Efficiency Analysis of Fuel Cell Components with Ionic Poly-Arylether Composite Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SA8 Solution
2.2. Preparation of Polyethylene Glycol Solution
2.3. Preparation of SA8 and PEG Film-Forming Solution
2.4. Preparation of sPEEK Solution
2.5. Preparation of SA8 and sPEEK Film-Forming Solution
2.6. Preparation of SA8, PEG and sPEEK Film-Forming Solution
2.7. Preparation of Fuel Cell Components
2.8. Instrument Measurement
3. Results
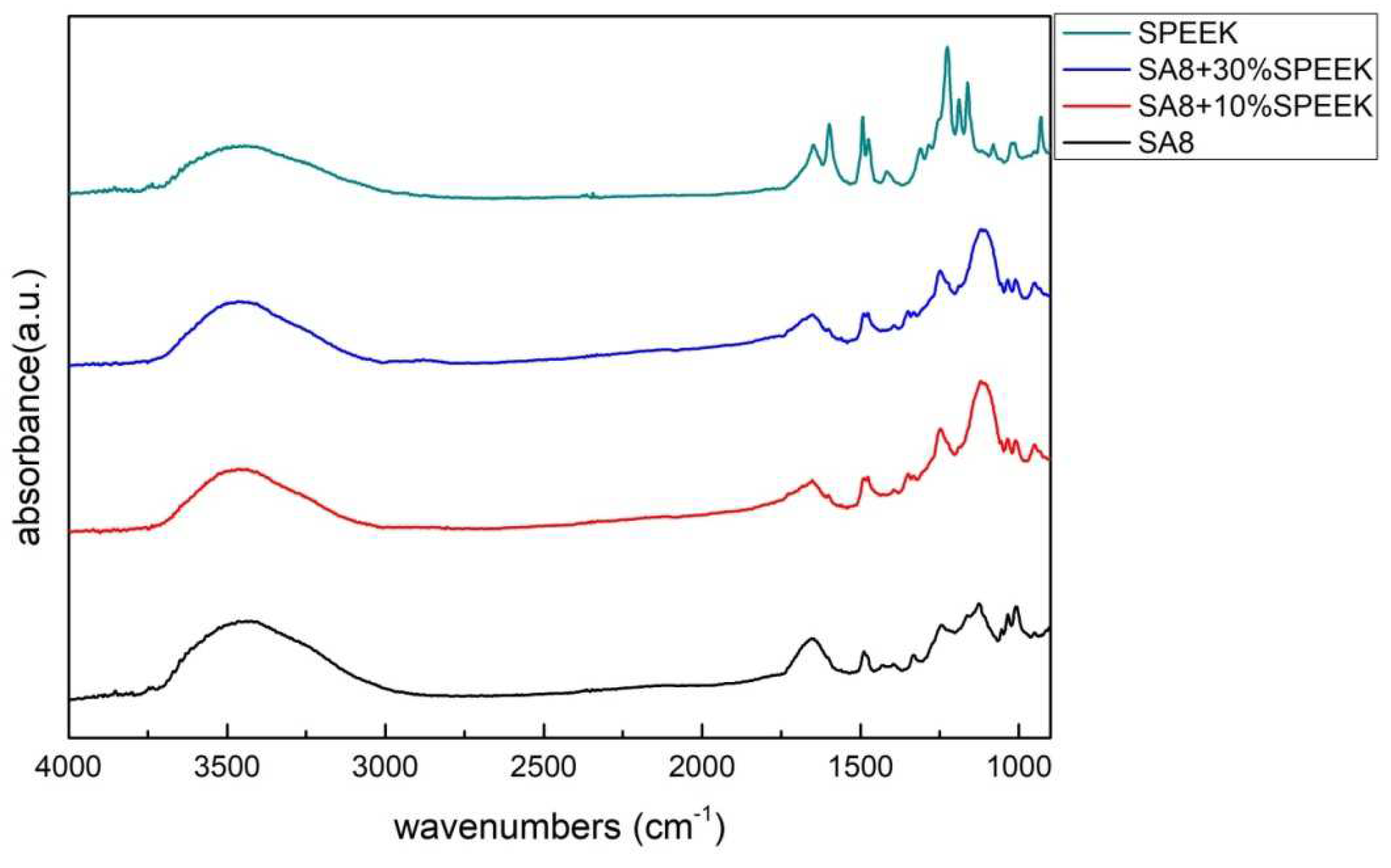
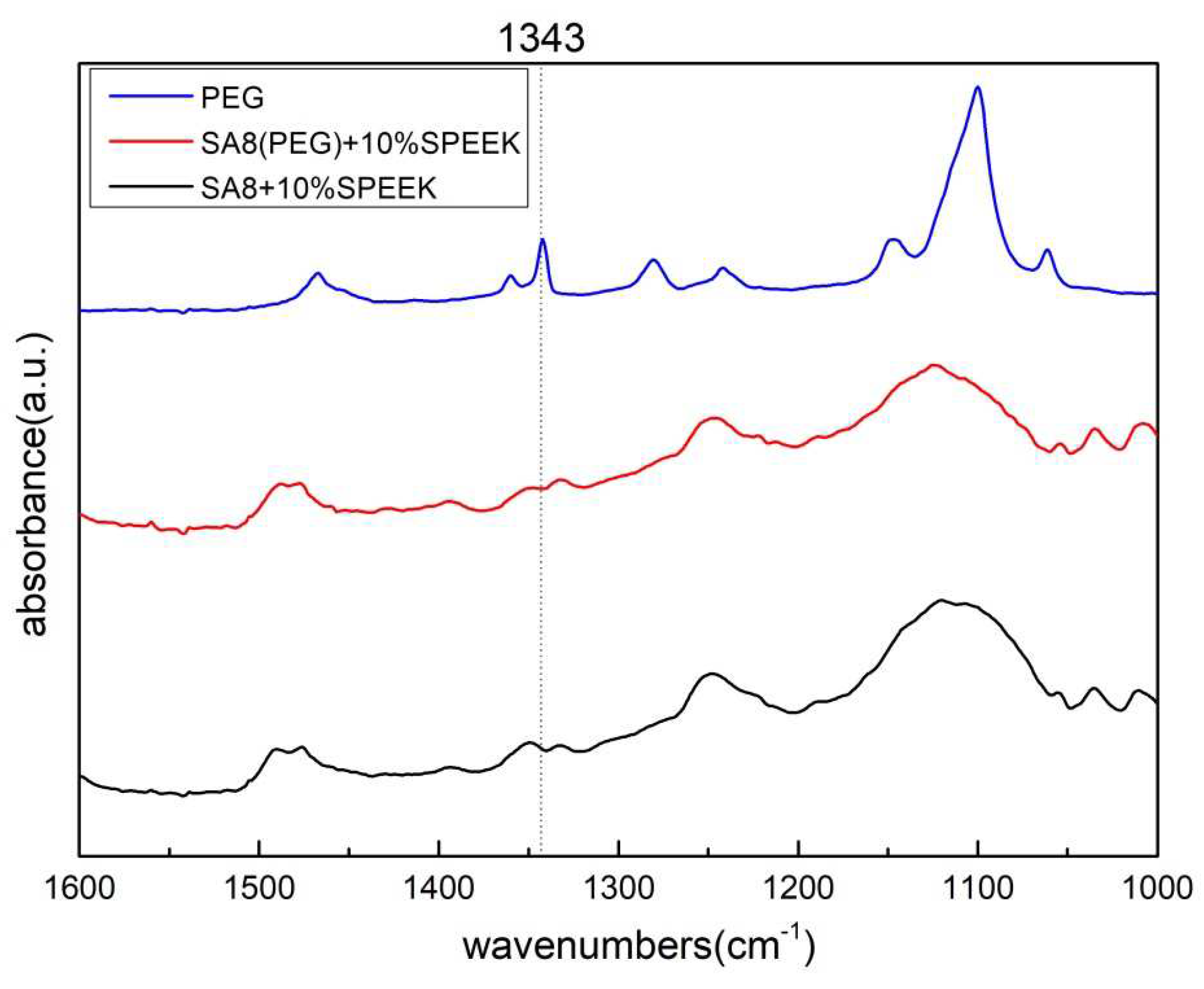
