A Comparative Analysis of the Effect of Carbonaceous Nanoparticles on the Physicochemical Properties of Hybrid Polyethersulfone Ultrafiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide
2.3. MWCNTs and GO Characterization
2.4. Membrane Fabrication
2.5. Membrane Characterization
2.6. Ultrafiltration and Water Flux Assessment
3. Results and Discussion
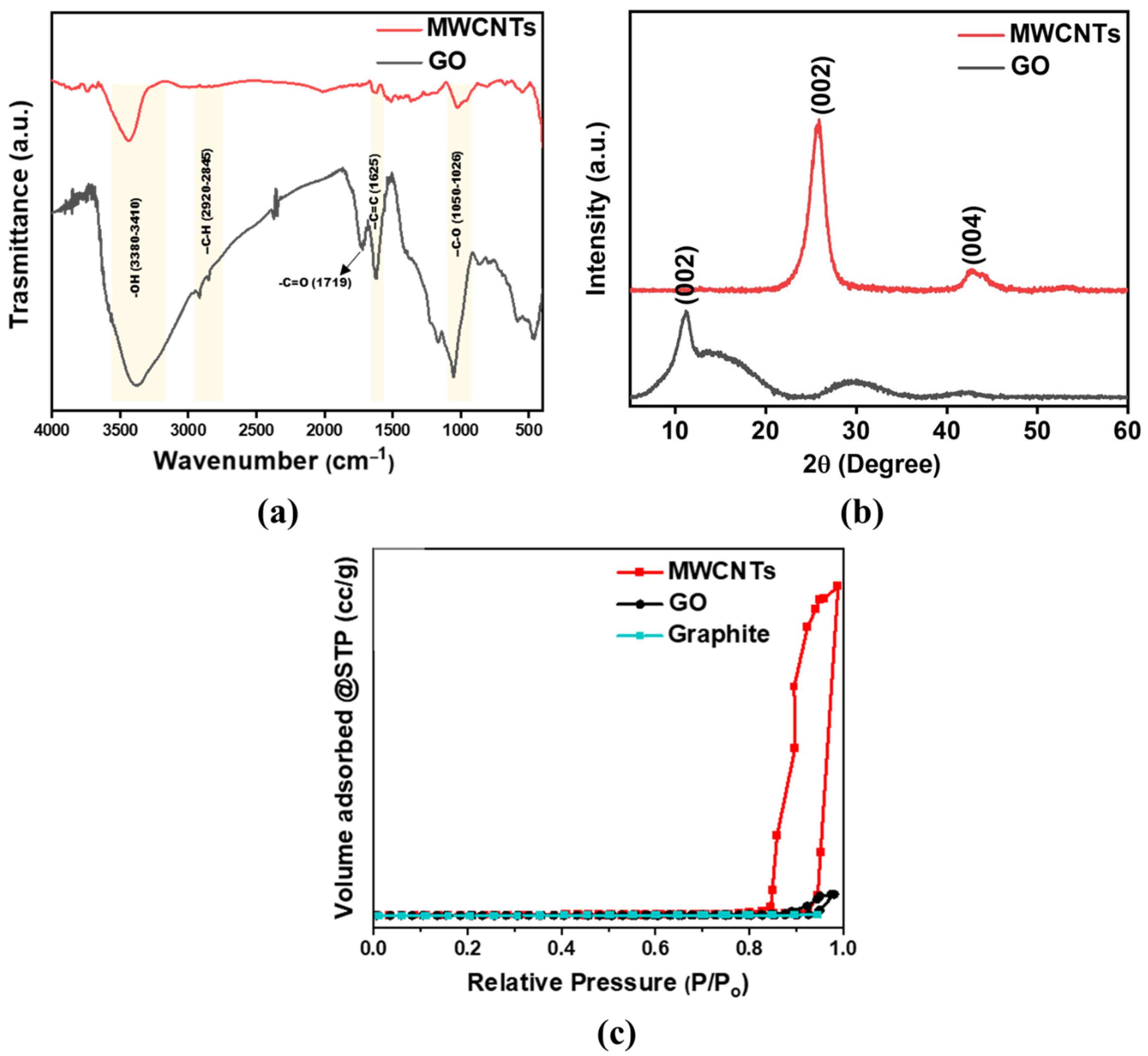
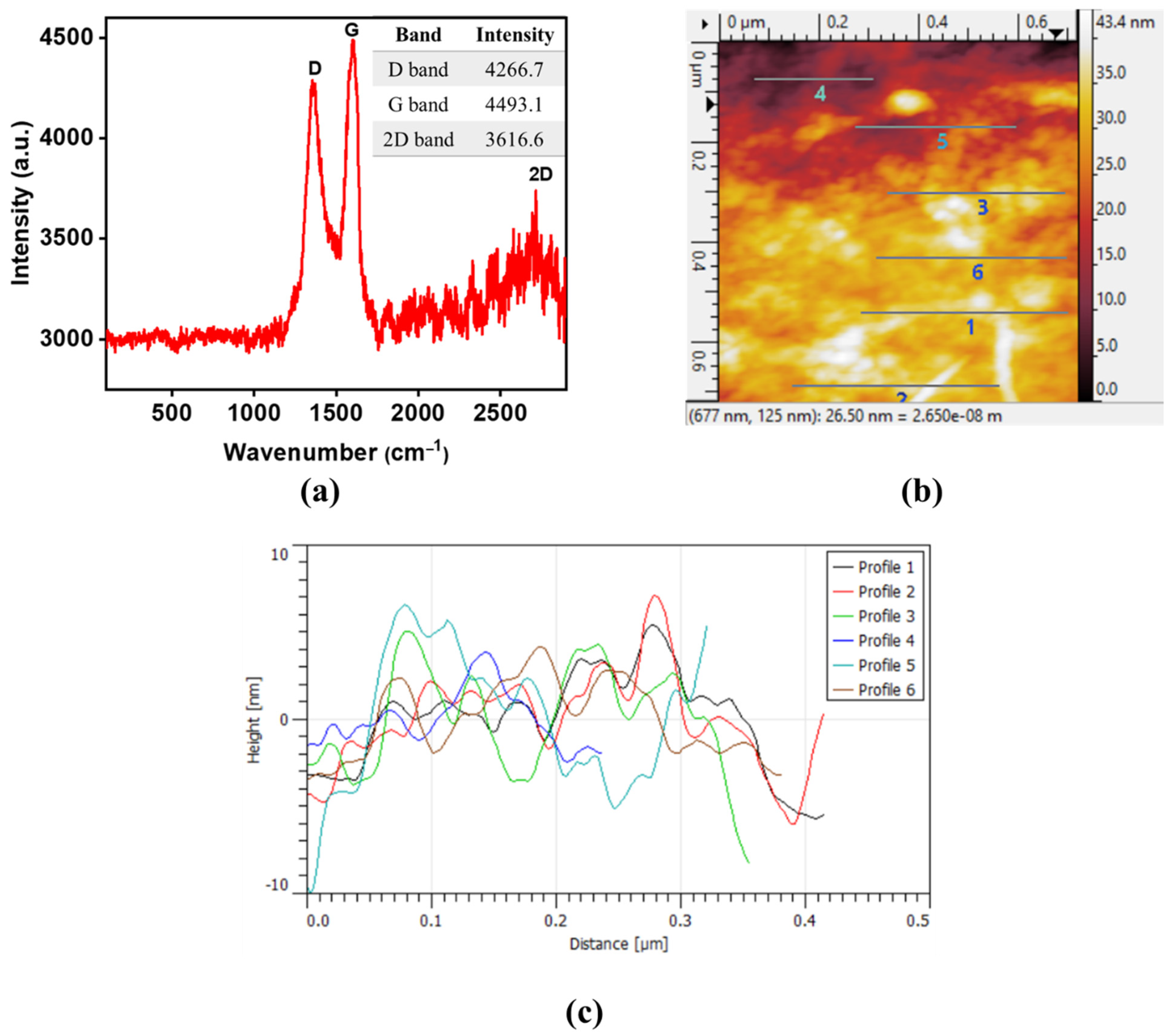
3.1. Characterization of MWCNTs and GO
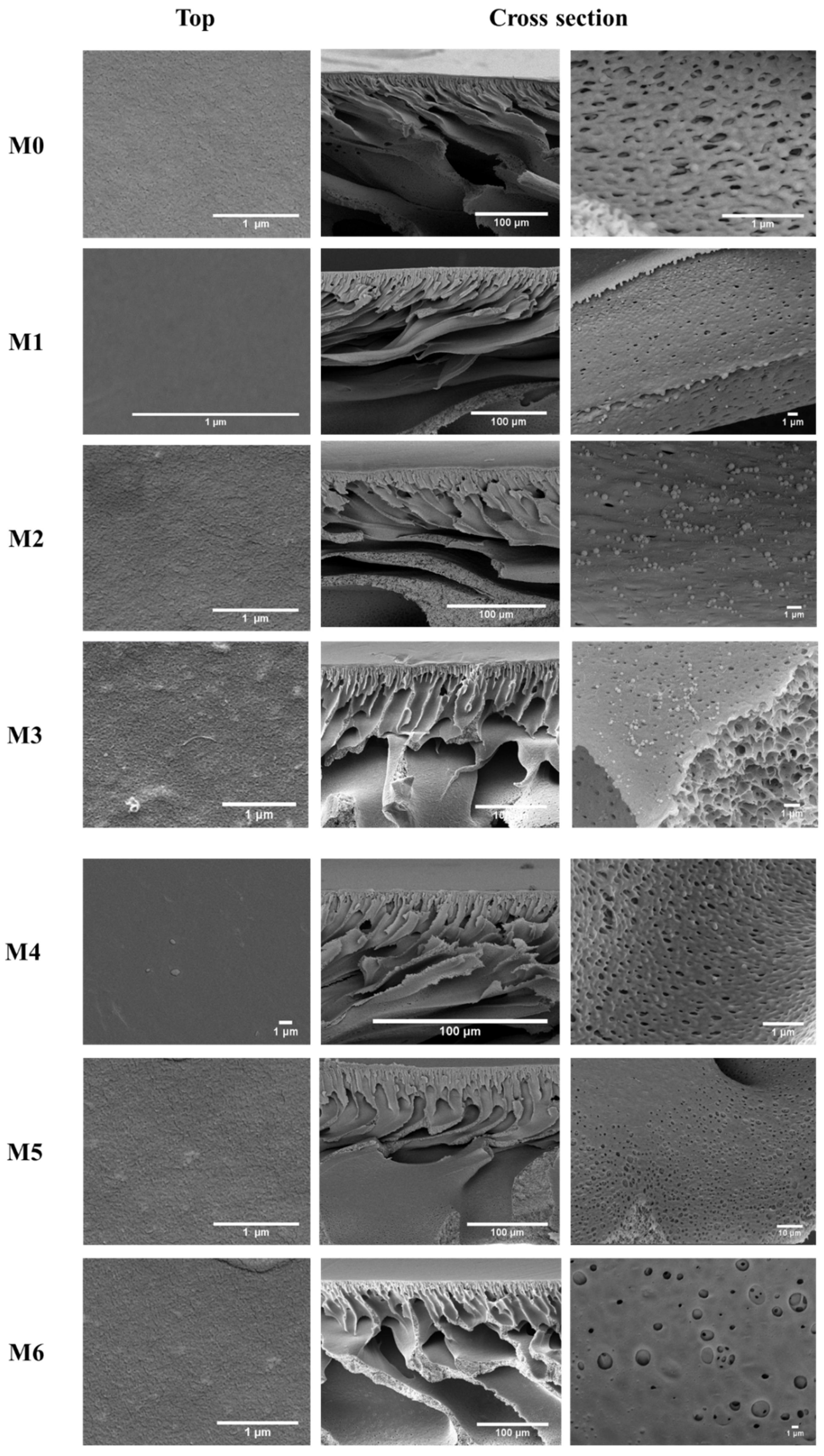
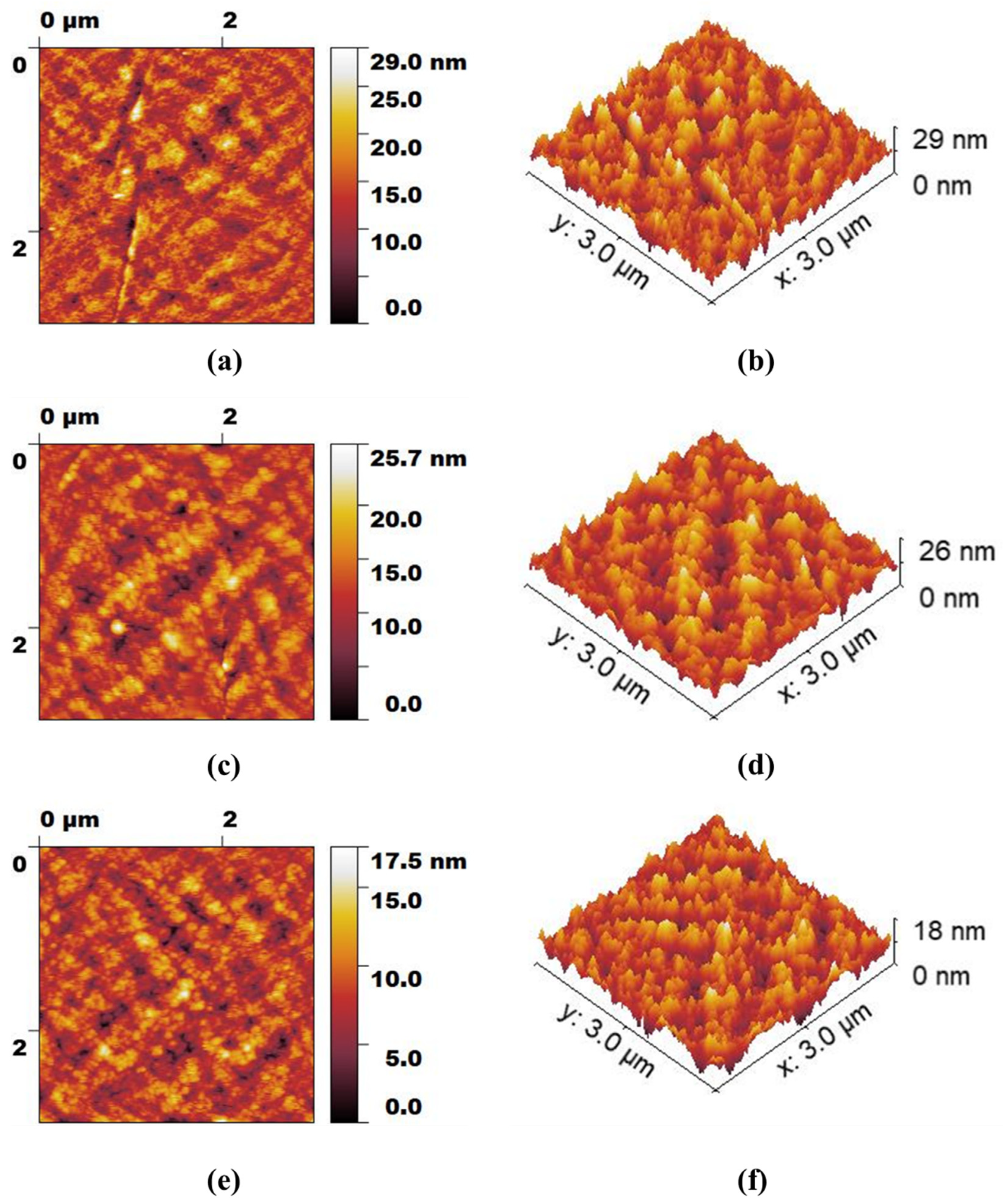
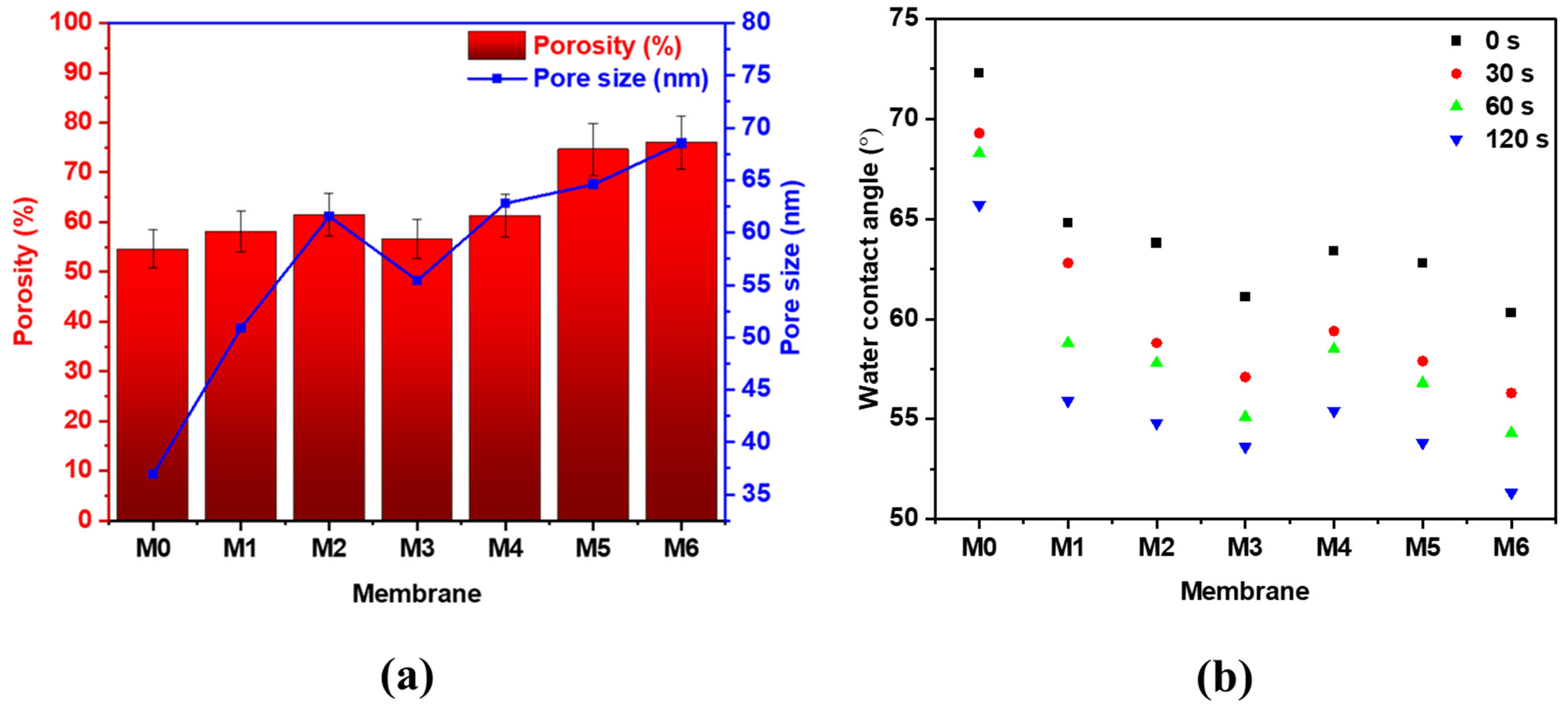
3.2. Membrane Characterization
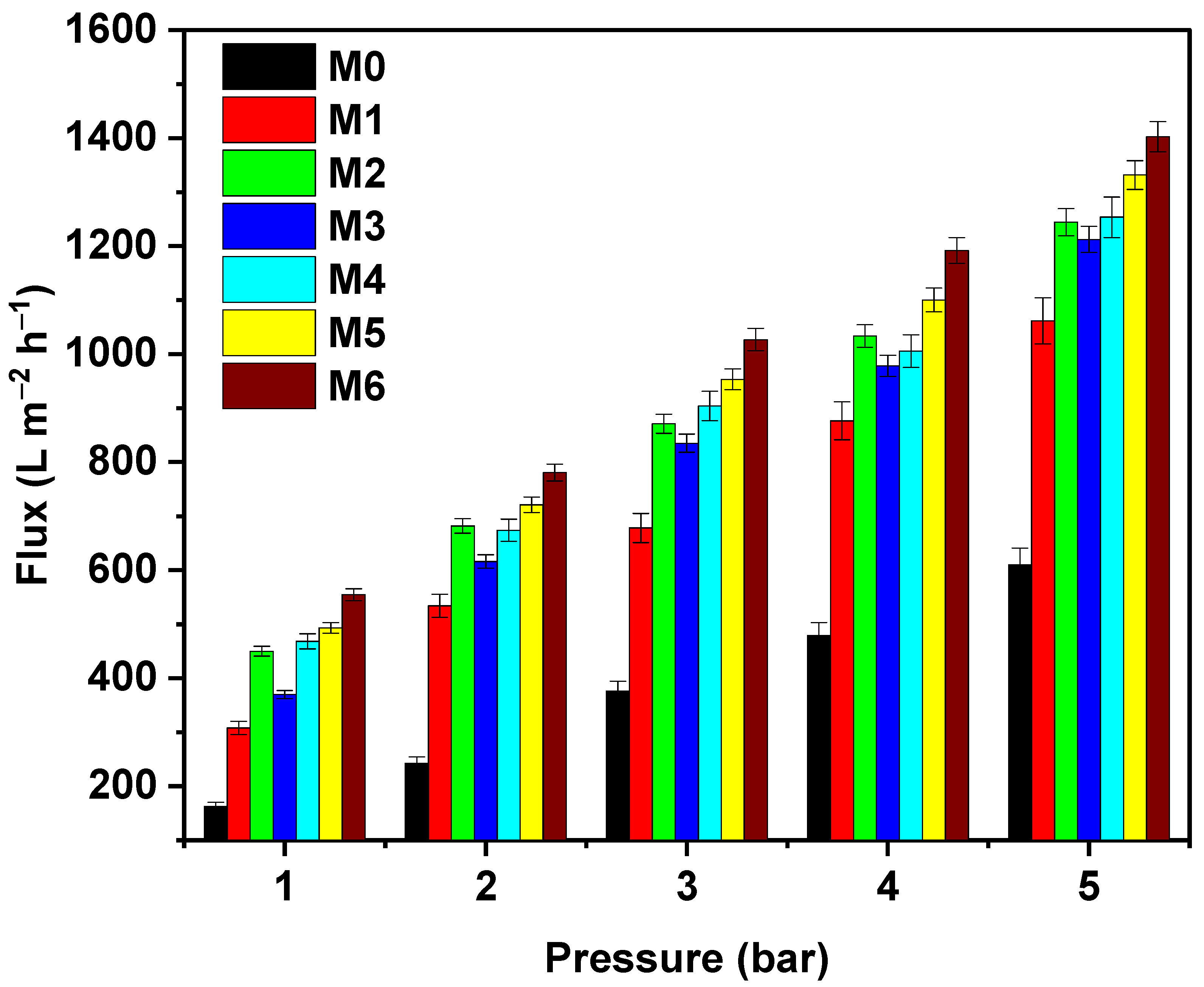
3.3. Membrane Performance
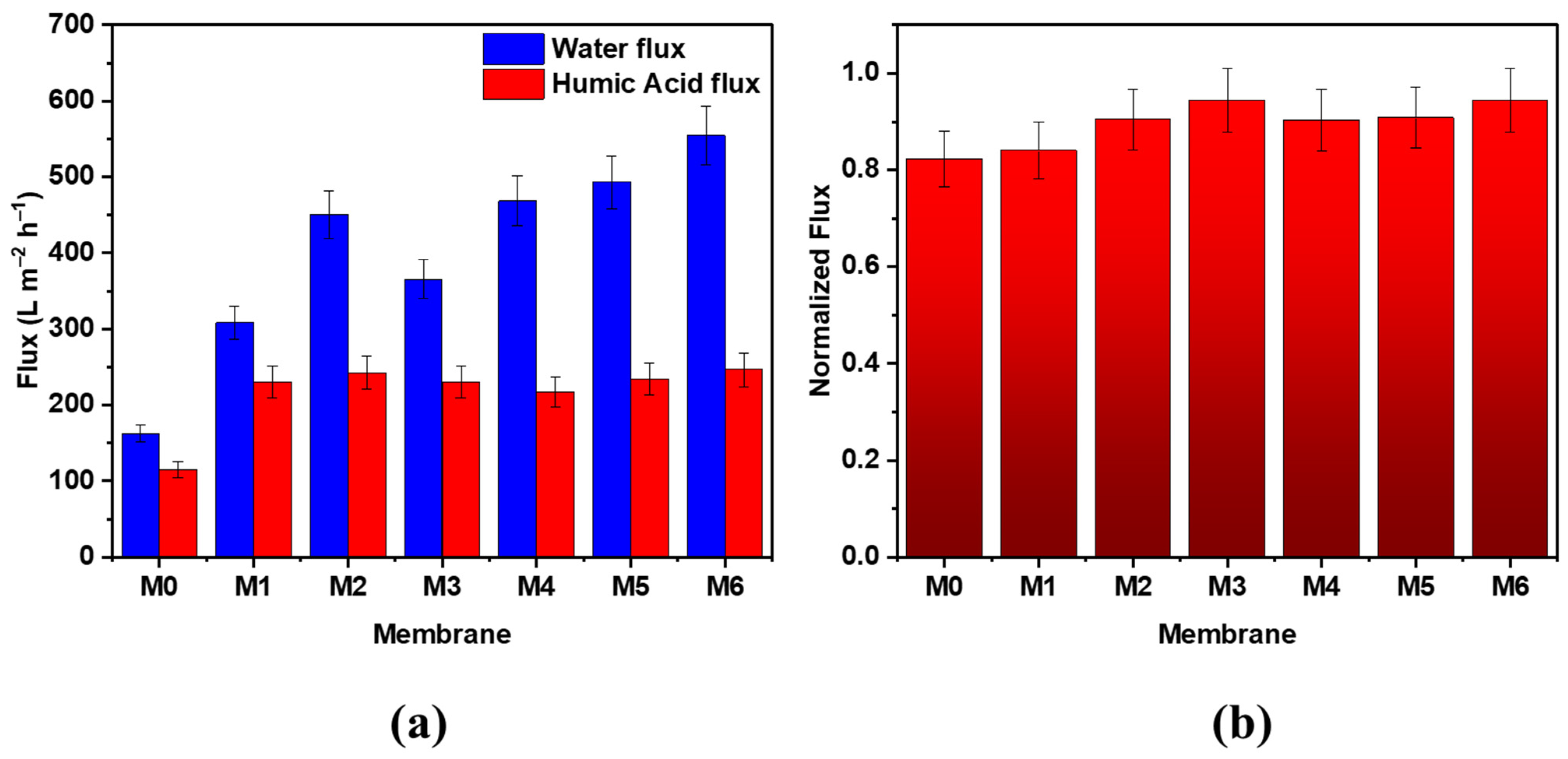
3.3.1. Membrane Fluxes during Filtration of Pure Water and HA Solutions
3.3.2. HA Filtration
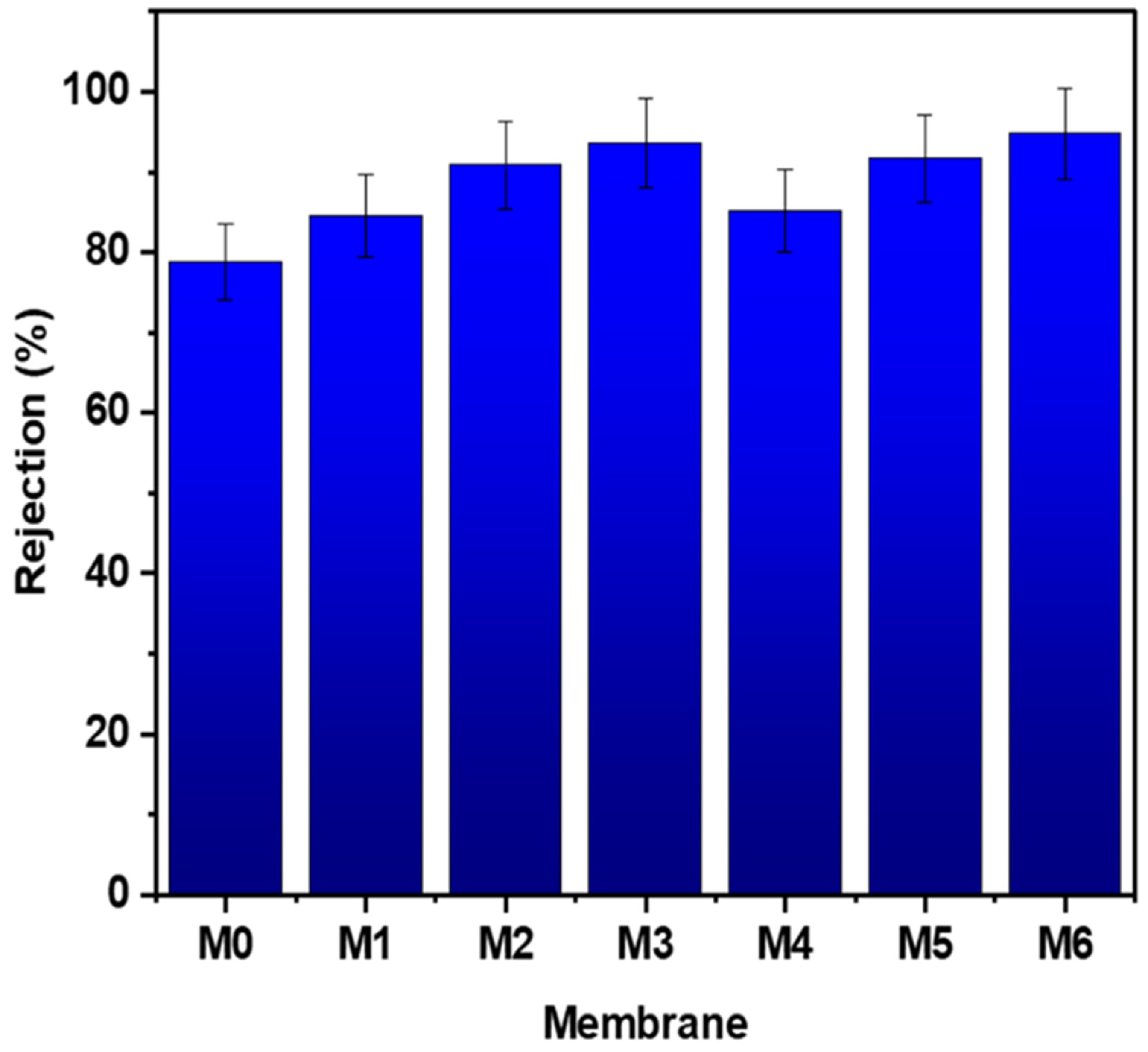
3.3.3. HA Rejection
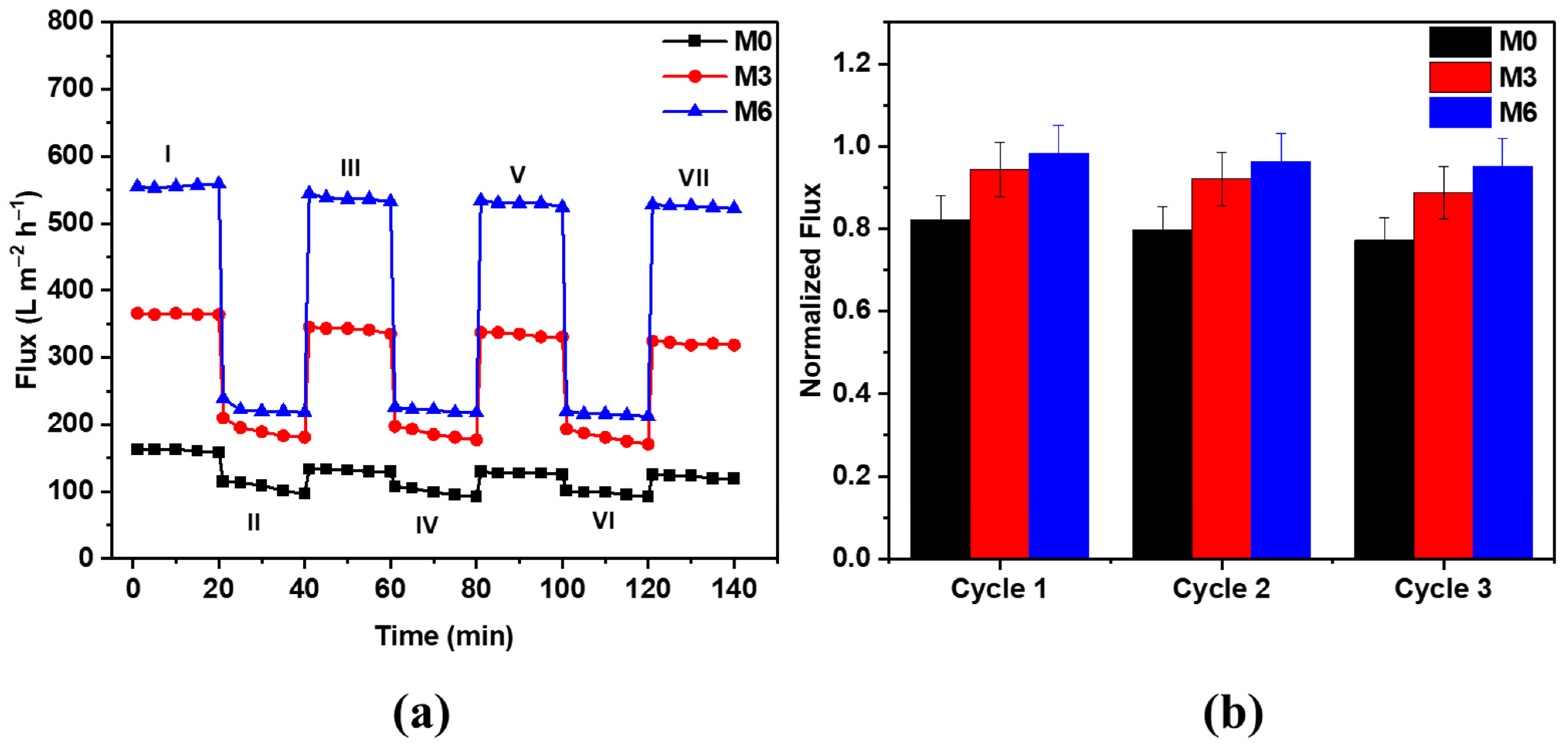
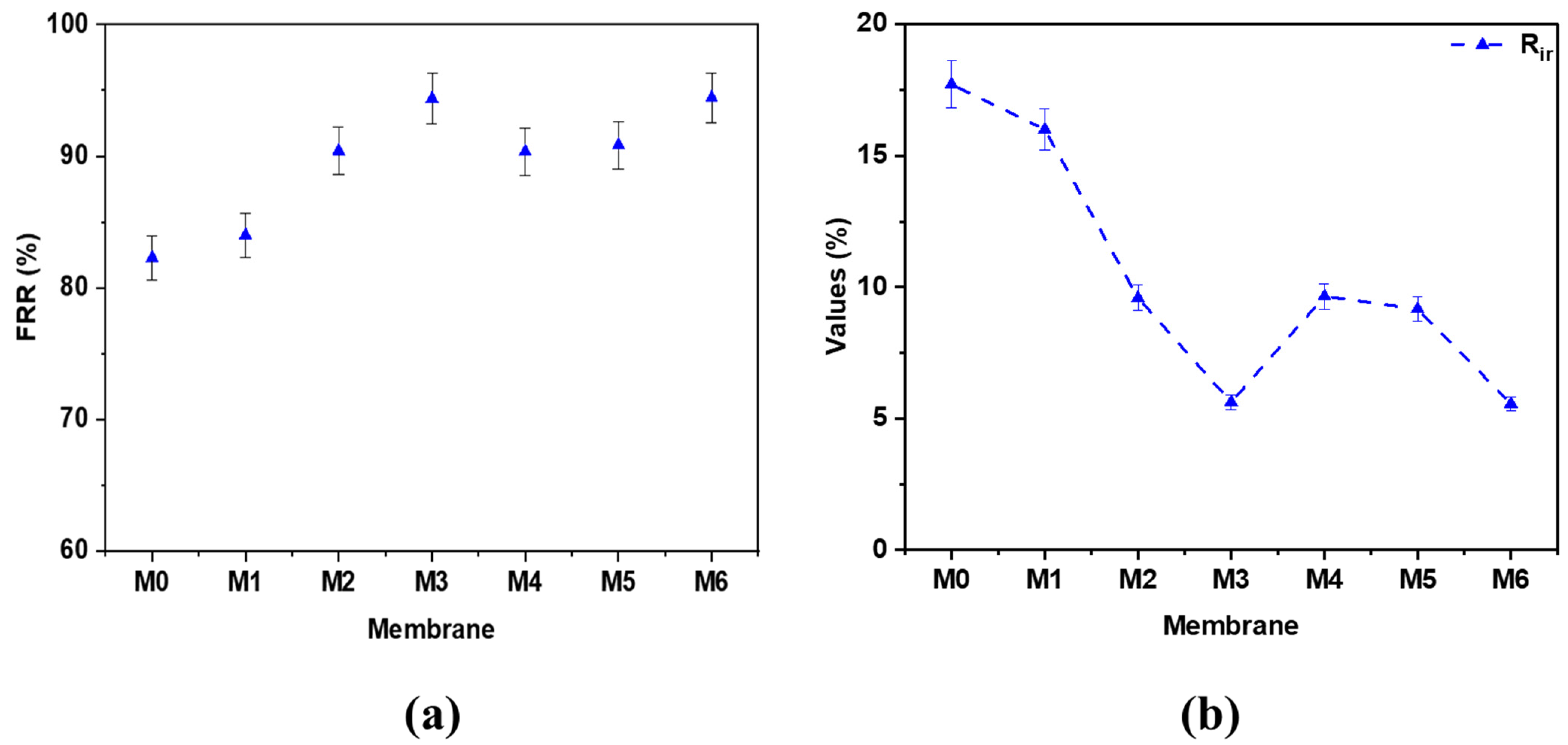
3.3.4. Flux Recovery of PES Hybrid Membranes
3.3.5. Comparative Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almanassra, I.W.; Kochkodan, V.; Mckay, G.; Atieh, M.A.; Al-Ansari, T. Kinetic and Thermodynamic Investigations of Surfactants Adsorption from Water by Carbide-Derived Carbon. J. Environ. Sci. Heal. Part A Toxic/Hazard. Subst. Environ. Eng. 2021, 56, 1206–1220. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.D.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhou, J.; Liu, F.; Xu, B.M.; Pan, Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes 2022, 12, 519. [Google Scholar] [CrossRef]
- Sabri, S.; Najjar, A.; Manawi, Y.; Eltai, N.O.; Al-Thani, A.; Atieh, M.A.; Kochkodan, V. Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum. Membranes 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siagian, U.W.R.; Khoiruddin, K.; Wardani, A.K.; Aryanti, P.T.P.; Widiasa, I.N.; Qiu, G.; Ting, Y.P.; Wenten, I.G. High-Performance Ultrafiltration Membrane: Recent Progress and Its Application for Wastewater Treatment. Curr. Pollut. Rep. 2021, 7, 448–462. [Google Scholar] [CrossRef]
