Graphene Oxide-Carbon Nanotube (GO-CNT) Hybrid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Sorption Studies
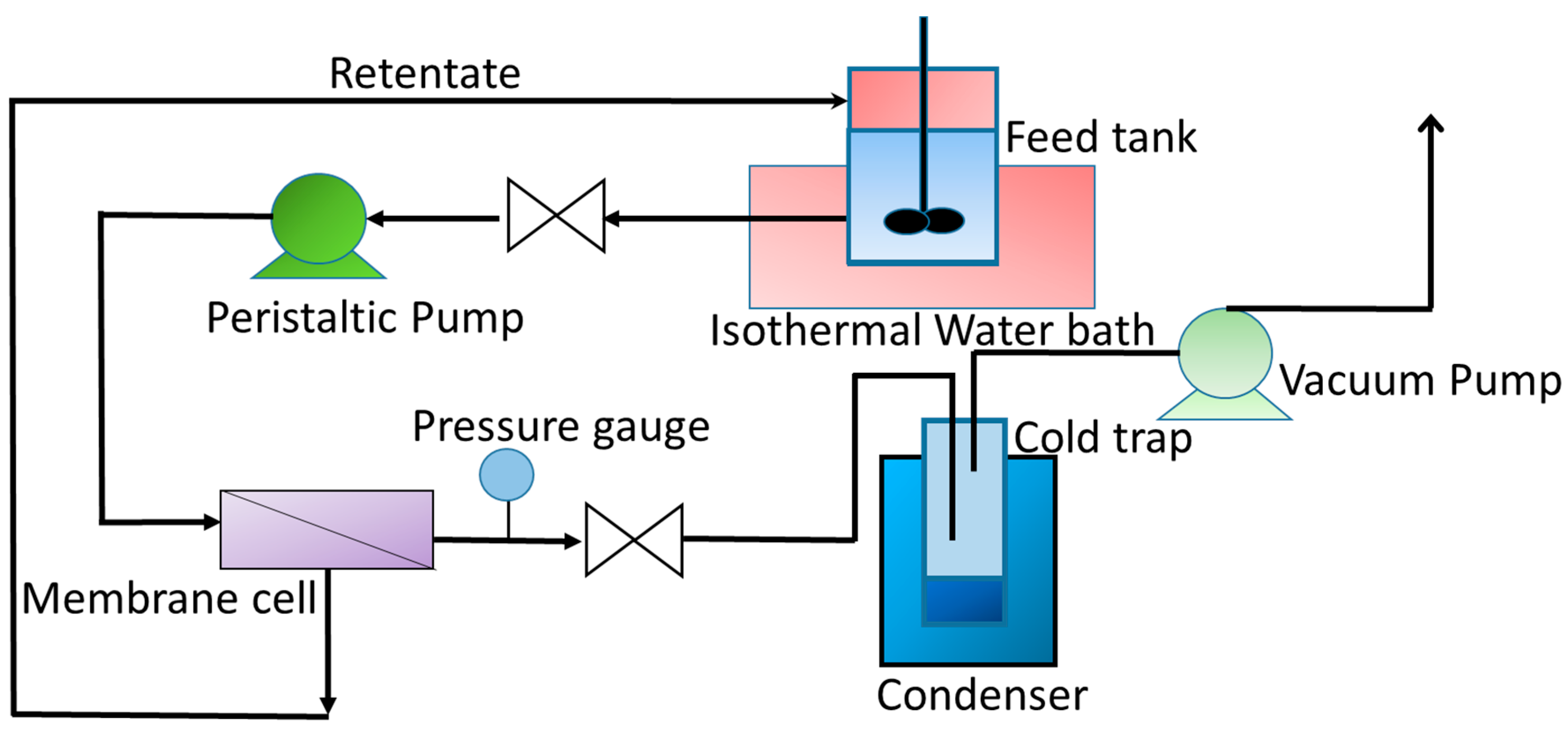
3. Pervaporation Experiments
4. Results and Discussion
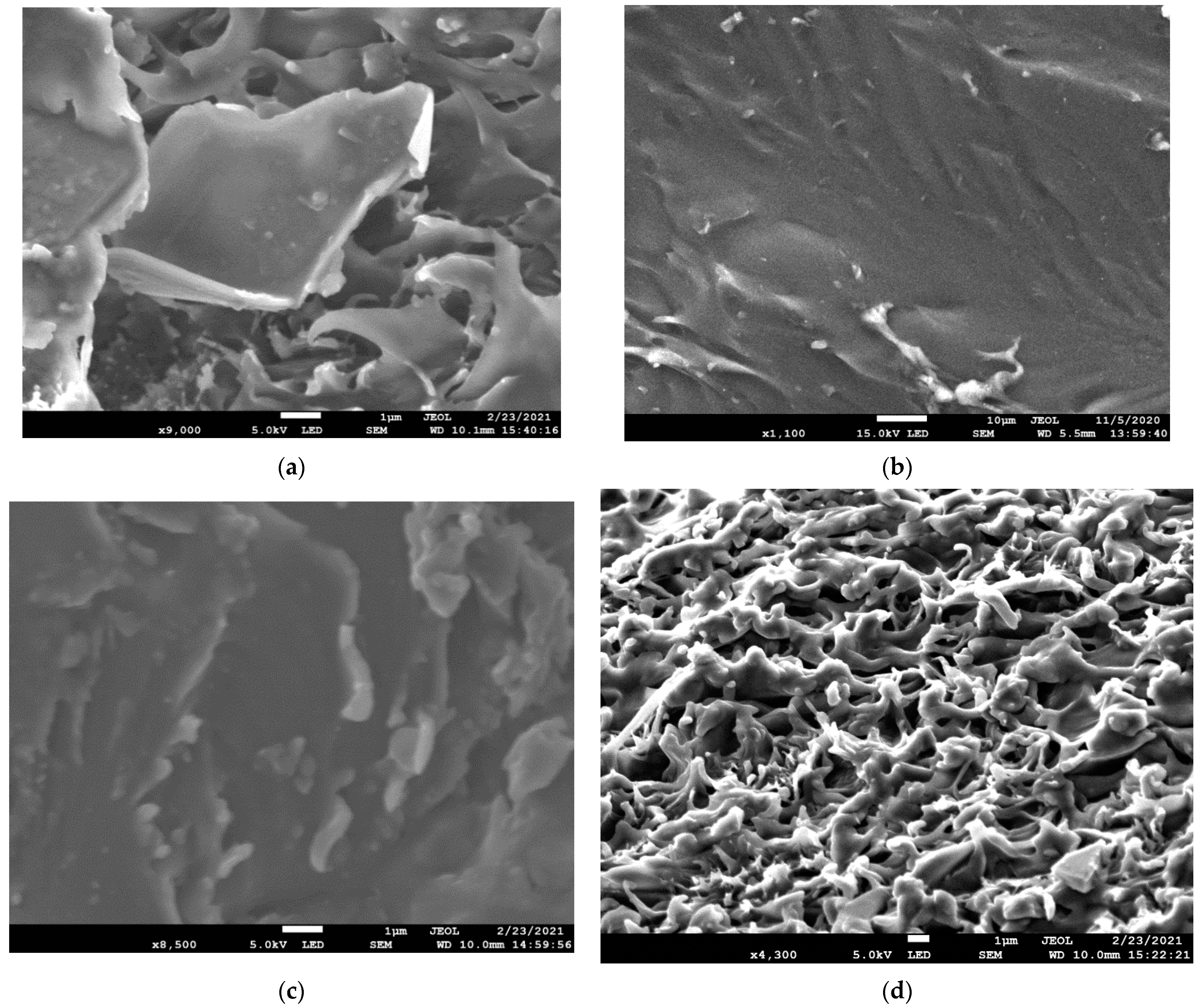
4.1. Characterization of the Crosslinked PVA Membranes
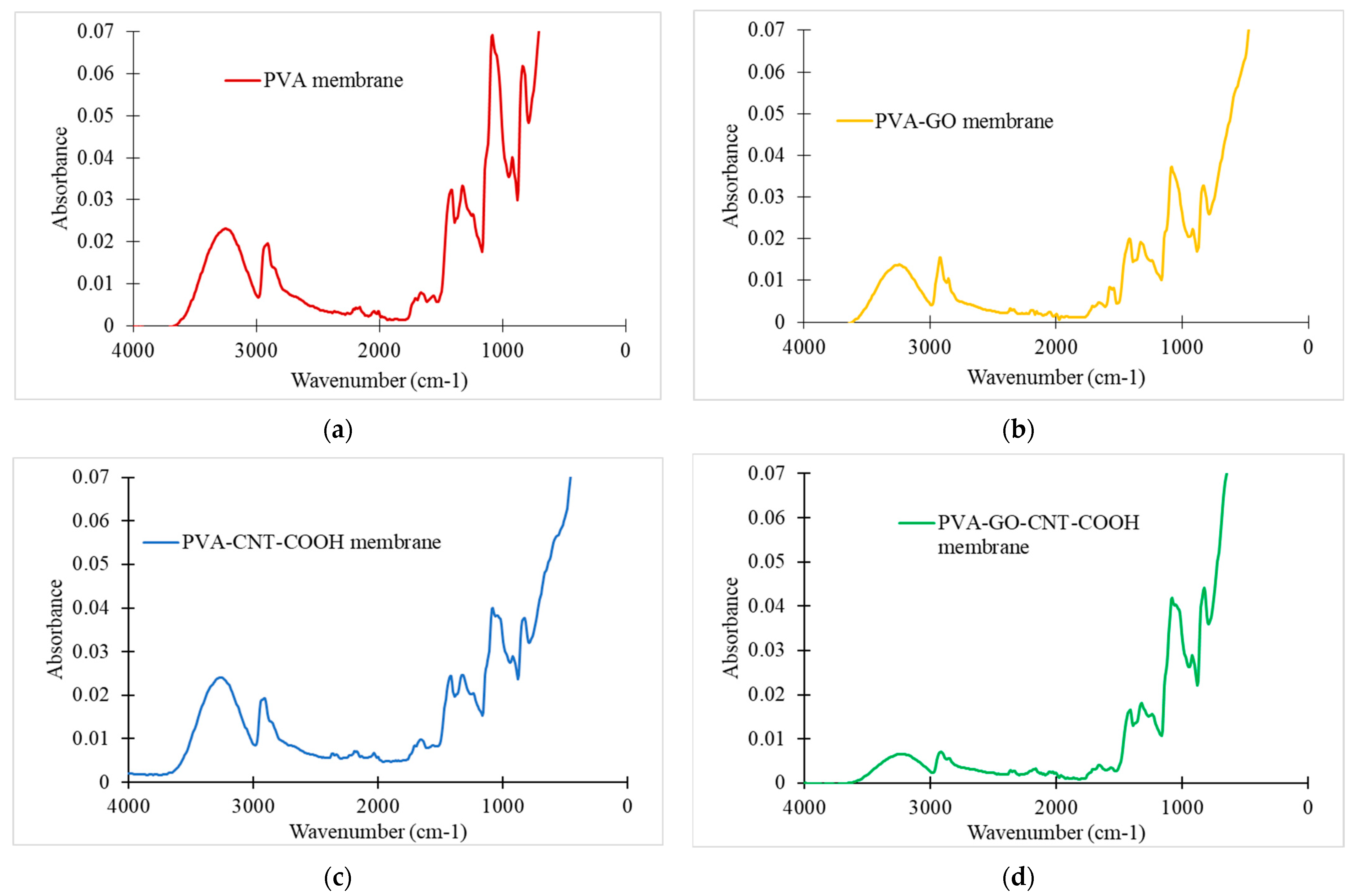
4.1.1. FTIR Analysis
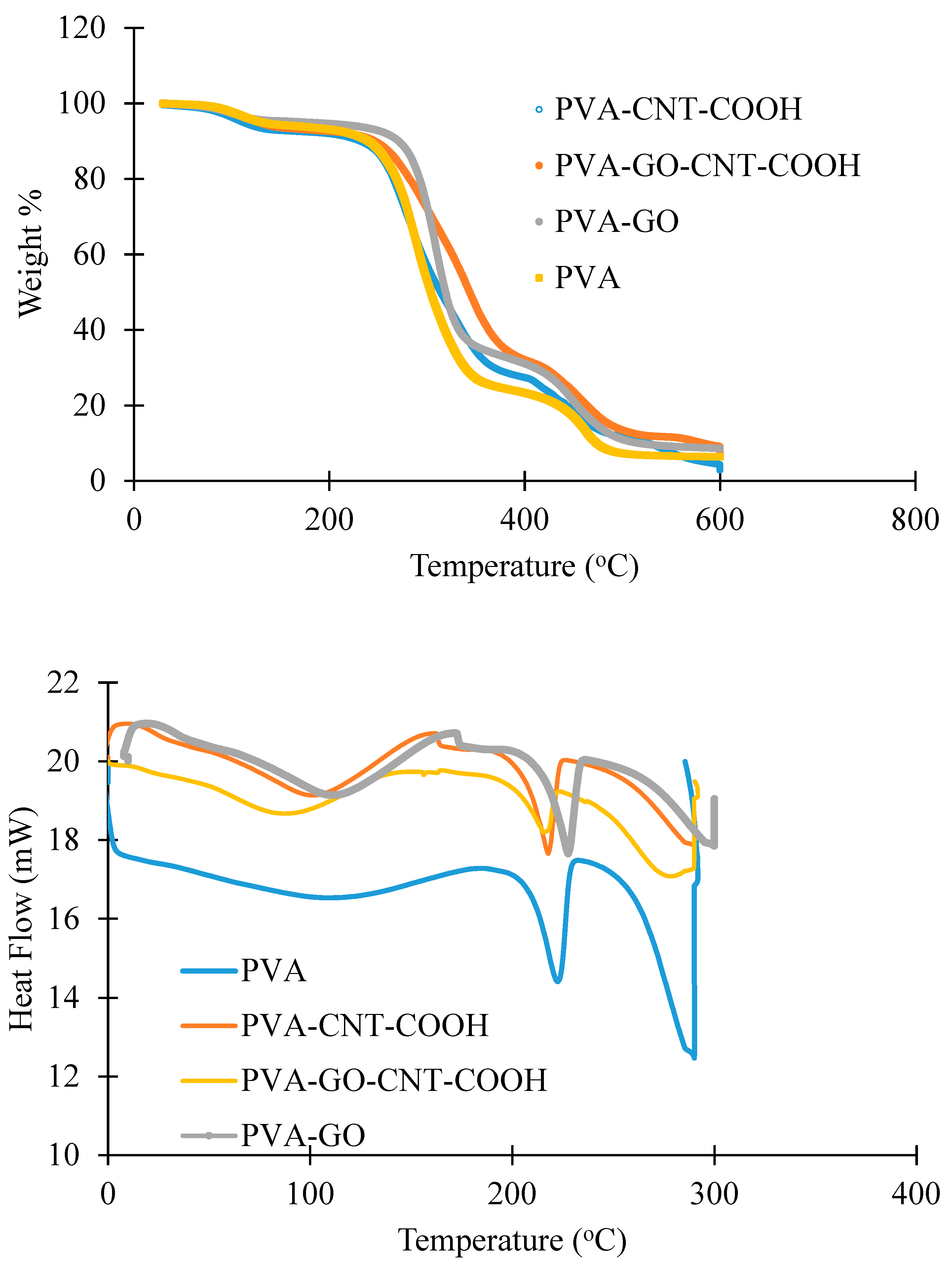
4.1.2. TGA Studies
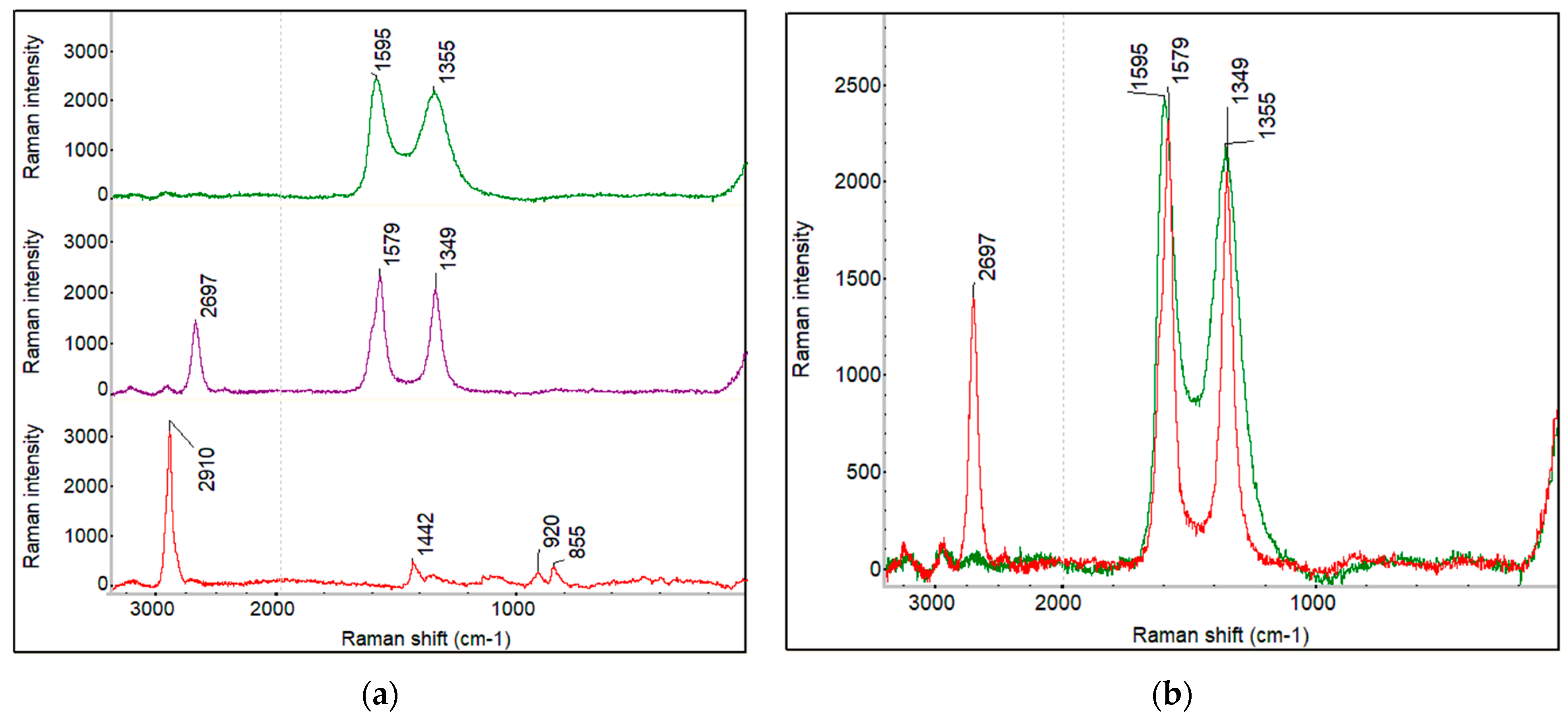
4.1.3. Raman Spectra and Raman Imaging of Fabricated Membranes
Optical and Raman Imaging of PVA Membrane
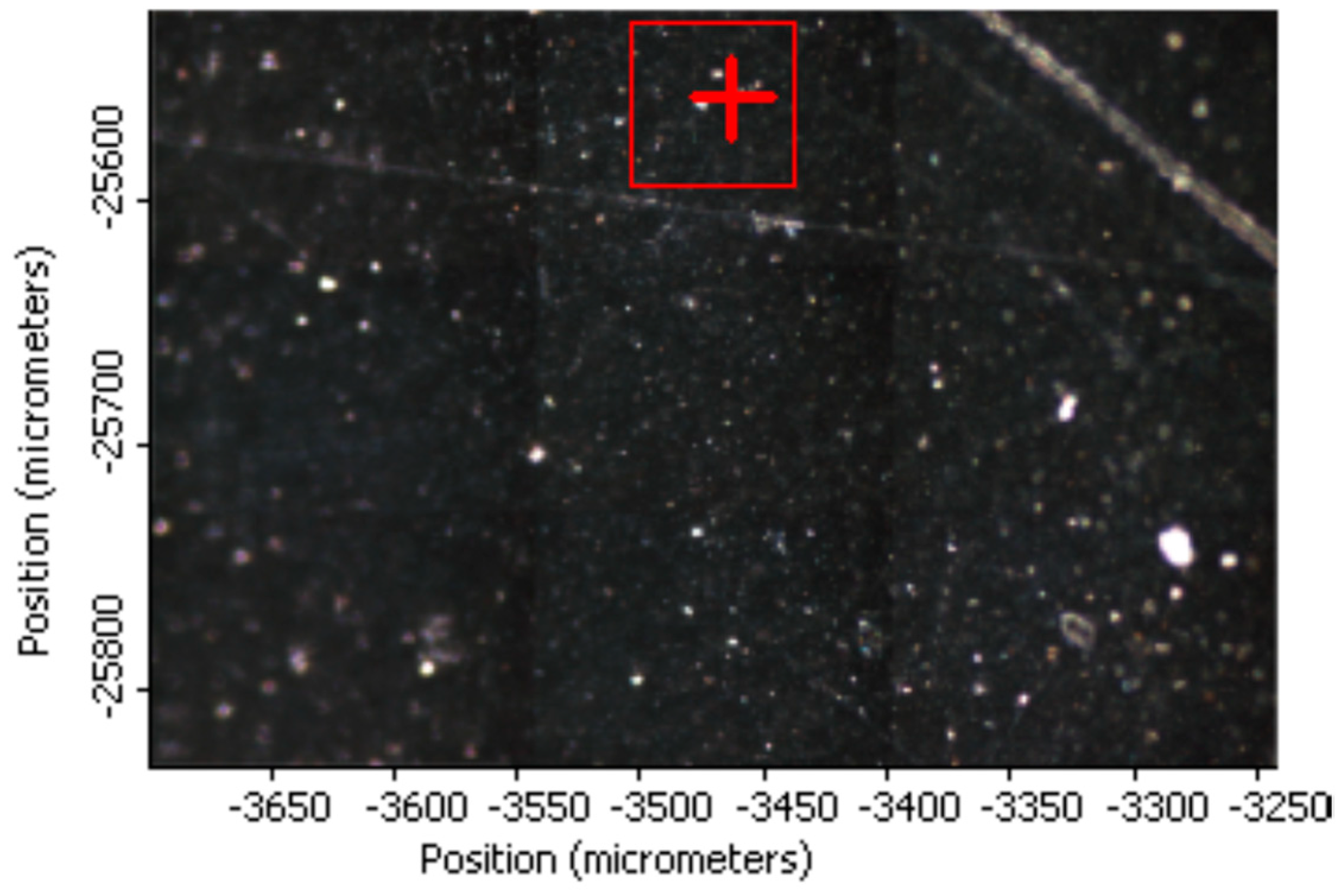
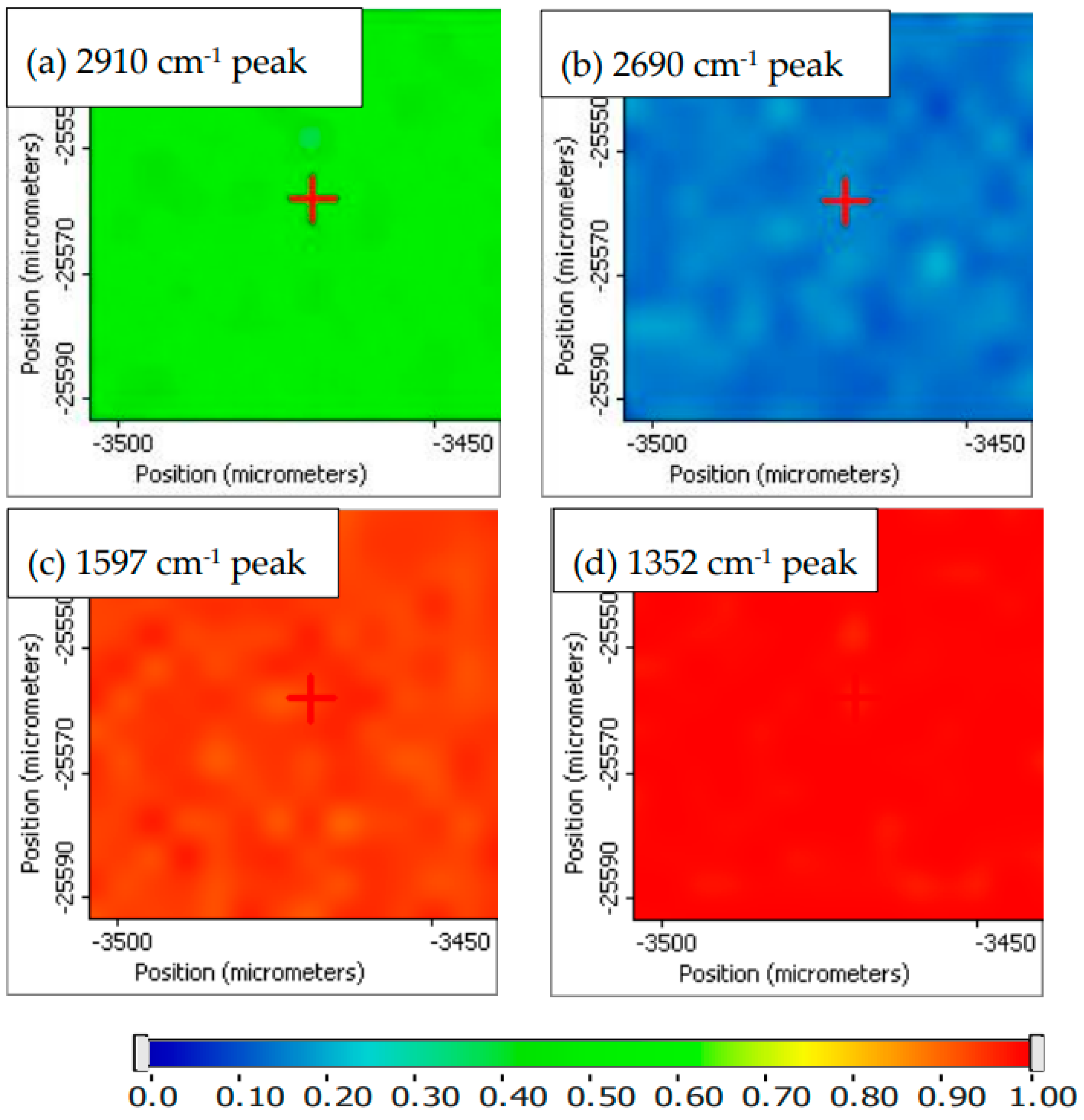
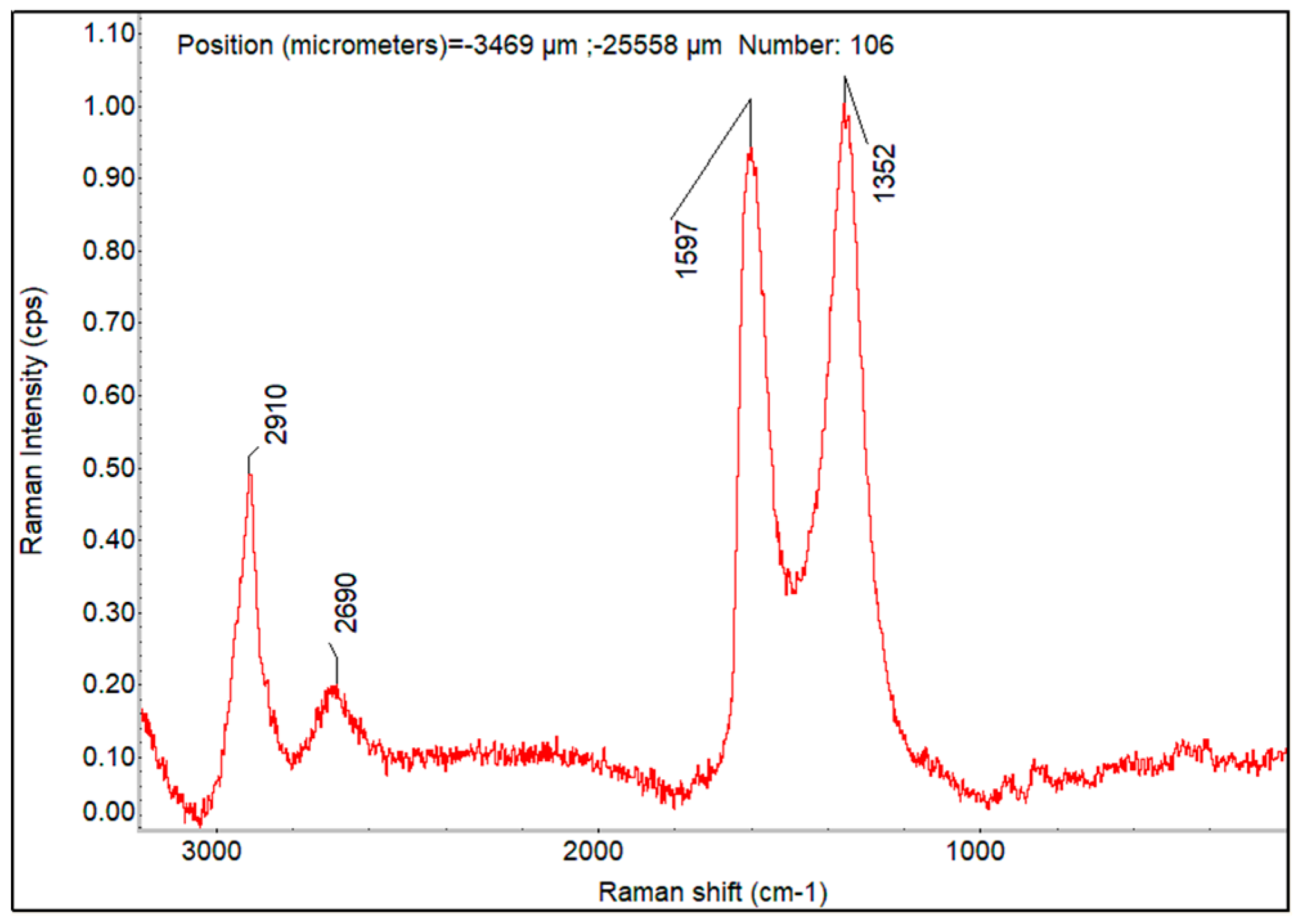
Raman Imaging of PVA_GO_CNT_COOH
4.1.4. Contact Angle Measurements
5. Pervaporation Experiments Results
5.1. Pervaporation
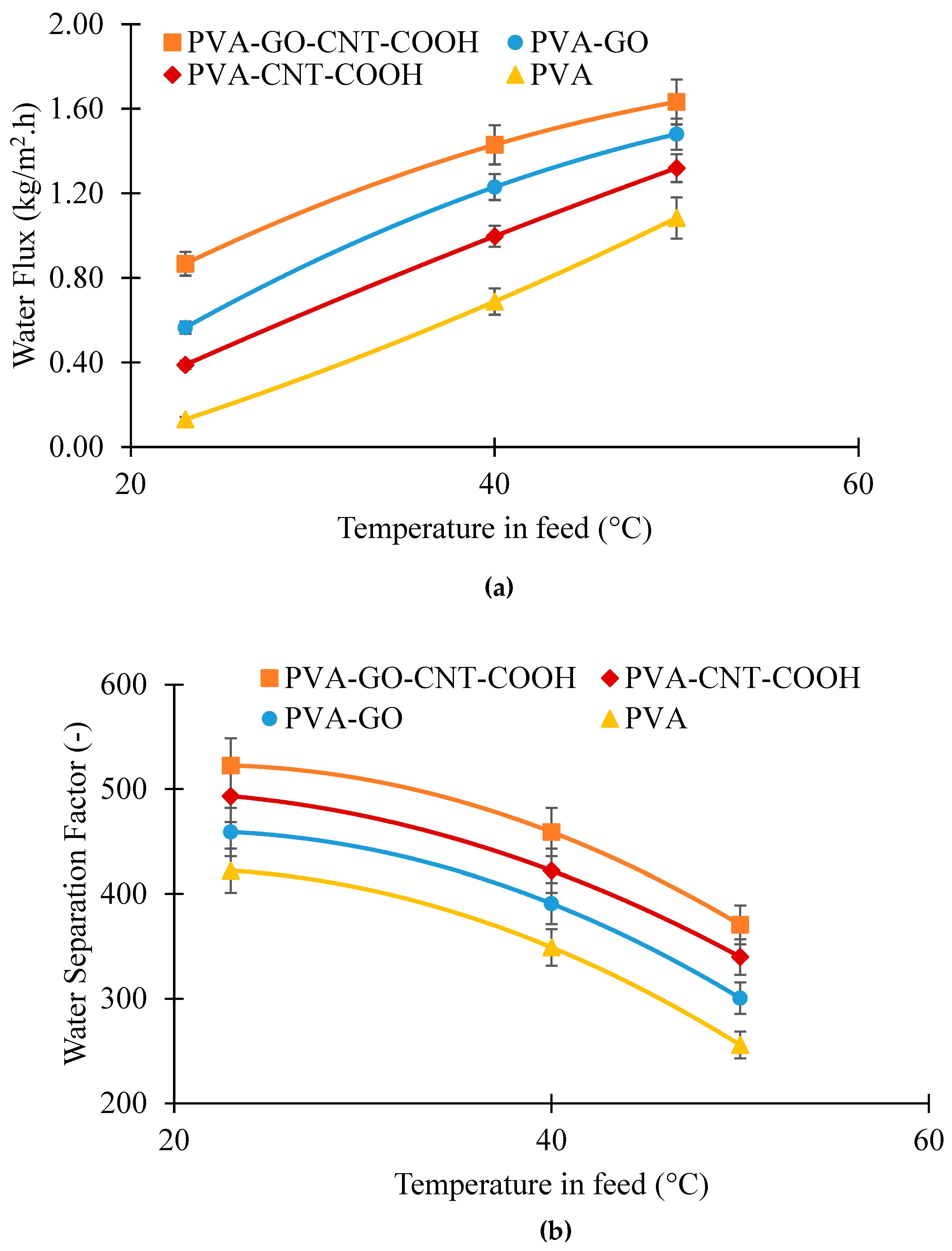
Effect of Ethanol–Water Solution Temperature on Pervaporation Performance of PVA, PVA_GO, PVA_CNT_COOH and PVA_GO_CNT_COOH Membranes
5.2. Membrane Swelling and Preferential Sorption
5.3. Model Calculation of the Theoretical Flux and Diffusion Coefficient
6. Proposed Mechanism
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amillastre, E.; Aceves-Lara, C.-A.; Uribelarrea, J.-L.; Alfenore, S.; Guillouet, S.E. Dynamic model of temperature impact on cell viability and major product formation during fed-batch and continuous ethanolic fermentation in Saccharomyces cerevisiae. Bioresour. Technol. 2012, 117, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Govindaswamy, S.; Vane, L.M. Kinetics of growth and ethanol production on different carbon substrates using genetically engineered xylose-fermenting yeast. Bioresour. Technol. 2007, 98, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Madson, P.W.; Lococo, D.B. Recovery of volatile products from dilute high-fouling process streams. Appl. Biochem. Biotechnol. 2000, 84, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Bin, T.; Nakao, S.-i. Selective ethanol extraction from fermentation broth using a silicalite membrane. Sep. Purif. Technol. 2002, 27, 59–66. [Google Scholar] [CrossRef]
- Carton, A.; Benito, G.G.; Rey, J.; de La Fuente, M. Selection of adsorbents to be used in an ethanol fermentation process. Adsorption isotherms and kinetics. Bioresour. Technol. 1998, 66, 75–78. [Google Scholar] [CrossRef]
- Bowen, T.C.; Vane, L.M. Ethanol, acetic acid, and water adsorption from binary and ternary liquid mixtures on high-silica zeolites. Langmuir 2006, 22, 3721–3727. [Google Scholar] [CrossRef]
- Bothun, G.D.; Knutson, B.L.; Strobel, H.J.; Nokes, S.E.; Brignole, E.A.; Díaz, S. Compressed solvents for the extraction of fermentation products within a hollow fiber membrane contactor. J. Supercrit. Fluids 2003, 25, 119–134. [Google Scholar] [CrossRef]
- Boudreau, T.M.; Hill, G.A. Improved ethanol–water separation using fatty acids. Process Biochem. 2006, 41, 980–983. [Google Scholar] [CrossRef]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod. Biorefining 2008, 2, 553–588. [Google Scholar] [CrossRef]
