargel, İ. Production of 2-hydroxyethyl Methacrylate-g-poly(Ethylene Terephthalate) Nanofibers by Electrospinning and Evaluation of the Properties of the Obtained Nanofibers. J. Appl. Polym. Sci. 2020, 137, 49257. [Google Scholar] [CrossRef]
- Wu, C.-S.; Wu, D.-Y.; Wang, S.-S. Characterization and Functionality of Nanocomposite Mats Containing Polyester, Seashell, and Silica Aerogel Using an Electrospinning Fabrication Approach. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Danti, S.; Anand, S.; Azimi, B.; Milazzo, M.; Fusco, A.; Ricci, C.; Zavagna, L.; Linari, S.; Donnarumma, G.; Lazzeri, A.; et al. Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response. Pharmaceutics 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-J.; Chang, Y.-S.; Lin, Y.-Z.; Jiang, D.-H.; Chen, W.-H.; Lin, W.-Y.; Chen, C.-W.; Rwei, S.-P.; Kuo, C.-C. Green Electrospun Nanofiber Membranes Filter Prepared from Novel Biomass Thermoplastic Copolyester: Morphologies and Filtration Properties. J. Taiwan Inst. Chem. Eng. 2020, 106, 206–214. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A. Fabrication of Polypyrrole-Graphitic Carbon Nitride Nanocomposite Containing Hyper-Cross-Linked Polyamide Photoresponsive Membrane with Self-Cleaning Properties for Water Decontamination and Desalination Applications. J. Water Process Eng. 2022, 47, 102721. [Google Scholar] [CrossRef]
- Lu, D.; Babaniamansour, P.; Williams, A.; Opfar, K.; Nurick, P.; Escobar, I.C. Fabrication and Evaporation Time Investigation of Water Treatment Membranes Using Green Solvents and Recycled Polyethylene Terephthalate. J. Appl. Polym. Sci. 2022, 139, e52823. [Google Scholar] [CrossRef]
- Cadore, Í.R.; Ambrosi, A.; Cardozo, N.S.M.; Tessaro, I.C. Poly(Ethylene Terephthalate) Phase Inversion Membranes: Thermodynamics and Effects of a Poor Solvent on the Membrane Characteristics. Polym. Eng. Sci. 2022, 62, 1847–1858. [Google Scholar] [CrossRef]
- Cadore, Í.R.; Ambrosi, A.; Cardozo, N.S.M.; Tessaro, I.C. Phase Separation Behavior of Poly(Ethylene Terephthalate)/(Trifluoroacetic Acid/Dichloromethane)/Water System for Wet Phase Inversion Membrane Preparation. J. Appl. Polym. Sc.i 2019, 136, 47263. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Bidaki, A. Preparation of Polyethylene Terephthalate/Xanthan Nanofiltration Membranes Using Recycled Bottles for Removal of Diltiazem from Aqueous Solution. J. Clean. Prod. 2021, 314, 128082. [Google Scholar] [CrossRef]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(Ethylene Terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic Thin Film Composite Nanofiltration Membranes Derived Solely from Sustainable Sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Park, S.-H.; Yang, C.; Ayaril, N.; Szekely, G. Solvent-Resistant Thin-Film Composite Membranes from Biomass-Derived Building Blocks: Chitosan and 2,5-Furandicarboxaldehyde. ACS Sustain. Chem. Eng. 2022, 10, 998–1007. [Google Scholar] [CrossRef]
- Kusumocahyo, S.P.; Ambani, S.K.; Kusumadewi, S.; Sutanto, H.; Widiputri, D.I.; Kartawiria, I.S. Utilization of Used Polyethylene Terephthalate (PET) Bottles for the Development of Ultrafiltration Membrane. J. Environ. Chem. Eng. 2020, 8, 104381. [Google Scholar] [CrossRef]
- Kusumocahyo, S.P.; Ambani, S.K.; Marceline, S. Improved Permeate Flux and Rejection of Ultrafiltration Membranes Prepared from Polyethylene Terephthalate (PET) Bottle Waste. Sustain. Environ. Res. 2021, 31, 19. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Číhal, P.; Dendisová, M.; Randová, A.; Bouša, D.; Shaliutina-Kolešová, A.; Sofer, Z.; Friess, K. Fabrication of a PVDF Membrane with Tailored Morphology and Properties via Exploring and Computing Its Ternary Phase Diagram for Wastewater Treatment and Gas Separation Applications. RSC Adv. 2020, 10, 40373–40383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, X.; Zhao, L. Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes. Membranes 2014, 4, 81–95. [Google Scholar] [CrossRef]
- Kesting, R.E. Microporous Polyester Membranes and Polymer Assisted Phase Inversion Process for Their Manufacture. U.S. Patent 3,957,651, 18 May 1976. [Google Scholar]
- Gronwald, O.; Weber, M.; Muhlbach, K. Process for Producing Microporous Polyester Membranes for Electronic. Applications. Patent No. PCT/EP2015/062538, 17 December 2015. [Google Scholar]
- Realpe, Á.; Romero, K.A.; Acevedo, M.T. Síntesis de Membranas de Intercambio Protónico a Partir de Mezcla de Poliéster Insaturado y Látex Natural, Para Su Uso En Celdas de Combustible. Inf. Tecnol. 2015, 26, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Sancaktar, E. Effect of PET Support Membrane Thickness on Water Permeation Behavior of Thermally Responsive PNIPAM-g-PET Membranes. J. Memb. Sci. 2020, 610, 118304. [Google Scholar] [CrossRef]