- Kallem, P.; Othman, I.; Ouda, M.; Hasan, S.W.; AlNashef, I.; Banat, F. Polyethersulfone Hybrid Ultrafiltration Membranes Fabricated with Polydopamine Modified ZnFe2O4 Nanocomposites: Applications in Humic Acid Removal and Oil/Water Emulsion Separation. Process Saf. Environ. Prot. 2021, 148, 813–824. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Ooi, B.S.; Ismail, S. Recent Development in Additives Modifications of Polyethersulfone Membrane for Flux Enhancement. Chem. Eng. J. 2013, 223, 246–267. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Mahmoudi, E.; Johnson, D.J.; Mohammad, A.W.; Atieh, M.A. Characterization and Separation Performance of a Novel Polyethersulfone Membrane Blended with Acacia Gum. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Alkhouzaam, A.; Qiblawey, H. Novel Polysulfone Ultrafiltration Membranes Incorporating Polydopamine Functionalized Graphene Oxide with Enhanced Flux and Fouling Resistance. J. Membr. Sci. 2021, 620, 118900. [Google Scholar] [CrossRef]
- Algamdi, M.S.; Alsohaimi, I.H.; Lawler, J.; Ali, H.M.; Aldawsari, A.M.; Hassan, H.M.A. Fabrication of Graphene Oxide Incorporated Polyethersulfone Hybrid Ultrafiltration Membranes for Humic Acid Removal. Sep. Purif. Technol. 2019, 223, 17–23. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Monash, P.; Velu, S.; Banat, F.; Naushad, M.; Arthanareeswaran, G.; Loke Show, P. Effective Treatment of Dye Polluted Wastewater Using Nanoporous CaCl2 Modified Polyethersulfone Membrane. Process Saf. Environ. Prot. 2019, 124, 266–278. [Google Scholar] [CrossRef]
- Low, Z.X.; Wang, Z.; Leong, S.; Razmjou, A.; Dumée, L.F.; Zhang, X.; Wang, H. Enhancement of the Antifouling Properties and Filtration Performance of Poly(Ethersulfone) Ultrafiltration Membranes by Incorporation of Nanoporous Titania Nanoparticles. Ind. Eng. Chem. Res. 2015, 54, 11188–11198. [Google Scholar] [CrossRef]
- Kallem, P.; Bharath, G.; Rambabu, K.; Srinivasakannan, C.; Banat, F. Improved Permeability and Antifouling Performance of Polyethersulfone Ultrafiltration Membranes Tailored by Hydroxyapatite/Boron Nitride Nanocomposites. Chemosphere 2021, 268, 129306. [Google Scholar] [CrossRef] [PubMed]