- Kunnakorn, D.; Rirksomboon, T.; Siemanond, K.; Aungkavattana, P.; Kuanchertchoo, N.; Chuntanalerg, P.; Hemra, K.; Kulprathipanja, S.; James, R.; Wongkasemjit, S. Techno-economic comparison of energy usage between azeotropic distillation and hybrid system for water–ethanol separation. Renew. Energy 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Toutianoush, A.; Krasemann, L.; Tieke, B. Polyelectrolyte multilayer membranes for pervaporation separation of alcohol/water mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2002, 198, 881–889. [Google Scholar] [CrossRef]
- Chen, W.-J.; Aranda, P.; Martin, C.R. Pervaporation separation of ethanol/water mixtures by polystyrenesulfonate/alumina composite membranes. J. Membr. Sci. 1995, 107, 199–207. [Google Scholar] [CrossRef]
- Huang, R.; Yeom, C. Pervaporation separation of aqueous mixtures using crosslinked poly (vinyl alcohol)(PVA). II. Permeation of ethanol-water mixtures. J. Membr. Sci. 1990, 51, 273–292. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, K.-H.; Kim, S.Y. Pervaporation separation of water from ethanol through polyimide composite membranes. J. Membr. Sci. 2000, 169, 81–93. [Google Scholar] [CrossRef]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of organic–organic mixtures by pervaporation—A review. J. Membr. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Cao, S.; Shi, Y.; Chen, G. Properties and pervaporation characteristics of chitosan–poly (N-vinyl-2-pyrrolidone) blend membranes for MeOH–MTBE. J. Appl. Polym. Sci. 1999, 74, 1452–1458. [Google Scholar] [CrossRef]
- An, Q.; Qian, J.; Sun, H.; Wang, L.; Zhang, L.; Chen, H. Compatibility of PVC/EVA blends and the pervaporation of their blend membranes for benzene/cyclohexane mixtures. J. Membr. Sci. 2003, 222, 113–122. [Google Scholar] [CrossRef]
- Li, L.; Xiao, Z.; Zhang, Z.; Tan, S. Pervaporation of acetic acid/water mixtures through carbon molecular sieve-filled PDMS membranes. Chem. Eng. J. 2004, 97, 83–86. [Google Scholar] [CrossRef]
- Naidu, B.V.K.; Bhat, S.; Sairam, M.; Wali, A.; Sawant, D.; Halligudi, S.; Mallikarjuna, N.; Aminabhavi, T. Comparison of the pervaporation separation of a water–acetonitrile mixture with zeolite-filled sodium alginate and poly (vinyl alcohol)–polyaniline semi-interpenetrating polymer network membranes. J. Appl. Polym. Sci. 2005, 96, 1968–1978. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.-H.; Zhang, A.-S.; Xu, L.-H.; Lu, J.-J.; Zhao, Z.-P. Novel MOF-capped halloysite nanotubes/PDMS mixed matrix membranes for enhanced n-butanol permselective pervaporation. J. Membr. Sci. 2020, 595, 117543. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly (vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Lue, S.J.; Lee, D.-T.; Chen, J.-Y.; Chiu, C.-H.; Hu, C.-C.; Jean, Y.; Lai, J.-Y. Diffusivity enhancement of water vapor in poly (vinyl alcohol)–fumed silica nano-composite membranes: Correlation with polymer crystallinity and free-volume properties. J. Membr. Sci. 2008, 325, 831–839. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, Q.L.; Zhu, A.M.; Zhang, Q.G. Dehydration of acetic acid by pervaporation using SPEK-C/PVA blend membranes. J. Membr. Sci. 2008, 320, 416–422. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lin, Y.; Chen, C. Separation of dimethyl carbonate/methanol mixtures by pervaporation with poly (acrylic acid)/poly (vinyl alcohol) blend membranes. J. Membr. Sci. 2007, 305, 238–246. [Google Scholar] [CrossRef]