- Voss, H.; Therre, J.; Kaltenborn, N.; Richter, H.; Voigt, I. Process for Producing Carbon Membranes. U.S. Patent No. US8608828, 5 April 2012. [Google Scholar]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Dzidziguri, E.L.; Sheverdiyev, K.A.; Karpacheva, G.P. Conversion of Polyethylene Terephthalate Waste in the Presence of Cobalt Compound into Highly-Porous Metal-Carbon Nanocomposite (c-PET-Co). Compos. Commun. 2022, 33, 101200. [Google Scholar] [CrossRef]





| Disadvantage | Primary and Secondary Recycling | Incineration | Tertiary (Chemical) Recycling |
|---|---|---|---|
| Deterioration of polymer properties | + | + | - |
| Moisture sensitivity | + | - | - |
| Negative effect of additives on properties | + | - | +/- 1 |
| Low process rate | - | - | + |
| The need to regenerate liquid components | - | - | + |
| Toxicity of the components | - | + | + |
| Method | Agents | References |
|---|---|---|
| Grafting | Polyvinyl alcohol, glutaraldehyde (binding agent), hydrochloric acid (catalyst) | [83] |
| Acrylic acid, N-vinylimidazole | [84] | |
| Oxidation | Hydrogen peroxide | [84,85] |
| UV treatment | [86] | |
| Plasma treatment | [86,87] | |
| Steam treatment | [87] 1 |
| Coating Type | Substances | Properties | Applications | References |
|---|---|---|---|---|
| Polyelectrolyte | Polyaniline | Electrical conductivity and other electrophysical properties | Detection of the charged molecules | [95,96] |
| Polypyrrole | [95] | |||
| Poly(2-acrylamido glycolic acid) | [97] | |||
| Poly(N-acetyl dehydroalanine) | [97] | |||
| Methacrylic acid/poly(allylamine) | Heavy ions detection | [98] | ||
| Nanoparticles | Titanium | Electrical conductivity, chemical and thermal stability | Production of the sensitive electrodes | [99,100,101] |
| Titanium dioxide | Catalytic activity | Preparation of the catalysts | [99,100,102] | |
| Cuprous oxide | [103] | |||
| Zinc oxide | [103,104] | |||
| Gold | [105] | |||
| Silver | Detection of acetaminophen in water | [106] | ||
| Co0·5 Ni0·5 FeCrO4 | Magnetic properties | Gas adsorption or separation, water purification | [107] | |
| Adsorption properties | ||||
| Metal–organic framework | [108,109] | |||
| Pd | Hydrogen purification | [110,111] | ||
| Tubes and wires | Ag nanowires | SERS analysis | [112] | |
| Nickel/gold microtubes | [113,114] | |||
| Gold microtubes | [113,114] | |||
| Catalytic activity | Preparation of the catalysts | [115,116,117] | ||
| Silver microtubes | [118,119,120,121] | |||
| Copper microtubes | [122,123,124] | |||
| Adsorption properties | Removal of the arsenic compounds | [125] | ||
| Carbon nanotubes | Gas adsorption or separation, water purification | [107,108] | ||
| Lightweight, electrical conductivity, low specific heat | Production of acoustic membranes, production of batteries, protection against electromagnetic interference | [126] | ||
| Fe/Ni nanotubes | Targeted delivery of drugs and proteins | [127] | ||
| Fe/Co nanotubes | [128] | |||
| Fe nanotubes | Preparation of lithium-ion batteries | [129] |
| Component Type | Substances | References |
|---|---|---|
| Solvent | Trifluoroacetic acid, dichloromethane | [159,160] |
| Trifluoroacetic acid | [161,162] | |
| 1,1,1,3,3,3-Hexafluoro-2-propanol | [162] | |
| Phenol, 100 °C | [165,166] | |
| m-Cresol, 100 °C | [165] | |
| Dimethyl sulfoxide, 100 °C | [165] | |
| Non-solvent | Water | [160,161,162] |
| Methanol | [161,162] | |
| Ethanol | [162,165,166] | |
| n-Propanol | [165] | |
| n-Butanol | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirshanov, K.; Toms, R.; Aliev, G.; Naumova, A.; Melnikov, P.; Gervald, A. Recent Developments and Perspectives of Recycled Poly(ethylene terephthalate)-Based Membranes: A Review. Membranes 2022, 12, 1105. https://doi.org/10.3390/membranes12111105
Kirshanov K, Toms R, Aliev G, Naumova A, Melnikov P, Gervald A. Recent Developments and Perspectives of Recycled Poly(ethylene terephthalate)-Based Membranes: A Review. Membranes. 2022; 12(11):1105. https://doi.org/10.3390/membranes12111105
Chicago/Turabian StyleKirshanov, Kirill, Roman Toms, Gadir Aliev, Alina Naumova, Pavel Melnikov, and Alexander Gervald. 2022. "Recent Developments and Perspectives of Recycled Poly(ethylene terephthalate)-Based Membranes: A Review" Membranes 12, no. 11: 1105. https://doi.org/10.3390/membranes12111105
APA StyleKirshanov, K., Toms, R., Aliev, G., Naumova, A., Melnikov, P., & Gervald, A. (2022). Recent Developments and Perspectives of Recycled Poly(ethylene terephthalate)-Based Membranes: A Review. Membranes, 12(11), 1105. https://doi.org/10.3390/membranes12111105