- Kallem, P.; Ouda, M.; Bharath, G.; Hasan, S.W.; Banat, F. Enhanced Water Permeability and Fouling Resistance Properties of Ultrafiltration Membranes Incorporated with Hydroxyapatite Decorated Orange-Peel-Derived Activated Carbon Nanocomposites. Chemosphere 2022, 286, 131799. [Google Scholar] [CrossRef] [PubMed]
- Najjar, A.; Sabri, S.; Al-Gaashani, R.; Atieh, M.A.; Kochkodan, V. Antibiofouling Performance by Polyethersulfone Membranes Cast with Oxidized Multiwalled Carbon Nanotubes and Arabic Gum. Membranes. 2019, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Ouda, M.; Hai, A.; Krishnamoorthy, R.; Govindan, B.; Othman, I.; Kui, C.C.; Choi, M.Y.; Hasan, S.W.; Banat, F. Surface Tuned Polyethersulfone Membrane Using an Iron Oxide Functionalized Halloysite Nanocomposite for Enhanced Humic Acid Removal. Environ. Res. 2022, 204, 112113. [Google Scholar] [CrossRef]
- Lewis, J.; Al-sayaghi, M.A.Q.; Buelke, C.; Alshami, A. Activated Carbon in Mixed-Matrix Membranes. Sep. Purif. Rev. 2021, 50, 1–31. [Google Scholar] [CrossRef]
- Van Der Bruggen, B. Chemical Modification of Polyethersulfone Nanofiltration Membranes: A Review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Biswas, P.; Fortner, J.D. Graphene Oxides as Nanofillers in Polysulfone Ultrafiltration Membranes: Shape Matters. J. Membr. Sci. 2019, 581, 453–461. [Google Scholar] [CrossRef]
- Seetharaman, S.; Raghu, S.C.; Velan, M.; Ramya, K.; Mahabadi, K.A. Comparison of the Performance of Reduced Graphene Oxide and Multiwalled Carbon Nanotubes Based Sulfonated Polysulfone Membranes for Electrolysis Application. Polym. Compos. 2015, 36, 475–481. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic Effects of Oxidized Carbon Nanotubes and Graphene Oxide on Fouling Control and Anti-Fouling Mechanism of Polyvinylidene Fluoride Ultrafiltration Membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (Go) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Z.; Mai, W.; Min, C.; Zhou, B.; Shan, M.; Li, Y.; Yang, C.; Wang, Z.; Qian, X. Improved Hydrophilicity, Permeability, Antifouling and Mechanical Performance of PVDF Composite Ultrafiltration Membranes Tailored by Oxidized Low-Dimensional Carbon Nanomaterials. J. Mater. Chem. A 2013, 1, 3101–3111. [Google Scholar] [CrossRef]