- Ruiz, J.; Mantecón, A.; Cadiz, V. Synthesis and swelling characteristics of acid-containing poly (vinyl alcohol) hydrogels. J. Appl. Polym. Sci. 2001, 81, 1444–1450. [Google Scholar] [CrossRef]
- Alghezawi, N.; Şanlı, O.; Aras, L.; Asman, G. Separation of acetic acid–water mixtures through acrylonitrile grafted poly (vinyl alcohol) membranes by pervaporation. Chem. Eng. Process. Process Intensif. 2005, 44, 51–58. [Google Scholar] [CrossRef]
- Li, G.; Zhang, W.; Yang, J.; Wang, X. Time-dependence of pervaporation performance for the separation of ethanol/water mixtures through poly (vinyl alcohol) membrane. J. Colloid Interface Sci. 2007, 306, 337–344. [Google Scholar] [CrossRef]
- Praptowidodo, V.S. Influence of swelling on water transport through PVA-based membrane. J. Mol. Struct. 2005, 739, 207–212. [Google Scholar] [CrossRef]
- Huang, Z.; Guan, H.-M.; lee Tan, W.; Qiao, X.-Y.; Kulprathipanja, S. Pervaporation study of aqueous ethanol solution through zeolite-incorporated multilayer poly (vinyl alcohol) membranes: Effect of zeolites. J. Membr. Sci. 2006, 276, 260–271. [Google Scholar] [CrossRef]
- Süer, M.G.; BaC, N.; Yilmaz, L. Gas permeation characteristics of polymer-zeolite mixed matrix membranes. J. Membr. Sci. 1994, 91, 77–86. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Zeolite K-LTL-loaded sodium alginate mixed matrix membranes for pervaporation dehydration of aqueous–organic mixtures. J. Membr. Sci. 2007, 306, 173–185. [Google Scholar] [CrossRef]
- Flynn, E.; Keane, D.; Tabari, P.; Morris, M. Pervaporation performance enhancement through the incorporation of mesoporous silica spheres into PVA membranes. Sep. Purif. Technol. 2013, 118, 73–80. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Hu, S.Y.; Zhang, Y.; Lawless, D.; Feng, X. Composite membranes comprising of polyvinylamine-poly (vinyl alcohol) incorporated with carbon nanotubes for dehydration of ethylene glycol by pervaporation. J. Membr. Sci. 2012, 417, 34–44. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Chung, T.S.; Rajagopalan, R. Matrimid®/MgO mixed matrix membranes for pervaporation. AIChE J. 2007, 53, 1745–1757. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Enhanced membrane distillation of organic solvents from their aqueous mixtures using a carbon nanotube immobilized membrane. J. Membr. Sci. 2018, 568, 134–140. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Microwave Induced Membrane Distillation for Enhanced Ethanol–Water Separation on a Carbon Nanotube Immobilized Membrane. Ind. Eng. Chem. Res. 2019, 58, 18313–18319. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Nanocarbon-immobilized membranes for separation of tetrahydrofuran from water via membrane distillation. ACS Appl. Nano Mater. 2020, 3, 6344–6353. [Google Scholar] [CrossRef]