3.1. Fourier-Transform Infrared Spectroscopy Analysis [20]
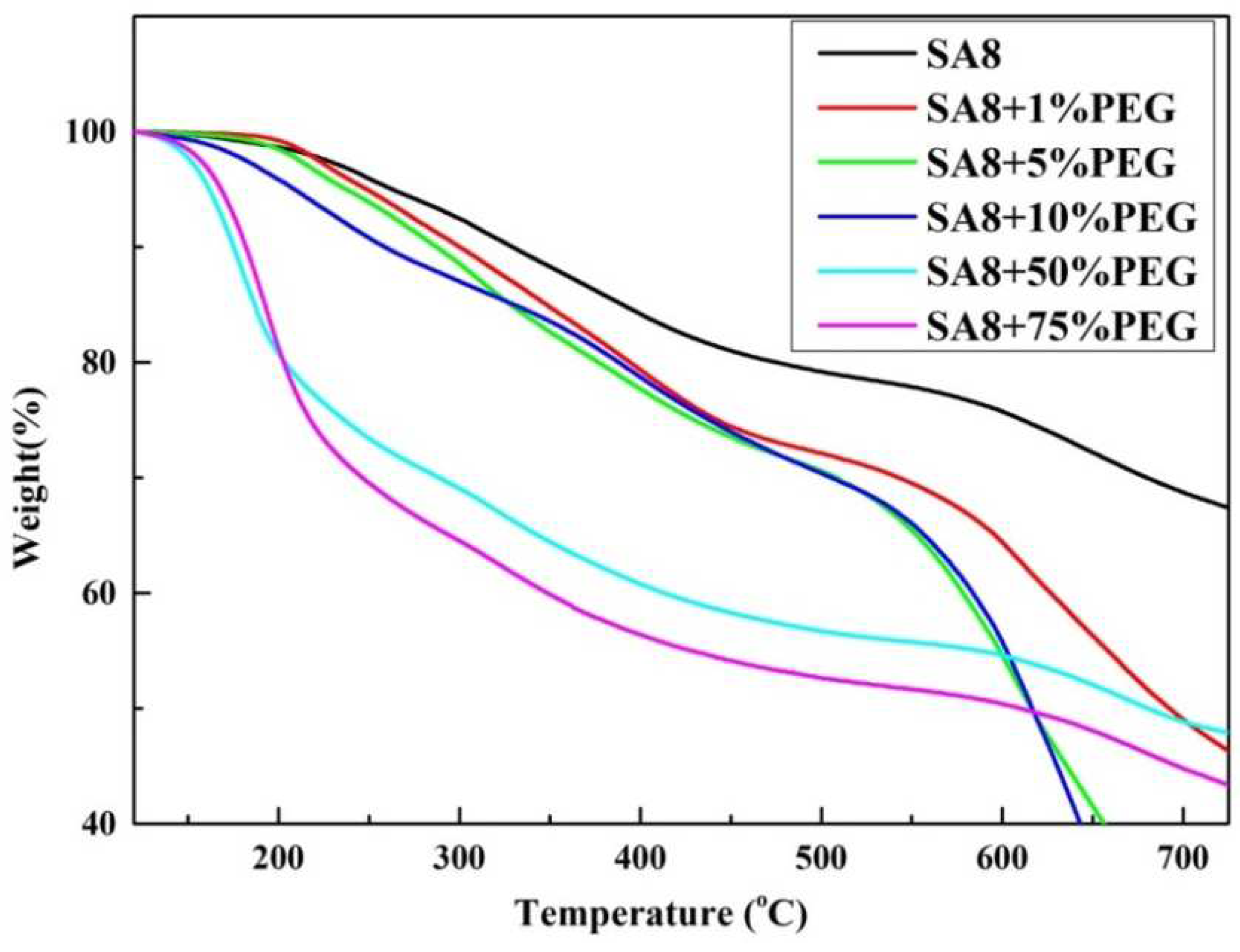
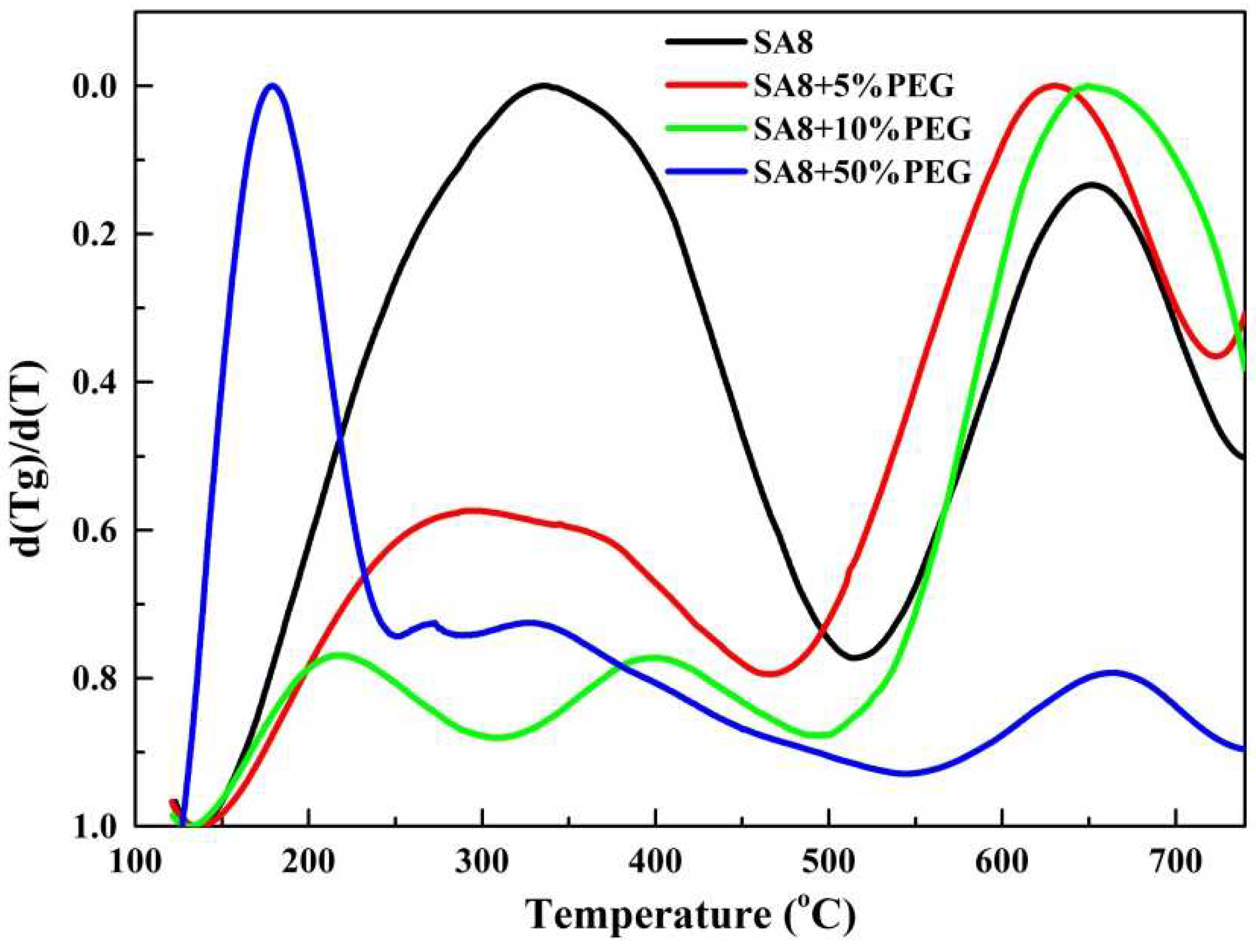
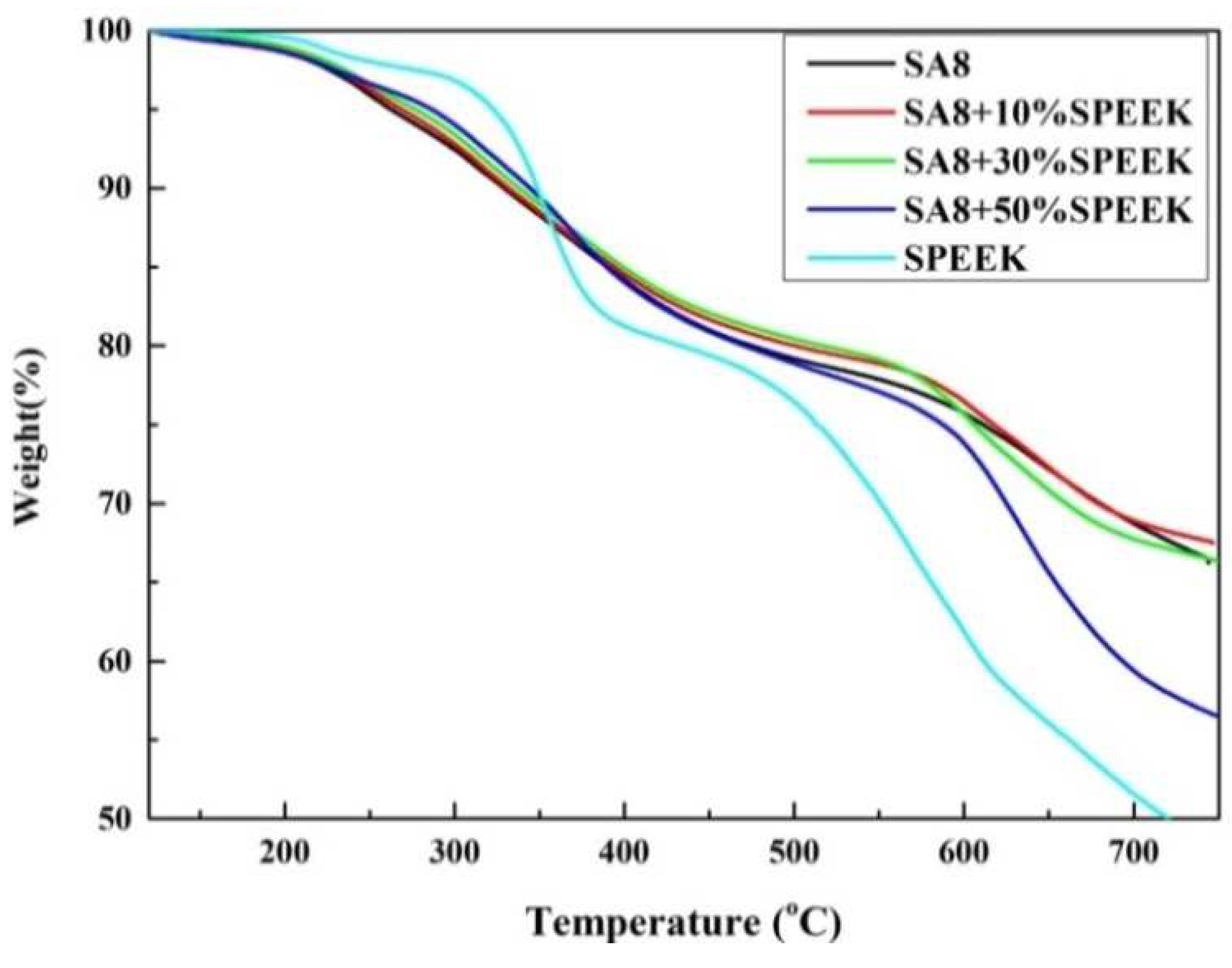
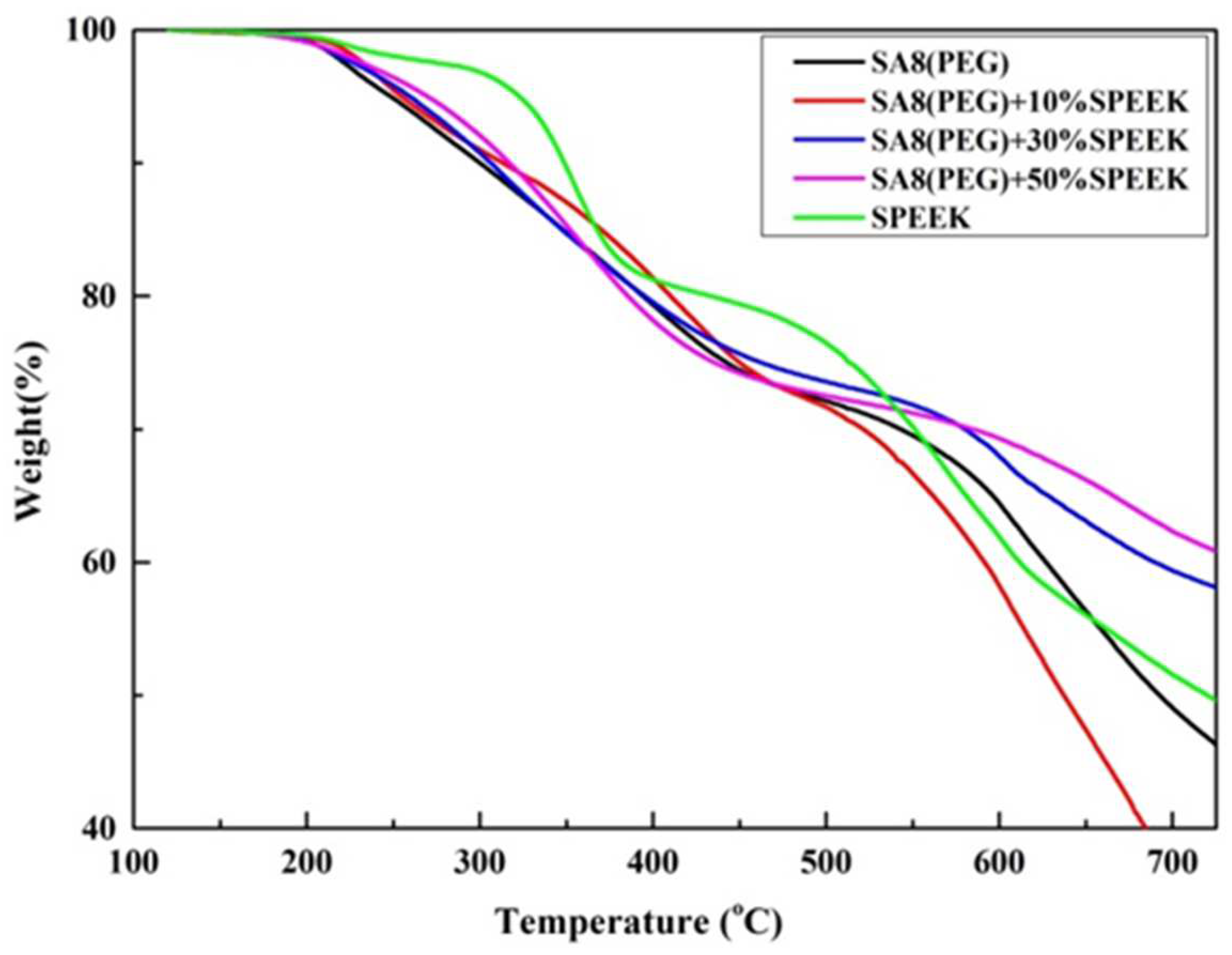
3.2. Thermogravimetric Analysis of Thermal Stability
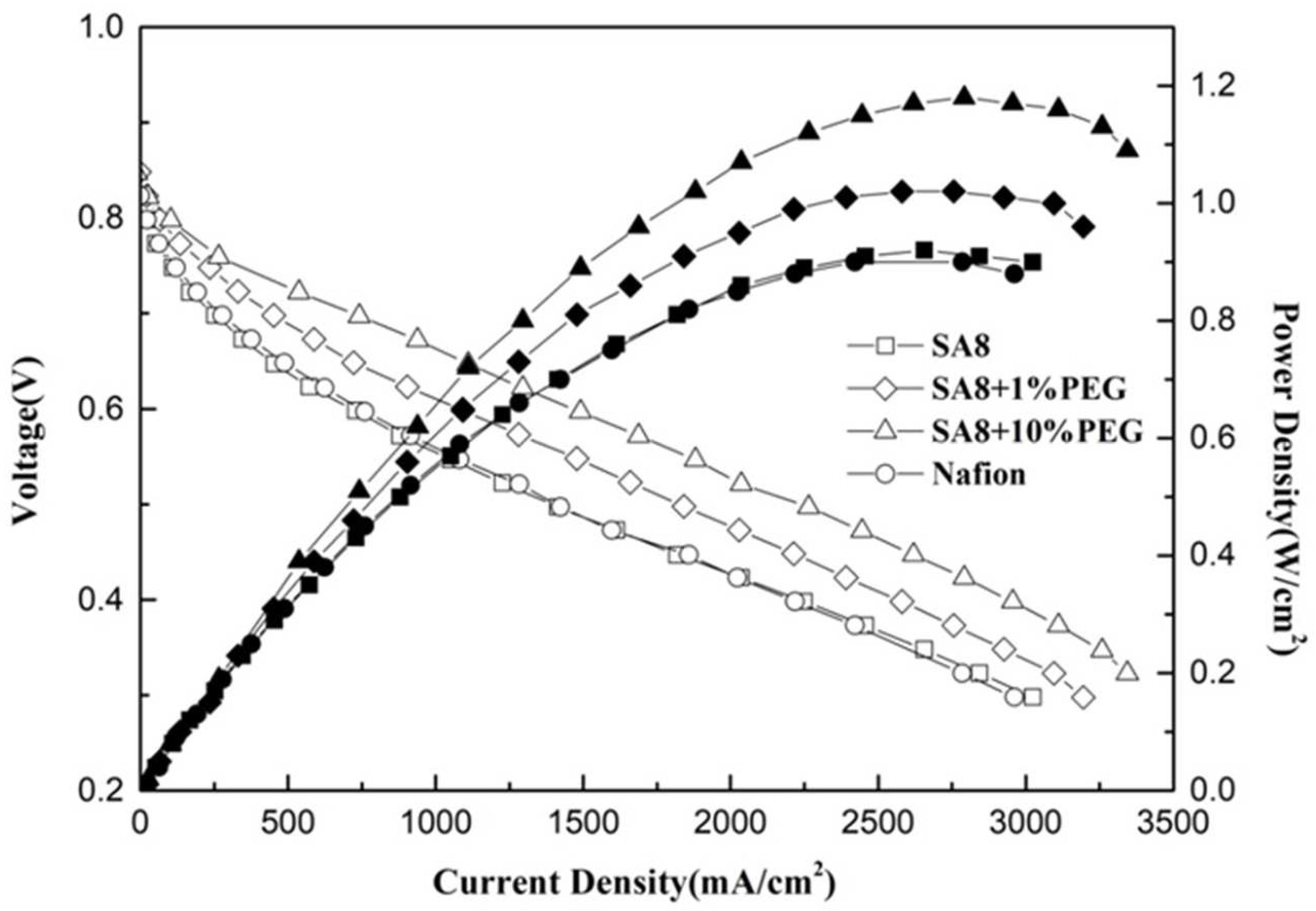