- Shevate, R.; Kumar, M.; Karunakaran, M.; Hedhili, M.N.; Peinemann, K.V. Polydopamine/Cysteine Surface Modified Isoporous Membranes with Self-Cleaning Properties. J. Membr. Sci. 2017, 529, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of Metal-Organic Framework Hybrid Nanocomposites Based on GO and CNT with High Adsorption Capacity for Dye Removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Sun, L.; Wang, M.; Li, W.; Luo, S.; Wu, Y.; Ma, C.; Liu, S. Carbon Material–Immobilized Ionic Liquids Were Applied on Absorption of Hg2+ from Water Phase. Environ. Sci. Pollut. Res. 2020, 27, 26882–26904. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [Green Version]
- Moosa, A.A.; Abed, M.S. Graphene Preparation and Graphite Exfoliation. Turk. J. Chem. 2021, 45, 493–519. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Lee, D.J.; Park, S.S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 590. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, H.D.; Nguyen, V.C.; Ngo, T.T.T.; Le, D.Q.; Nguyen, X.N.; Phan, N.M. Synthesis of Multi-Layer Graphene Films on Copper Tape by Atmospheric Pressure Chemical Vapor Deposition Method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 35012. [Google Scholar] [CrossRef]
- Hwangbo, Y.; Lee, C.K.; Mag-Isa, A.E.; Jang, J.W.; Lee, H.J.; Lee, S.B.; Kim, S.S.; Kim, J.H. Interlayer Non-Coupled Optical Properties for Determining the Number of Layers in Arbitrarily Stacked Multilayer Graphenes. Carbon 2014, 77, 454–461. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Sagadevan, V. Polyethylene Glycol and Iron Oxide Nanoparticles Blended Polyethersulfone Ultrafiltration Membrane for Enhanced Performance in Dye Removal Studies. E-Polymers 2015, 15, 151–159. [Google Scholar] [CrossRef]