- Gupta, I.; Azizighannad, S.; Farinas, E.T.; Mitra, S. Antiviral properties of select carbon nanostructures and their functionalized analogs. Mater. Today Commun. 2021, 29, 102743. [Google Scholar] [CrossRef]
- Gupta, I.; Chakraborty, J.; Roy, S.; Farinas, E.T.; Mitra, S. Nanocarbon immobilized membranes for generating bacteria and endotoxin free water via membrane distillation. Sep. Purif. Technol. 2021, 259, 118133. [Google Scholar] [CrossRef]
- Gupta, I.; Chakraborty, J.; Roy, S.; Farinas, E.T.; Mitra, S. Synergistic Effects of Microwave Radiation and Nanocarbon Immobilized Membranes in the Generation of Bacteria-Free Water via Membrane Distillation. Ind. Eng. Chem. Res. 2021, 61, 1453–1463. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, Y.; Wen, R.; Guo, Y.-H.; Su, J.-F.; Matsuura, T. Multilayer poly (vinyl alcohol)–zeolite 4A composite membranes for ethanol dehydration by means of pervaporation. Sep. Purif. Technol. 2006, 51, 126–136. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsu, C.-Y.; Su, Y.-H.; Lai, J.-Y. Chitosan-silica complex membranes from sulfonic acid functionalized silica nanoparticles for pervaporation dehydration of ethanol-water solutions. Biomacromolecules 2005, 6, 368–373. [Google Scholar] [CrossRef]
- Georgakilas, V.; Bourlinos, A.; Gournis, D.; Tsoufis, T.; Trapalis, C.; Mateo-Alonso, A.; Prato, M. Multipurpose organically modified carbon nanotubes: From functionalization to nanotube composites. J. Am. Chem. Soc. 2008, 130, 8733–8740. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Dave, H.K.; Nath, K. Graphene oxide incorporated novel polyvinyl alcohol composite membrane for pervaporative recovery of acetic acid from vinegar wastewater. J. Water Process Eng. 2016, 14, 124–134. [Google Scholar] [CrossRef]
- Wang, N.; Ji, S.; Li, J.; Zhang, R.; Zhang, G. Poly (vinyl alcohol)–graphene oxide nanohybrid “pore-filling” membrane for pervaporation of toluene/n-heptane mixtures. J. Membr. Sci. 2014, 455, 113–120. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Low temperature recovery of acetone–butanol–ethanol (ABE) fermentation products via microwave induced membrane distillation on carbon nanotube immobilized membranes. Sustain. Energy Fuels 2020, 4, 3487–3499. [Google Scholar] [CrossRef]
- Huang, K.; Liu, G.; Lou, Y.; Dong, Z.; Shen, J.; Jin, W. A graphene oxide membrane with highly selective molecular separation of aqueous organic solution. Angew. Chem. 2014, 126, 7049–7052. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I.; Son, Y.-W. Origin of anomalous water permeation through graphene oxide membrane. Nano Lett. 2013, 13, 3930–3935. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Yang, L.; Wang, T.-J.; Liu, B. Separation performance of graphene oxide membrane in aqueous solution. Ind. Eng. Chem. Res. 2016, 55, 4803–4810. [Google Scholar] [CrossRef]