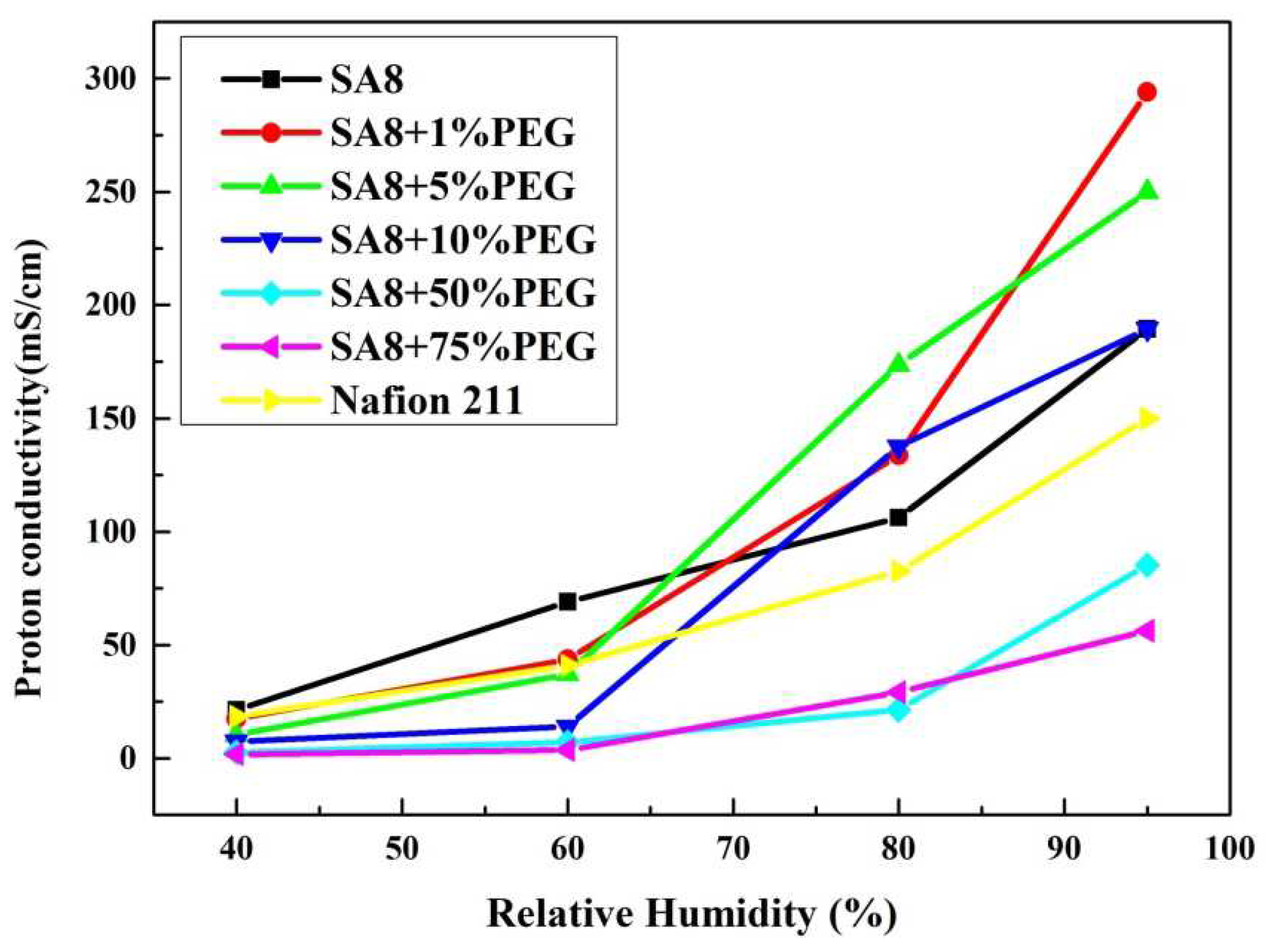
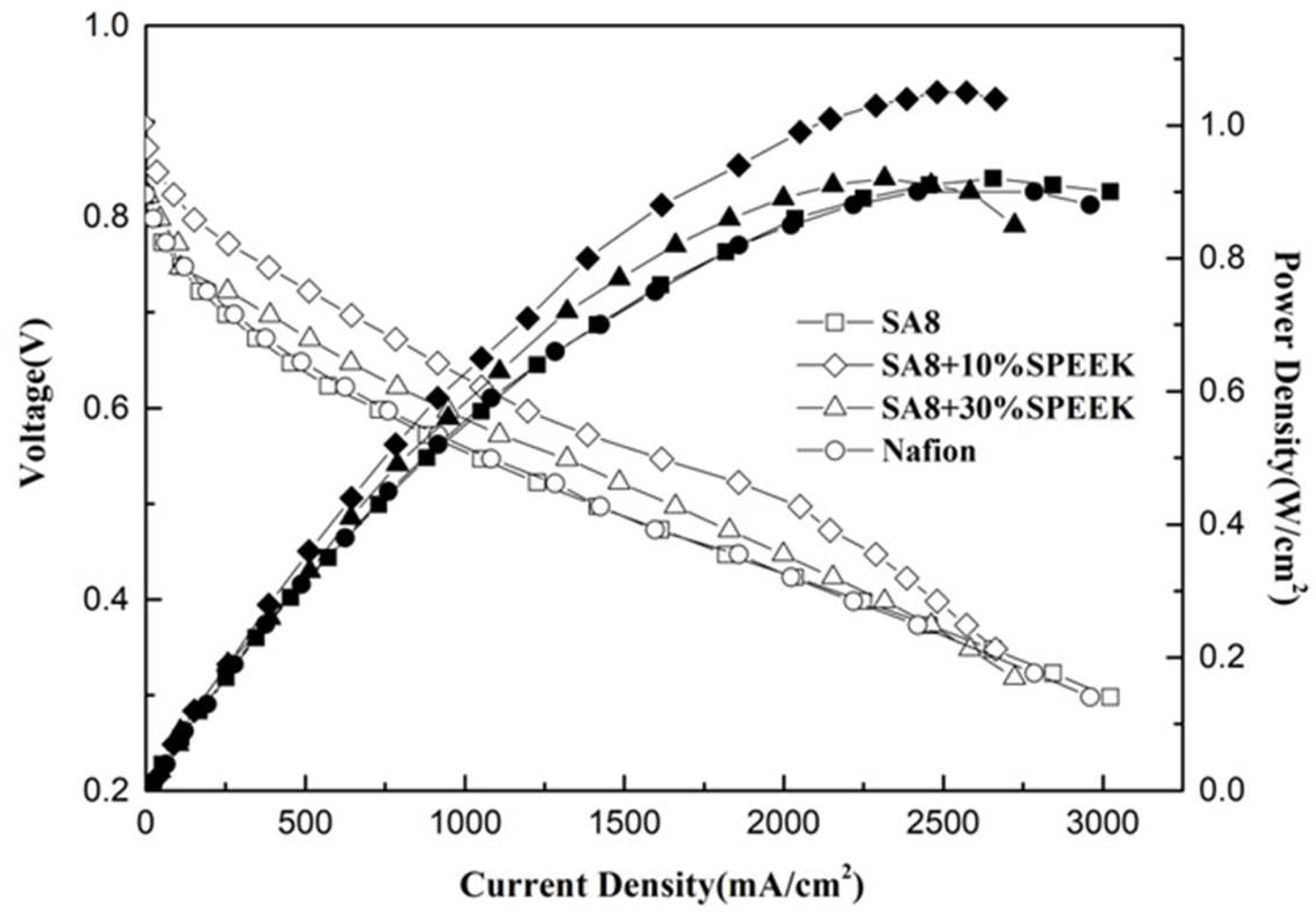
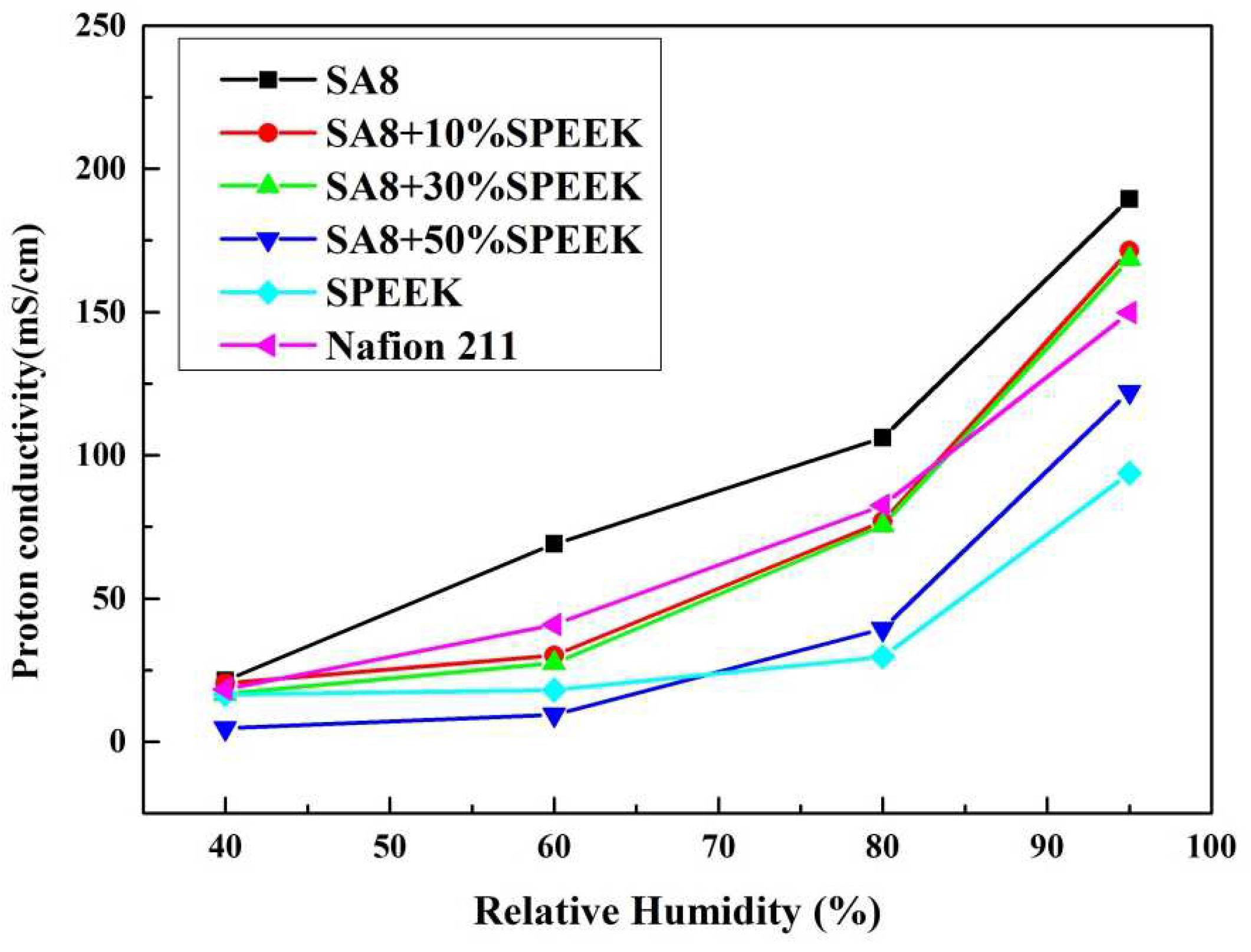
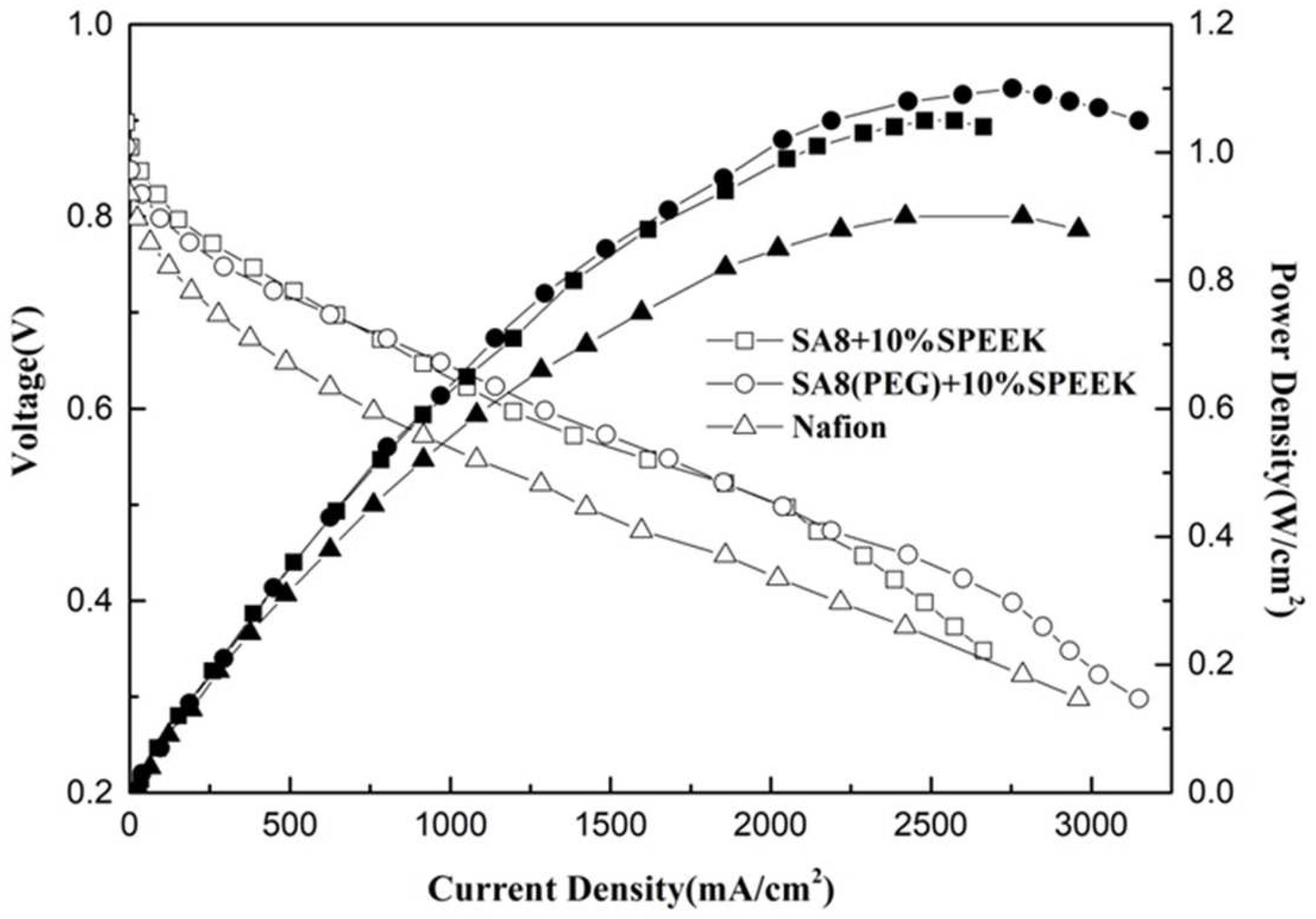
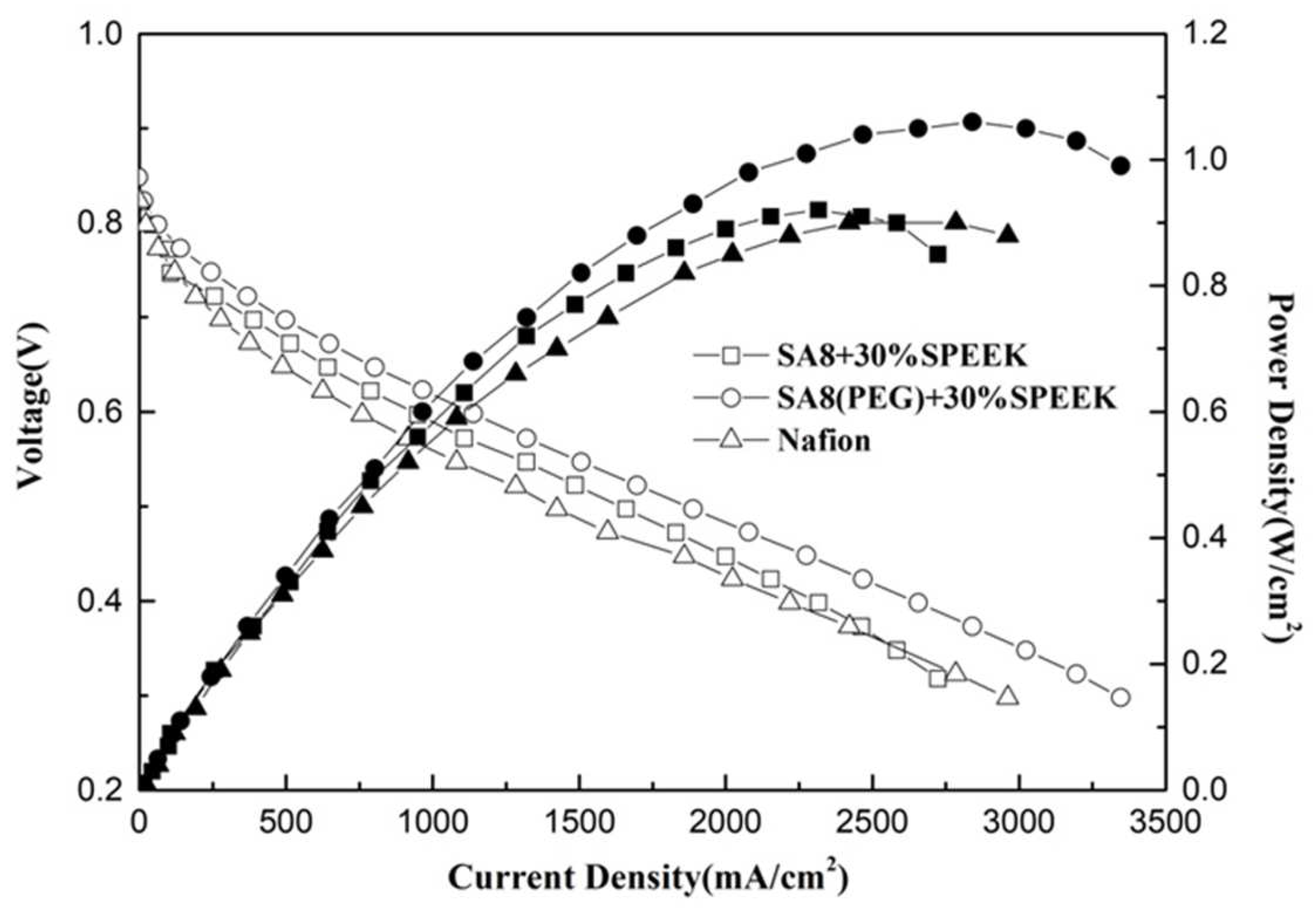
3.3. Fuel Cell Component