- Behdarvand, F.; Valamohammadi, E.; Tofighy, M.A.; Mohammadi, T. Polyvinyl Alcohol/Polyethersulfone Thin-Film Nanocomposite Membranes with Carbon Nanomaterials Incorporated in Substrate for Water Treatment. J. Environ. Chem. Eng. 2021, 9, 104650. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a Novel Antifouling Mixed Matrix PES Membrane by Embedding Graphene Oxide Nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, G. Fabrication of High-Performance Ultrafiltration Membranes Using Zwitterionic Carbon Nanotubes and Polyethersulfone. High Perform. Polym. 2018, 30, 602–611. [Google Scholar] [CrossRef]
- Mehrparvar, A.; Rahimpour, A.; Jahanshahi, M. Modified Ultrafiltration Membranes for Humic Acid Removal. J. Taiwan Inst. Chem. Eng. 2014, 45, 275–282. [Google Scholar] [CrossRef]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon Nanotube Blended Polyethersulfone Membranes for Fouling Control in Water Treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Han, M.J.; Nam, S.T. Thermodynamic and Rheological Variation in Polysulfone Solution by PVP and Its Effect in the Preparation of Phase Inversion Membrane. J. Membr. Sci. 2002, 202, 55–61. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel Functionalized Carbon Nanotubes for Improving the Surface Properties and Performance of Polyethersulfone (PES) Membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Ye, W.; Liu, H.; Lin, F.; Lin, J.; Zhao, S.; Yang, S.; Hou, J.; Zhou, S.; Van Der Bruggen, B. High-Flux Nanofiltration Membranes Tailored by Bio-Inspired Co-Deposition of Hydrophilic g-C3N4 Nanosheets for Enhanced Selectivity towards Organics and Salts. Environ. Sci. Nano 2019, 6, 2958–2967. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Fang, X.; Bakzhan, K.; Wang, L.; Van der Bruggen, B. A Synergetic Analysis Method for Antifouling Behavior Investigation on PES Ultrafiltration Membrane with Self-Assembled TiO2 Nanoparticles. J. Colloid Interface Sci. 2016, 469, 164–176. [Google Scholar] [CrossRef]
- Wang, X.; Feng, M.; Liu, Y.; Deng, H.; Lu, J. Fabrication of Graphene Oxide Blended Polyethersulfone Membranes via Phase Inversion Assisted by Electric Field for Improved Separation and Antifouling Performance. J. Membr. Sci. 2019, 577, 41–50. [Google Scholar] [CrossRef]
