The Structure Stability of Metal Diffusion Membrane-Filters in the Processes of Hydrogen Absorption/Desorption
Abstract
:1. Introduction
2. Samples and Experiment
3. Results and Discussion
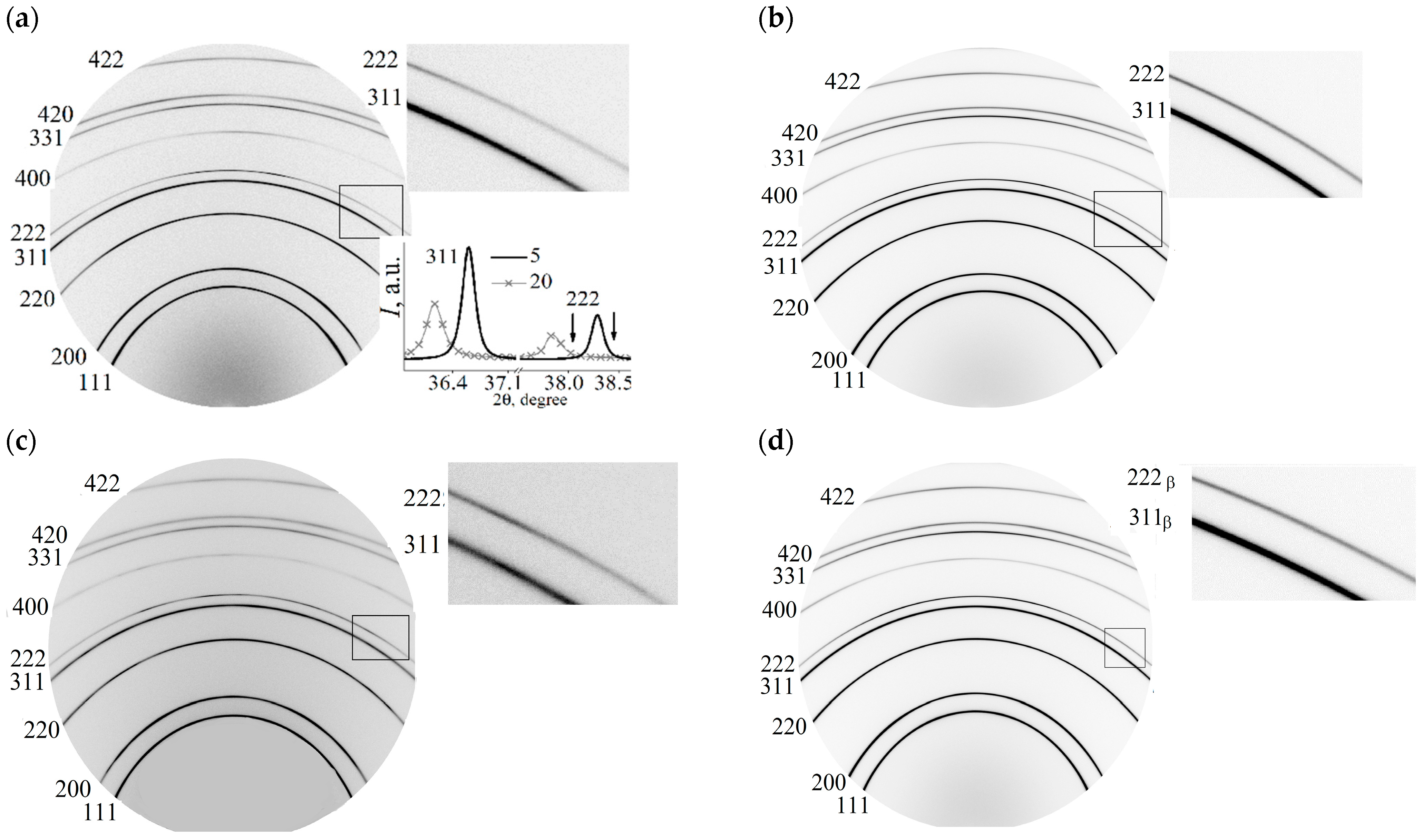
3.1. X-ray Diffraction
3.2. SEM and EDXS Analysis Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, B.D.; Chen, A. The role of palladium in hydrogen economy. Mater. Today 2011, 14, 282–290. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Burkhanov, G.S.; Gorina, N.B.; Kolchugina, N.B.; Roshan, N.R. Pd based alloy membranes for separation of high purity hydrogen from hydrogen-containing gas mixtures. Platinum Met. Rev. 2011, 55, 3–12. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-based membranes for hydrogen separation: Review of alloying elements and their influence on membrane properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Rosseau, L.R.S.; Medrano, J.A.; Bhardwaj, R.; Goetheer, E.L.V.; Filot Ivo, A.W.; Gallucci, F.; van Sint, A.M. On the potential of gallium- and indium-based liquid metal membranes for hydrogen separation. Membranes 2022, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, S.V.; Kannykin, S.V.; Penkina, T.N.; Roshan, N.R.; Chustov, E.M.; Burkhanov, G. Palladium–lead alloys for the purification of hydrogen-containing gas mixtures and the separation of hydrogen from them. Russ. Metall. 2017, 1, 54–59. [Google Scholar] [CrossRef]
- Rahimpoura, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process. Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Mascarenhas, M.D.M.; Tomaz, G.R.; Meneses, G.M.S.D.; Rodrigues, M.T.P.; Pereira, V.O.D.M.; Corassa, R.B. Influence of nanoxides on diffusivity and solubility of hydrogen in Pd-based alloys. Mater. Res. 2018, 20, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Alimov, V.N.; Busnyuk, A.O.; Notkin, M.E.; Peredistov, E.Y.; Livshits, A.I. Hydrogen transport through V–Pd alloy membranes: Hydrogen solution, permeation and diffusion. J. Membr. Sci. 2015, 481, 54–62. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.-S. Pd/Ag alloy as an application for hydrogen sensing. Int. J. Hydrogen Energy 2017, 42, 25446–25452. [Google Scholar] [CrossRef]
- Akimova, O.V.; Veligzhanin, A.A.; Svetogorov, R.D. Kinetics of the structure evolution of diffusion membrane filters of the Pd–Y system after hydrogenation. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2020, 14, 867–874. [Google Scholar] [CrossRef]