- Gupta, I.; Azizighannad, S.; Farinas, E.T.; Mitra, S. Synergistic Antiviral Effects of Metal Oxides and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 11957. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Praveen, V.K.; Das, G.; Ajayaghosh, A. Hybrid materials of 1D and 2D carbon allotropes and synthetic π-systems. NPG Asia Mater. 2018, 10, 107–126. [Google Scholar] [CrossRef]
- Sa, K.; Mahakul, P.C.; Subramanyam, B.; Raiguru, J.; Das, S.; Alam, I.; Mahanandia, P. Effect of reduced graphene oxide-carbon nanotubes hybrid nanofillers in mechanical properties of polymer nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 338, 012055. [Google Scholar]
- Mani, V.; Devadas, B.; Chen, S.-M. Direct electrochemistry of glucose oxidase at electrochemically reduced graphene oxide-multiwalled carbon nanotubes hybrid material modified electrode for glucose biosensor. Biosens. Bioelectron. 2013, 41, 309–315. [Google Scholar] [CrossRef]
- Mahakul, P.C.; Sa, K.; Das, B.; Subramaniam, B.; Saha, S.; Moharana, B.; Raiguru, J.; Dash, S.; Mukherjee, J.; Mahanandia, P. Preparation and characterization of PEDOT: PSS/reduced graphene oxide–carbon nanotubes hybrid composites for transparent electrode applications. J. Mater. Sci. 2017, 52, 5696–5707. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Penkova, A. Novel PDMS-b-PPO Membranes Modified with Graphene Oxide for Efficient Pervaporation Ethanol Dehydration. Membranes 2022, 12, 832. [Google Scholar] [CrossRef]
- Burts, K.S.; Plisko, T.V.; Prozorovich, V.G.; Melnikova, G.B.; Ivanets, A.I.; Bildyukevich, A.V. Modification of Thin Film Composite PVA/PAN Membranes for Pervaporation Using Aluminosilicate Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7215. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Prozorovich, V.; Melnikova, G.; Ivanets, A.; Bildyukevich, A. Development and Study of PVA–SiO2/poly (AN-co-MA) Dynamic Nanocomposite Membranes for Ethanol Dehydration via Pervaporation. Membr. Membr. Technol. 2022, 4, 101–110. [Google Scholar] [CrossRef]
- Cao, T.; Li, J.; Li, C.; Zhang, N.; Cai, P.; Wang, N.; An, Q.-F. POSS-graphene oxide nanocomposite membranes for ethanol permselective pervaporation. Microporous Mesoporous Mater. 2022, 331, 111635. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhou, S.; Xue, A.; Zhang, Y.; Zhao, Y.; Zhong, J.; Zhang, Q. Graphitic carbon nitride nanosheets embedded in poly (vinyl alcohol) nanocomposite membranes for ethanol dehydration via pervaporation. Sep. Purif. Technol. 2017, 188, 24–37. [Google Scholar] [CrossRef]
- Tumnantong, D.; Srisamrid, K.; Poompradub, S.; Prasassarakich, P. Preparation of poly (methyl methacrylate)-silica nanocomposites via DMP-assisted RAFT polymerization and NR/PMMA-RAFT-SiO2 hybrid membrane for pervaporation. Eur. Polym. J. 2022, 168, 111088. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.J.; Fozer, D.; Haaz, E.; Valentínyi, N.; Nagy, T.; Keri, O.; Bakos, L.P.; Szilágyi, I.M.; Mizsey, P. Effect of silver-nanoparticles generated in poly (vinyl alcohol) membranes on ethanol dehydration via pervaporation. Chin. J. Chem. Eng. 2019, 27, 1595–1607. [Google Scholar] [CrossRef]
- Nguyen, H.H.; To, L.A.; Ngo, N.P.D. Fabrication and characterization of graphene/graphene oxide based poly (vinyl alcohol) nanocomposite membranes for pervaporation dehydration of ethanol. Can Tho Univ. J. Sci. 2016, 36–45. [Google Scholar] [CrossRef]
- Chen, Y.; Mitra, S. Fast microwave-assisted purification, functionalization and dispersion of multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 5770–5775. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N.R. Polymeric nanocomposite membranes for next generation pervaporation process: Strategies, challenges and future prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef]
- Singha, N.; Kar, S.; Ray, S.; Ray, S. Separation of isopropyl alcohol–water mixtures by pervaporation using crosslink IPN membranes. Chem. Eng. Process. Process Intensif. 2009, 48, 1020–1029. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S. Dehydration of acetic acid, alcohols, and acetone by pervaporation using acrylonitrile-maleic anhydride copolymer membrane. Sep. Sci. Technol. 2005, 40, 1583–1596. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y. Preparation and performance of asymmetric polyetherimide membranes for isopropanol dehydration by pervaporation. J. Membr. Sci. 1996, 109, 165–172. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; de la Iglesia, O.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly (vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef]
- Yeom, C.-K.; Lee, K.-H. Pervaporation separation of water-acetic acid mixtures through poly (vinyl alcohol) membranes crosslinked with glutaraldehyde. J. Membr. Sci. 1996, 109, 257–265. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Fuentes, C.A.; Zhang, D.; Van Vuure, A.W.; Seo, J.W.; Seveno, D. Wettability of carbon nanotube fibers. Carbon 2017, 122, 128–140. [Google Scholar] [CrossRef]
- Shirazi, Y.; Tofighy, M.A.; Mohammadi, T. Synthesis and characterization of carbon nanotubes/poly vinyl alcohol nanocomposite membranes for dehydration of isopropanol. J. Membr. Sci. 2011, 378, 551–561. [Google Scholar] [CrossRef]
- Petkieva, D.; Ozerin, A.; Kurkin, T.; Golubev, E.; Ivan’kova, E.; Zelenetskii, A. Carbonization of oriented poly (vinyl alcohol) fibers impregnated with potassium bisulfate. Carbon Lett. 2020, 30, 637–650. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lee, Y.-J.; Chiu, S.-J.; Lee, K.-T.; Chien, W.-C.; Lin, C.-T.; Huang, C.-A. Preparation of a PVA/HAP composite polymer membrane for a direct ethanol fuel cell (DEFC). J. Appl. Electrochem. 2008, 38, 1329–1337. [Google Scholar] [CrossRef]
- Sharma, S.; Prakash, J.; Pujari, P. Effects of the molecular level dispersion of graphene oxide on the free volume characteristics of poly (vinyl alcohol) and its impact on the thermal and mechanical properties of their nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 29201–29209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, X.; Cao, B.; Li, P. Fabrication of high-performance PVA/PAN composite pervaporation membranes crosslinked by PMDA for wastewater desalination. Pet. Sci. 2018, 15, 146–156. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Qian, X.; Li, N.; Wang, Q.; Ji, S. Chitosan/graphene oxide mixed matrix membrane with enhanced water permeability for high-salinity water desalination by pervaporation. Desalination 2018, 438, 83–96. [Google Scholar] [CrossRef]