- Najjar, A.; Sabri, S.; Al-Gaashani, R.; Kochkodan, V.; Atieh, M.A. Enhanced Fouling Resistance and Antibacterial Properties of Novel Graphene Oxide-Arabic Gum Polyethersulfone Membranes. Appl. Sci. 2019, 9, 513. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, M.; Mohammadi, T.; Kasiri, N.; Tofighy, M.A. Fabrication of Magnetic Field Induced Mixed Matrix Membranes Containing GO/Fe3O4 nanohybrids with Enhanced Antifouling Properties for Wastewater Treatment Applications. J. Environ. Chem. Eng. 2021, 9, 105675. [Google Scholar] [CrossRef]
- Kong, S.; Lim, M.; Shin, H.; Baik, J.H.; Lee, J.C. High-Flux and Antifouling Polyethersulfone Nanocomposite Membranes Incorporated with Zwitterion-Functionalized Graphene Oxide for Ultrafiltration Applications. J. Ind. Eng. Chem. 2020, 84, 131–140. [Google Scholar] [CrossRef]
- Lemos, H.G.; Ragio, R.A.; Conceição, A.C.S.; Venancio, E.C.; Mierzwa, J.C.; Subtil, E.L. Assessment of Mixed Matrix Membranes (MMMs) Incorporated with Graphene Oxide (GO) for Co-Treatment of Wastewater and Landfill Leachate (LFL) in a Membrane Bioreactor (MBR). Chem. Eng. J. 2021, 425, 131772. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Jabbarvand Behrouz, S.; Vatanpour, V.; Haddadi Gharamaleki, S.; Orooji, Y.; Safarpour, M. Development of MoS2/O-MWCNTs/PES Blended Membrane for Efficient Removal of Dyes, Antibiotic, and Protein. Sep. Purif. Technol. 2022, 280, 119822. [Google Scholar] [CrossRef]
- Pang, W.Y.; Ahmad, A.L.; Zaulkiflee, N.D. Antifouling and Antibacterial Evaluation of ZnO/MWCNT Dual Nanofiller Polyethersulfone Mixed Matrix Membrane. J. Environ. Manag. 2019, 249, 109358. [Google Scholar] [CrossRef] [PubMed]
- Gumbi, N.N.; Hu, M.; Mamba, B.B.; Li, J.; Nxumalo, E.N. Macrovoid-Free PES/SPSf/O-MWCNT Ultrafiltration Membranes with Improved Mechanical Strength, Antifouling and Antibacterial Properties. J. Membr. Sci. 2018, 566, 288–300. [Google Scholar] [CrossRef]