Performance Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozano, L.D.; Carter, K.R.; Lee, V.Y.; Miller, R.D.; DiPietro, R.; Scott, J.C. Electroluminescent devices based on cross-linked polymer blends. J. Appl. Phys. 2003, 94, 3061. [Google Scholar] [CrossRef]
- Gutru, R.; Peera, S.G.; Bhat, S.D.; Sahu, A.K. Synthesis of sulfonated poly(bis(phenoxy)phosphazene) based blend membranes and its effect as electrolyte in fuel cells. Solid State Ion. 2016, 296, 127–136. [Google Scholar] [CrossRef]
- Zheng, C.; Greer, H.F.; Chiang, C.Y.; Zhou, W. Microstructural study of the formation mechanism of metal–organic framework MOF-5. CrystEngComm 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 3, 221–291. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzu, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly(arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447. [Google Scholar] [CrossRef]
- Hsieh, T.L.; Guo, W.H.; Chang, M.Y.; Huang, W.Y.; Wen, H.Y. Electric Field-Assisted Filling of Sulfonated Polymers in ePTFE Backing Material for Fuel Cell. Membranes 2022, 12, 974. [Google Scholar] [CrossRef]
- Deguchi, Y.; Kono, M.; Koizumi, Y.; Izato, Y.I.; Miyake, A. