Morphological Analysis of Poly(4,4′-oxydiphenylene-pyromellitimide)-Based Organic Solvent Nanofiltration Membranes Formed by the Solution Method
Abstract
1. Introduction
2. Materials and Methods
2.1. The Object under the Study
2.2. Materials
2.3. Synthesis of coPUIs
2.4. PUI Films Obtaining
2.5. AFM Investigation
2.6. X-ray Diffraction (XRD)
2.7. Filtration Experiments
2.8. Sorption and Swelling Studies
3. Results and Discussion
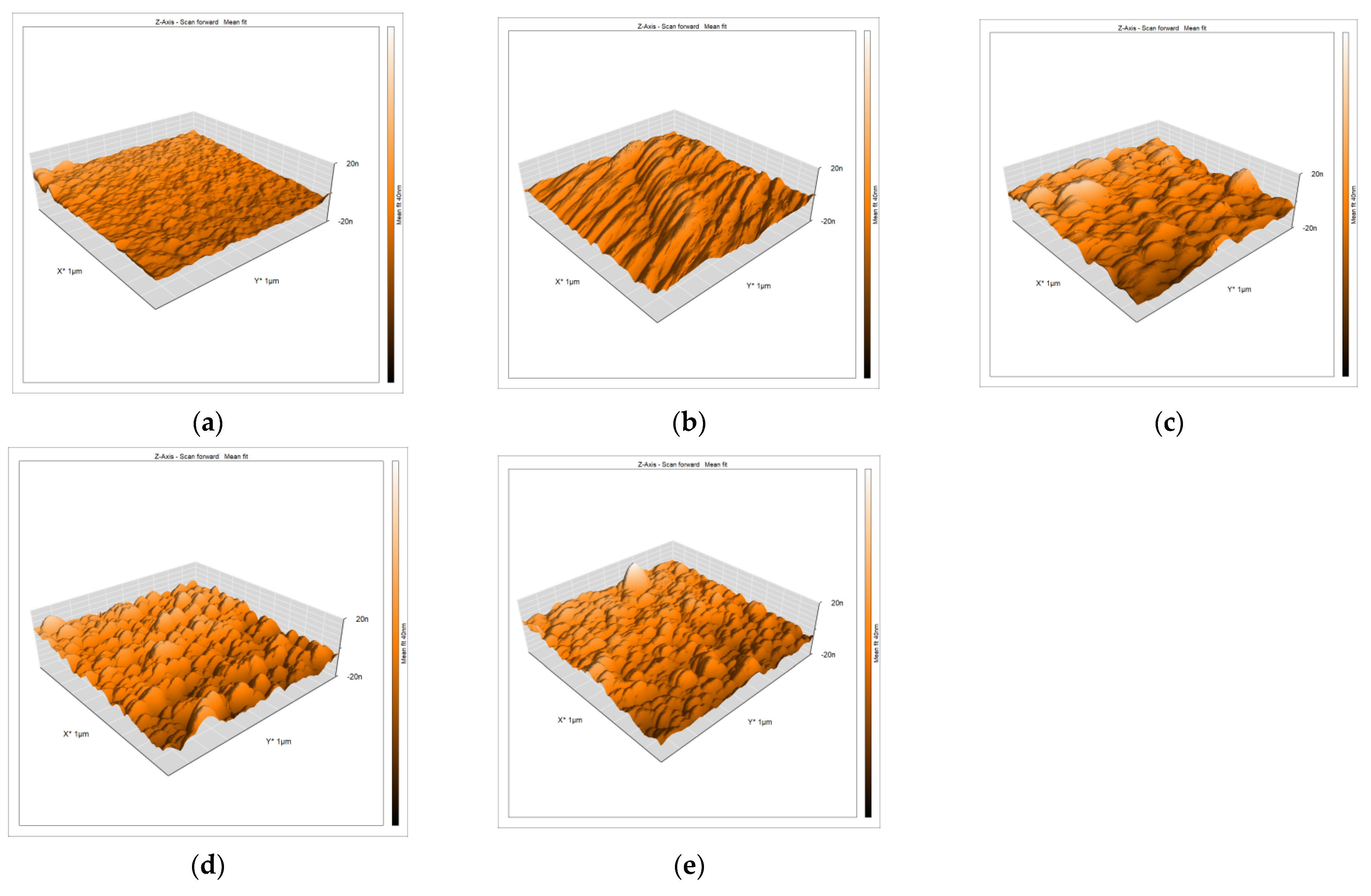
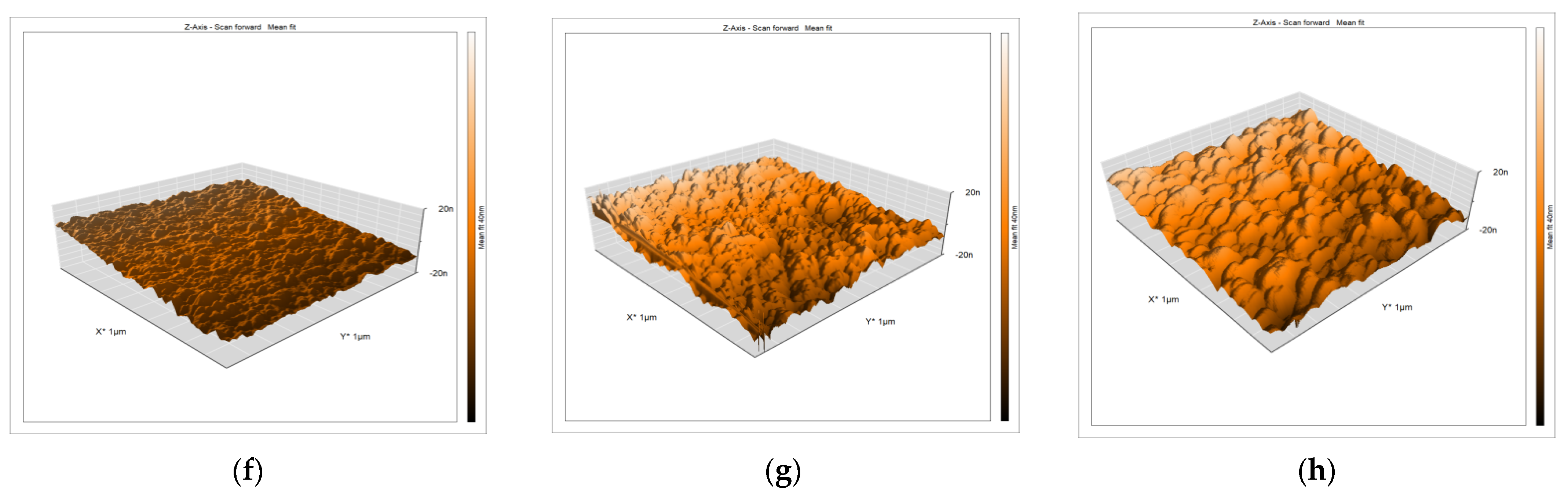
3.1. AFM Investigation
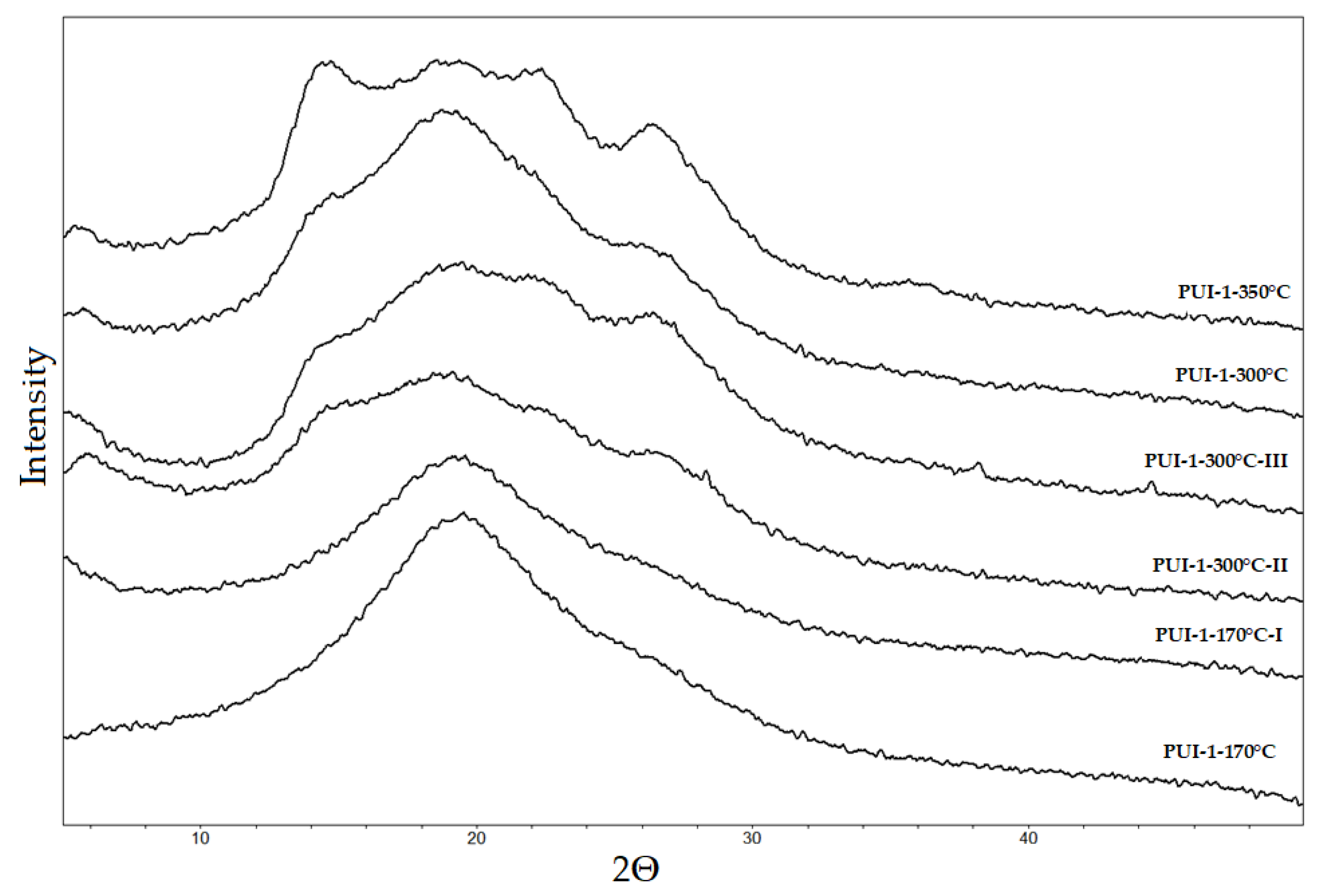
3.2. X-ray Diffraction (XRD) Investigation
3.3. Sorption and Filtration Properties of Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahlqvist, G.P.; McGeough, C.P.; Senanayake, C.; Armstrong, J.D.; Yadaw, A.; Roy, S.; Ahmad, S.; Snead, D.R.; Jamison, T.F. Progress Toward a Large-Scale Synthesis of Molnupiravir (MK-4482, EIDD-2801) from Cytidine. ACS Omega 2021, 6, 10396–10402. [Google Scholar] [CrossRef]
- Rossi, F.V.; Gentili, D.; Marcantoni, E. Metal-Promoted Heterocyclization: A Heterosynthetic Approach to Face a Pandemic Crisis. Molecules 2021, 26, 2620. [Google Scholar] [CrossRef]
- am Ende, D.J.; am Ende, M.T. (Eds.) Chemical Engineering in the Pharmaceutical Industry: Active Pharmaceutical Ingredients, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; ISBN 978-1-119-28586-1. [Google Scholar]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735. [Google Scholar] [CrossRef]
- Tsarkov, S.M. Nanofiltration of Organic Media. In Membranes and Membrane Technologies; Yaroslavtsev, A.B., Ed.; World Scientific: Singapore, 2013; pp. 540–580. [Google Scholar]
- Galizia, M.; Bye, K.P. Advances in Organic Solvent Nanofiltration Rely on Physical Chemistry and Polymer Chemistry. Front. Chem. 2018, 6, 511. [Google Scholar] [CrossRef] [PubMed]