- Hegab, H.M.; Elaraby, A.; Ibrahim, Y.; Elmekawy, A.; Marzooqi, F.; Aljundi, I.H.; Hasan, S.W. Designing of Amino Silica Covalently Functionalized Carboxylic Multi-Wall Carbon Nanotubes-Based Polyethersulfone Membranes for Enhancing Oily Wastewater Treatment. J. Environ. Chem. Eng. 2022, 10, 108667. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.; Shan, B.; Xie, C.; Liu, C.; Cui, F.; Li, G. Preparation and Characterization of SLS-CNT/PES Ultrafiltration Membrane with Antifouling and Antibacterial Properties. J. Membr. Sci. 2018, 548, 459–469. [Google Scholar] [CrossRef]

| Membrane | PES (wt.%) | PVP (wt.%) | Nanoadditives | Nanoadditive Loading (wt.%) |
|---|---|---|---|---|
| M0 | 16 | 2 | N/A | N/A |
| M1 | 16 | 2 | MWCNTs | 0.1 |
| M2 | 16 | 2 | MWCNTs | 0.5 |
| M3 | 16 | 2 | MWCNTs | 1.0 |
| M4 | 16 | 2 | GO | 0.1 |
| M5 | 16 | 2 | GO | 0.5 |
| M6 | 16 | 2 | GO | 1.0 |
| Membrane | φ (%) | (°) | |
|---|---|---|---|
| M0 | 74.62 | 72.3 | 94.9 |
| M1 | 78.76 | 64.8 | 103.8 |
| M2 | 79.19 | 63.8 | 104.9 |
| M3 | 79.73 | 61.1 | 107.9 |
| M4 | 79.72 | 63.4 | 105.4 |
| M5 | 80.08 | 62.8 | 106.1 |
| M6 | 81.65 | 60.3 | 108.9 |
| Membrane (wt.%) | Membrane Preparation Method | Physicochemical Properties | Water Flux (LMH) and Pollutant Rejection (%) | FRR (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Porosity (%) | Mean Pore Size (nm) | WCA (°) | |||||
| PES/GO (0.008) | Phase inversion assisted by a direct current electric field | 80 | 23.2 | 50.7 | 289.86 at 4 bar 86.58% Methyl red | 89.18 | [42] |
| PES/GA/GO (5) | Phase inversion | ~85 | 66.4 | ~58–59 | ~200 at 1 bar ~80–85% BSA | 75 | [43] |
| GO/Fe3O4/PES (2) | Phase inversion | ~75 | ~30 | 66.1 | 175.6 at 1 bar 98.0% BSA | 87.9 | [44] |
| M-GO/Fe3O4/PES (2) | Phase inversion | ~86 | ~32 | 53.9 | 252.7 at 1 bar 92.3% BSA | 80.1 | |
| CGO/PES (0.5) | Non-solvent-induced phase separation | N/A | 7.2 | 53.2 | 82.6 at 2 bar 99.8% BSA | 92.1 | [45] |
| PES-GO (0.6) | Phase inversion | 61.6 | N/A | 46.9 | 161 at 1 bar 97% HA 99.5% BSA | 85 | [46] |
| PES/GO (1) | Non-solvent-induced phase separation | 76 | 68.5 | 60.3 | 554.8 at 1 bar 94.8% HA | 94.4 | This work |
| MoS2/O-MWCNTs/PES (0.75) | Phase inversion | 81.7 | 25.1 | 49.6 | 192.2 at 3 bar 98.4% Reactive red 195 93.5% Reactive blue 19 99.0% BSA | 60.8 | [47] |
| PES/ZnO-MWCNTs (0.5) | Non-solvent-induced phase separation | 46.02 | 58.81 | 58.7 | 57.1 at 1 bar 88.5% HA | 77.18 | [48] |
| PES/SPSf/O-MWCNT (0.05) | Non-solvent-induced phase separation | 88 | 40.9 | ~50 | 553 at 1 bar 100% BSA | ~93 | [49] |
| SiO2-f-MWCNTs/PES (2) | Phase inversion | ~70 | ~65 | 55.3 | 293 at 0.7 bar 85.6% HA 97% oil | 99 | [50] |
| f-CNT/PES (2) | Phase inversion | 74 | ~86–87 | 57 | ~600 at 1 bar >95% BSA | >90 | [51] |
| F-MWCNTs/PES (1) | Phase inversion | N/A | N/A | 51.9 | ~185 at 3 bar ~86–87% BSA | 46 | [39] |
| PES/GA/OMWCNTs (3) | Phase inversion | ~82 | ~68 | ~64 | ~520 at 1 bar ~80% BSA | N/A | [15] |
| PES/MWCNTs (0.5) | Non-solvent-induced phase separation | 61.5 | 61.5 | 63.8 | 450 at 1 bar 90.8% HA | 90.4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaber, L.; Almanassra, I.W.; Backer, S.N.; Kochkodan, V.; Shanableh, A.; Atieh, M.A. A Comparative Analysis of the Effect of Carbonaceous Nanoparticles on the Physicochemical Properties of Hybrid Polyethersulfone Ultrafiltration Membranes. Membranes 2022, 12, 1143. https://doi.org/10.3390/membranes12111143
Jaber L, Almanassra IW, Backer SN, Kochkodan V, Shanableh A, Atieh MA. A Comparative Analysis of the Effect of Carbonaceous Nanoparticles on the Physicochemical Properties of Hybrid Polyethersulfone Ultrafiltration Membranes. Membranes. 2022; 12(11):1143. https://doi.org/10.3390/membranes12111143
Chicago/Turabian StyleJaber, Lubna, Ismail W. Almanassra, Sumina Namboorimadathil Backer, Viktor Kochkodan, Abdallah Shanableh, and Muataz Ali Atieh. 2022. "A Comparative Analysis of the Effect of Carbonaceous Nanoparticles on the Physicochemical Properties of Hybrid Polyethersulfone Ultrafiltration Membranes" Membranes 12, no. 11: 1143. https://doi.org/10.3390/membranes12111143
APA StyleJaber, L., Almanassra, I. W., Backer, S. N., Kochkodan, V., Shanableh, A., & Atieh, M. A. (2022). A Comparative Analysis of the Effect of Carbonaceous Nanoparticles on the Physicochemical Properties of Hybrid Polyethersulfone Ultrafiltration Membranes. Membranes, 12(11), 1143. https://doi.org/10.3390/membranes12111143