- Kyriakopoulou, H.P.; Karmiris-Obratański, P.; Tazedakis, A.S.; Daniolos, N.M.; Dourdounis, E.C.; Manolakos, D.E.; Pantelis, D. Investigation of Hydrogen Embrittlement Susceptibility and Fracture Toughness Drop after in situ Hydrogen Cathodic Charging for an X65 Pipeline Steel. Micromachines 2020, 11, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alefeld, G.; Völkl, J. (Eds.) Hydrogen in Metals I: Basic Properties; Springer: Berlin/Heidelberg, Germany, 1978; Volume 1, p. 432. ISBN 9783540358923. [Google Scholar]
- Akimova, O.V.; Svetogorov, R.D.; Gorbunov, S.V. Effect of Pb small additives on the phase composition and microstructure Pd-based membrane alloys. Key Eng. Mater. 2021, 910, 767–772. [Google Scholar] [CrossRef]
- Akimova, O.V.; Tereshina, I.S.; Kaminskaya, T.P. Effect of doping atoms in the surface morfohology of dence palladium-based diffusion membrane-filters. J. Phys. Conf. Ser. 2021, 2103, 012228. [Google Scholar] [CrossRef]
- Akimova, O.V.; Tereshina, I.S.; Kaminskaya, T.P.; Popov, V.V. Features of changing the surface relief of the diffusion membrane-filter as a result of interaction with hydrogen. Mater. Conf. Electron Beam Technol. X-ray Opt. Microelectron. 2021, 60. [Google Scholar]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Svetogorov, R.D. Dionis—Diffraction Open Integration Software; Certificate of State Registration of a Computer Program No 2018660965; LLC Scientific Electronic Library: Moscow, Russia, 2018. [Google Scholar]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Iveronova, V.I.; Revkevich, G.P. Theory of X-ray Scattering, 2nd ed.; MSU: Moscow, Russia, 1978; 278p. [Google Scholar]
- Fukai, Y.; Okuma, N. Formation of superabundant vacancies in Pd hydride under high hydrogen pressures. Phys. Rev. Lett. 1994, 73, 1640–1643. [Google Scholar] [CrossRef]
- Fukai, Y.; Sugimoto, H. Formation mechanism of defect metal hydrides containing superabundant vacancies. J. Phys. Condens. Matter 2007, 19, 436201. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filled aluminum and wolfram. Acta Metall. 1953, 1, 22–32. [Google Scholar] [CrossRef]
- Stokes, A.R.; Wilson, A.J.C. The diffraction of x rays by distorted crystal aggregates-I. Proc. Phys. Soc. 1944, 56, 174–181. [Google Scholar] [CrossRef]
- Kanaya, K.; Okayama, S. Penetration and energy loss theory of electrons in solid targets. J. Phys. D Appl. Phys. 1972, 5, 43–58. [Google Scholar] [CrossRef]