- Feng, B.; Xu, K.; Huang, A. Synthesis of graphene oxide/polyimide mixed matrix membranes for desalination. RSC Adv. 2017, 7, 2211–2217. [Google Scholar] [CrossRef]
- Panahian, S.; Raisi, A.; Aroujalian, A. Multilayer mixed matrix membranes containing modified-MWCNTs for dehydration of alcohol by pervaporation process. Desalination 2015, 355, 45–55. [Google Scholar] [CrossRef]
- Bartels-Caspers, C.; Tusel-Langer, E.; Lichtenthaler, R. Sorption isotherms of alcohols in zeolite-filled silicone rubber and in PVA-composite membranes. J. Membr. Sci. 1992, 70, 75–83. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering; McGraw-Hill: New York, NY, USA, 1993; Volume 5, p. 154. [Google Scholar]
- Qunhui, G.; Ohya, H.; Negishi, Y. Investigation of the permselectivity of chitosan membrane used in pervaporation separation II. Influences of temperature and membrane thickness. J. Membr. Sci. 1995, 98, 223–232. [Google Scholar] [CrossRef]
- Jiraratananon, R.; Chanachai, A.; Huang, R.; Uttapap, D. Pervaporation dehydration of ethanol–water mixtures with chitosan/hydroxyethylcellulose (CS/HEC) composite membranes: I. Effect of operating conditions. J. Membr. Sci. 2002, 195, 143–151. [Google Scholar] [CrossRef]
- Hauser, J.; Reinhardt, G.; Stumm, F.; Heintz, A. Experimental study of solubilities of water containing organic mixtures in polyvinylalcohol using gaschromatographic and infrared spectroscopic analysis. Fluid Phase Equilibria 1989, 49, 195–210. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S. Separation of organic mixtures by pervaporation using crosslinked and filled rubber membranes. J. Membr. Sci. 2006, 285, 108–119. [Google Scholar] [CrossRef]
- Jamali, S.H.; Wolff, L.; Becker, T.M.; Bardow, A.; Vlugt, T.J.; Moultos, O.A. Finite-size effects of binary mutual diffusion coefficients from molecular dynamics. J. Chem. Theory Comput. 2018, 14, 2667–2677. [Google Scholar] [CrossRef]
- Gao, C.; Shi, Q.; Dong, J. Adsorptive separation performance of 1-butanol onto typical hydrophobic zeolitic imidazolate frameworks (ZIFs). CrystEngComm 2016, 18, 3842–3849. [Google Scholar] [CrossRef]
- Sajjan, A.; Kumar, B.J.; Kittur, A.; Kariduraganavar, M. Novel approach for the development of pervaporation membranes using sodium alginate and chitosan-wrapped multiwalled carbon nanotubes for the dehydration of isopropanol. J. Membr. Sci. 2013, 425, 77–88. [Google Scholar] [CrossRef]
- Mattia, D.; Leese, H.; Lee, K.P. Carbon nanotube membranes: From flow enhancement to permeability. J. Membr. Sci. 2015, 475, 266–272. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhai, L.; Ying, Y.; Wang, Y.; Liu, G.; Dong, J.; Ng, D.Z.; Khan, S.A.; Zhao, D. Highly efficient CO2 capture by mixed matrix membranes containing three-dimensional covalent organic framework fillers. J. Mater. Chem. A 2019, 7, 4549–4560. [Google Scholar] [CrossRef]
- Peng, F.; Lu, L.; Hu, C.; Wu, H.; Jiang, Z. Significant increase of permeation flux and selectivity of poly (vinyl alcohol) membranes by incorporation of crystalline flake graphite. J. Membr. Sci. 2005, 259, 65–73. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Lee, H.D.; Shin, J.E.; Yoo, B.M.; Park, H.B. Water and ion sorption, diffusion, and transport in graphene oxide membranes revisited. J. Membr. Sci. 2017, 544, 425–435. [Google Scholar] [CrossRef]
- Roy, S.; Hussain, C.M.; Mitra, S. Carbon nanotube-immobilized super-absorbent membrane for harvesting water from the atmosphere. Environ. Sci. Water Res. Technol. 2015, 1, 753–760. [Google Scholar] [CrossRef]





| Membrane System | Conditions | Flux (g/m2.hr) | Separation Factor | Reference |
|---|---|---|---|---|
| PDMS-b-PPO Membranes Modified with Graphene Oxide | 4.4–70 wt.% water in water-ethanol mixture at 22 °C. | 80–90 | 72–34 | [59] |
| PVA/PAN-Aluminosilicate membrane | Ethanol-water (10 wt.%) at 35 °C | 51 | 141 | [60] |
| PVA–SiO2/poly(AN-co-MA) membrane | Ethanol-water (10 wt.%) at 34 °C | 44 | 72.8 | [61] |
| PVA-SiO2/PAN membrane | Ethanol-water (10 wt.%) at 60 °C | 390 | 20 | [32] |
| POSS-GO/PDMS membrane | Ethanol-water (5 wt.%) at RT | 1347 | 11.2 | [62] |
| CPVA-g-C3N4-4/PAN membrane | Ethanol-water (10 wt.%) at RT | 6332 | 30.7 | [63] |
| NR/PMMA-RAFT-SiO2 hybrid membrane | Ethanol-water (20 vol%) at RT | 2244 | 19 | [64] |
| AgNPs-PVA membrane | Ethanol-water (10 wt.%) at 40 °C | 127 | 43.6 | [65] |
| rGO/PVA membrane | Ethanol-water (20 wt.%) at 50 °C | 56 | 51.2 | [66] |
| PVA-GO-CNT-COOH hybrid membrane | Ethanol-water (10 wt.%) at 23 °C | 870 | 523 | This work |
| Membrane | Pristine PVA | PVA-GO | PVA-CNT-COOH | PVA-GO-CNT-COOH |
|---|---|---|---|---|
| Contact angle (°) | 69 | 65 | 75 | 71 |
| Membrane | D0,water (m2/s) | γ | D0,ethanol (m2/s) | β |
|---|---|---|---|---|
| PVA | 3.16 × 10−9 | 0.17 | 7.91 × 10−11 | 0.0001 |
| PVA-CNT-COOH | 2.06 × 10−8 | 0.38 | 2.06 × 10−10 | 0.00006 |
| PVA-GO | 1.58 × 10−8 | 0.4 | 3.95 × 10−10 | 0.0001 |
| PVA-GO-CNT-COOH | 2.37 × 10−8 | 0.31 | 3.16 × 10−10 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, O.; Roy, S.; Rao, L.; Mitra, S. Graphene Oxide-Carbon Nanotube (GO-CNT) Hybrid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol. Membranes 2022, 12, 1227. https://doi.org/10.3390/membranes12121227
Gupta O, Roy S, Rao L, Mitra S. Graphene Oxide-Carbon Nanotube (GO-CNT) Hybrid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol. Membranes. 2022; 12(12):1227. https://doi.org/10.3390/membranes12121227
Chicago/Turabian StyleGupta, Oindrila, Sagar Roy, Lingfen Rao, and Somenath Mitra. 2022. "Graphene Oxide-Carbon Nanotube (GO-CNT) Hybrid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol" Membranes 12, no. 12: 1227. https://doi.org/10.3390/membranes12121227
APA StyleGupta, O., Roy, S., Rao, L., & Mitra, S. (2022). Graphene Oxide-Carbon Nanotube (GO-CNT) Hybrid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol. Membranes, 12(12), 1227. https://doi.org/10.3390/membranes12121227