- See-Toh, Y.; Ferreira, F.; Livingston, A. The influence of membrane formation parameters on the functional performance of organic solvent nanofiltration membranes. J. Membr. Sci. 2007, 301, 3. [Google Scholar] [CrossRef]
- White, L.S. Transport properties of a polyimide solvent resistant nanofiltration membrane. J. Membr. Sci. 2002, 205, 191. [Google Scholar] [CrossRef]
- Guo, X.; Liu, D.; Han, T.; Huang, H.; Yang, Q.; Zhong, C. Preparation of thin film nanocomposite membranes with surface modified MOF for high flux organic solvent nanofiltration. AIChE J. 2017, 63, 1303. [Google Scholar] [CrossRef]
- Peyravi, M.; Jahanshahi, M.; Rahimpour, A.; Javadi, A.; Hajavi, S. Novel thin film nanocomposite membranes incorporated with functionalized TiO2 nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 2014, 241, 155–166. [Google Scholar] [CrossRef]
- Vanherck KVandezande, P.; Aldea, S.O.; Vankelecom, I.F.J. Cross-linked polyimide membranes for solvent resistant nanofiltration in aprotic solvents. J. Membr. Sci. 2008, 320, 468–476. [Google Scholar] [CrossRef]
- Ba, C.; Economy, J. Preparation of PMDA/ODA polyimide membrane for use as substrate in a thermally stable composite reverse osmosis membrane. J. Membr. Sci. 2010, 363, 140. [Google Scholar] [CrossRef]
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Anokhina, T.; Borisov, I.; Yushkin, A.; Vaganov, G.; Didenko, A.; Volkov, A. Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties. Polymers 2020, 12, 2785. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Zhu, T.; Hua, Z.; Chen, Q.; Yu, X. Blends of thermoplastic pol- yurethane and polyether–polyimide: Preparation and properties. Polymer 2001, 42, 1493–1500. [Google Scholar] [CrossRef]
- Kogiso, T.; Inoue, S. Synthesis and Properties of Elastic Polyurethane-Imide. J. Appl. Polym. Sci. 2010, 115, 242–248. [Google Scholar] [CrossRef]
- Krizan, T.D.; Bershtein, V.A.; Sukhanova, T.E.; Keating, M.Y.; Bursian, A.E.; Grigoriev, A.I.; Egorov, V.M.; Vylegzhanina, M.E.; Yakushev, P.N. Structure-property relationships as determined in PMDA-ODA polyimide processed by powder metallurgy techniques. In Polyimides and Other High Temperature Polymers; CRC Press: Boca Raton, FL, USA, 2005; pp. 89–110. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Sukhanova, T.E.; Krizan, T.D.; Keating, M.Y.; Grigoriev, A.I.; Egorov, V.M.; Yakushev, P.N.; Peschanskaya, N.N.; Vylegzhanina, M.E.; Bursian, A.E. Relationship of Processing Conditions to Structure and Properties in PMDA-ODA Polyimide. J. Macromol. Sci. Part B 2005, 44, 613–639. [Google Scholar] [CrossRef]
- Didenko, A.L.; Ivanov, A.G.; Smirnova, V.E.; Vaganov, G.V.; Anokhina, T.S.; Borisov, I.L.; Volkov, V.V.; Volkov, A.V.; Kudryavtsev, V.V. Selective Destruction of Soluble Polyurethaneimide as NovelApproach for Fabrication of Insoluble Polyimide Films. Polymers 2022, 14, 4130. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Inoue, S.-I. Thermoplasticity in Polyurethane-Imide Elastomers. Org. Polym. Mater. 2018, 8, 11. [Google Scholar] [CrossRef][Green Version]
- Jonquieres, A.; Clement, R.; Lochon, P. Permeability of block copolymers to vapors and liquid. Prog. Polym. Sci. 2002, 27, 1803–1877. [Google Scholar] [CrossRef]
- Solimando, X.; Babin, J.; Arnal-Herault, C.; Wang, M.; Barth, D.; Roizard, D.; Doillon-Halmenschlager, J.-R.; Poncot, M.; Royaud, I.; Alcouffe, P.D.; et al. Highly selective multi-block poly (ether-urea-imide) s for CO2/N2 separation: Structure-morphology- properties relationships. Polymer 2017, 131, 56. [Google Scholar] [CrossRef]
- Sang, X.; Wang, R.; Chen, X.; Zhang, L.; An, M.; Shen, Y. A Review on Synthesis and Property of Polyurethane-imide. Adv. Mater. Res. 2011, 284–286, 1746. [Google Scholar] [CrossRef]
- Ueda, T.; Nishio, T.; Inoue, S. Influences of Diamines on the Morphologies and the Chemical, Thermal, and Mechanical Properties of Polyurethane-Imide Elastomers. Open J. Org. Polym. Mater. 2017, 7, 47. [Google Scholar] [CrossRef]
- Masiulanis, B.; Hrouz, J.; Baldrian, J.; Ilavský, M.; Dušek, K. Dynamic Mechanical Behavior and Structure of Polyurethaneimides. J. Appl. Polym. Sci. 1987, 34, 1941. [Google Scholar] [CrossRef]
- Holden, G.; Kricheldorf, H.; Quirk, R. Thermoplastic Elastomers, 3rd ed.; Hanser Publication: Cincinnati, OH, USA, 2004; p. 540. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons International Right. Inc.: Hoboken, NJ, USA, 2014; p. 439. [Google Scholar] [CrossRef]
- Didenko, A.L.; Kuznetcov, D.A.; Smirnova, V.E.; Bogdanov, E.A.; Vaganov, G.V.; Ivanov, A.G.; Kobykhno, I.A.; Vasil’eva, E.S.; Tolochko, O.V.; Svetlichnyi, V.M.; et al. Synthesis, Heat Resistance, and Mechanical Properties of Cross-Linked Urethane–Imide Copolymers Containing Blocks of Two Structurally Diff erent Aliphatic Fragments (Polyether and Polyester) in the Backbone.Russ. J. Appl. Chem. 2021, 94, 1240–1259. [Google Scholar] [CrossRef]
- Didenko, A.L.; Kuznetcov, D.A.; Smirnova, V.E.; Popova, E.N.; Vaganov, G.V.; Ivanov, A.G.; Svetlichnyi, V.M.; Yudin, V.E.; Kudryavtsev, V.V. Co-poly (urethane-imide) s based on poly[di(ethylene glycol) adipate] and their compositions with thermoplas- tic polyimide: Synthesis and properties. Russ. Chem. Bull. 2020, 69, 369–377. [Google Scholar] [CrossRef]
- Didenko, A.L.; Kuznetsov, D.A.; Vaganov, G.V.; Smirnova, V.E.; Popova, E.N.; Ivanov, A.G.; Svetlichnyi, V.M.; Yudin, V.E.; Kudryavtsev, V.V. Multiblock Copoly (urethane–imide)s with the Properties of Thermoplastic Elastomers. Polym. Sci. Ser. C 2020, 62, 90–110. [Google Scholar] [CrossRef]
- de Visser, A.C.; Driessen, A.A.; Wolke, J.G.C. Segmented copolyether-imides, 2. Die Makromol. Chem. Rapid Commun. 1980, 1, 177. [Google Scholar] [CrossRef]
- Yeganeh, H.; Shamekhi, M.A. Poly (urethane-imide), a New Generation of Thermoplastic Elastomers with Enhanced Thermal Stability. Polymer 2004, 45, 359. [Google Scholar] [CrossRef]
- Gerkin, R.M.; Hilker, B.L. Block Copolymers: Segmented: Encyclopedia of Materials: Science and Technology; Elsevier Ltd.: Amsterdam, The Netherlands, 2001; p. 10388. [Google Scholar] [CrossRef]
- Vasnev, V.A.; Kuchanov, S.I. Non-equilibrium Copolycondensation in Homogeneous Systems. Russ. Chem. Rev. 1973, 42, 1020–1033. [Google Scholar] [CrossRef]
- Russel, T.P. A small-angle X-ray scattering study of an aromatic polyimide. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 1105–1117. [Google Scholar] [CrossRef]
- Takahashi, N.; Yoon, D.Y.; Parris, W. Molecular order in condensed states of semiflexible poly(amic acid) and polyimide. Macromolecules 1984, 17, 2583. [Google Scholar] [CrossRef]


| Sample | Thermolysis Temperature, °C | Hydrolysis Blend Composition |
|---|---|---|
| PUI-1-170 °C | 170 | - |
| PUI-1-250 °C | 250 | - |
| PUI-1-300 °C | 300 | - |
| PUI-1-350 °C | 350 | - |
| PUI-1-370 °C | 370 | - |
| PUI-1-170 °C-I | 170 | CH3COOH + HCl 90:10 (oб) % |
| PUI-1-300 °C-II | 300 | CH3COOH + HCl 10:90 (oб) % |
| PUI-1-300 °C-III | 300 | C2HF3O2 + H2O 50:50 (oб) % |
| Sample | Solubility in DMF | SD, % | Ks, g/g | dav., nm | PDMF kg/m2 h bar | R, % Remasol Brilliant Blue R |
|---|---|---|---|---|---|---|
| PUI-1-170 °C | Insoluble | 38.7 | 0.51 | - | 0.01 | 95 |
| PUI-1-250 °C | 20.2 | 0.21 | - | Not permeable | ||
| PUI-1-300 °C | 5.0 | 0.12 | - | Not permeable | ||
| PUI-1-170 °C-I | 33.7 | 0.70 | 66 | 2.3 | 10 | |
| PUI-1-300 °C-II | 27.3 | 0.21 | - | Not permeable | ||
| PUI-1-300 °C-III | 21.1 | 0.25 | 82 | 7.5 | 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhanova, T.E.; Didenko, A.L.; Borisov, I.L.; Anokhina, T.S.; Ivanov, A.G.; Nesterova, A.S.; Kobykhno, I.A.; Yushkin, A.A.; Kudryavtsev, V.V.; Volkov, A.V. Morphological Analysis of Poly(4,4′-oxydiphenylene-pyromellitimide)-Based Organic Solvent Nanofiltration Membranes Formed by the Solution Method. Membranes 2022, 12, 1235. https://doi.org/10.3390/membranes12121235
Sukhanova TE, Didenko AL, Borisov IL, Anokhina TS, Ivanov AG, Nesterova AS, Kobykhno IA, Yushkin AA, Kudryavtsev VV, Volkov AV. Morphological Analysis of Poly(4,4′-oxydiphenylene-pyromellitimide)-Based Organic Solvent Nanofiltration Membranes Formed by the Solution Method. Membranes. 2022; 12(12):1235. https://doi.org/10.3390/membranes12121235
Chicago/Turabian StyleSukhanova, Tatyana E., Andrey L. Didenko, Ilya L. Borisov, Tatyana S. Anokhina, Aleksey G. Ivanov, Anna S. Nesterova, Ilya A. Kobykhno, Alexey A. Yushkin, Vladimir V. Kudryavtsev, and Alexey V. Volkov. 2022. "Morphological Analysis of Poly(4,4′-oxydiphenylene-pyromellitimide)-Based Organic Solvent Nanofiltration Membranes Formed by the Solution Method" Membranes 12, no. 12: 1235. https://doi.org/10.3390/membranes12121235
APA StyleSukhanova, T. E., Didenko, A. L., Borisov, I. L., Anokhina, T. S., Ivanov, A. G., Nesterova, A. S., Kobykhno, I. A., Yushkin, A. A., Kudryavtsev, V. V., & Volkov, A. V. (2022). Morphological Analysis of Poly(4,4′-oxydiphenylene-pyromellitimide)-Based Organic Solvent Nanofiltration Membranes Formed by the Solution Method. Membranes, 12(12), 1235. https://doi.org/10.3390/membranes12121235