- Akimova, O.V.; Avdyukhina, V.M.; Shchetinin, I.V. Elimination of a texture of the palladium-based alloy during prolonged relaxation after hydrogenation. In Proceedings of the IV International Conference “Fundamental Research and Innovative Technologies in Mechanical Engineering”, Moscow, Russia, 24–26 November 2015; pp. 21–23. [Google Scholar]
- Nazarov, R.; Hickel, T.; Neugebauer, J. Vacancy formation energies in fcc metals: Influence of exchange-correlation functionals and correction schemes. Phys. Rev. B 2012, 85, 144118. [Google Scholar] [CrossRef]
- Lu, G.; Kaxiras, E. Hydrogen embrittlement of aluminum: The crucial role of vacancies. Phys. Rev. Lett. 2005, 94, 155501. [Google Scholar] [CrossRef] [Green Version]
- Gong, T.; Yao, P.; Xiong, X.; Zhou, H.; Zhang, Z.; Xiao, Y.; Zhao, L.; Deng, M. Microstructure and tribological behavior of interfaces in Cu-SiO2 and Cu-Cr metal matrix composites. J. Alloys Compd. 2019, 786, 975–985. [Google Scholar] [CrossRef]
- Durussel, P.; Feschotte, P. The binary system Pb-Pd. J. Alloys Compd. 1996, 236, 195–202. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 87th ed.; Taylor & Francis: Oxford, UK, 2006; 2608p. [Google Scholar]






| Membranes | wt.% | <wt.%> | at.% | <at.%> | |
|---|---|---|---|---|---|
| 1 | Pd | 95.6//95.4//95.5//95.7//95.3 | 95.5 | 97.7//97.6//97.6//97.7//97.5 | 97.6 |
| Pb | 4.4//4.6//4.5//4.3//4.7 | 4.5 | 2.3//2.4//2.4//2.3//2.5 | 2.4 | |
| 2 | Pd | 86.3/86.2//86.3//86.2//86.8 | 86.4 | 92.5//92.4//92.5//92.4//92.7 | 92.5 |
| Pb | 13.7//13.8//13.7//13.8//13.2 | 13.6 | 7.5//7.6//7.5//7.6//7.3 | 7.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimova, O.V.; Svetogorov, R.D.; Ovcharov, A.V.; Roshan, N.R. The Structure Stability of Metal Diffusion Membrane-Filters in the Processes of Hydrogen Absorption/Desorption. Membranes 2022, 12, 1132. https://doi.org/10.3390/membranes12111132
Akimova OV, Svetogorov RD, Ovcharov AV, Roshan NR. The Structure Stability of Metal Diffusion Membrane-Filters in the Processes of Hydrogen Absorption/Desorption. Membranes. 2022; 12(11):1132. https://doi.org/10.3390/membranes12111132
Chicago/Turabian StyleAkimova, Olga V., Roman D. Svetogorov, Alexey V. Ovcharov, and Nataliya R. Roshan. 2022. "The Structure Stability of Metal Diffusion Membrane-Filters in the Processes of Hydrogen Absorption/Desorption" Membranes 12, no. 11: 1132. https://doi.org/10.3390/membranes12111132






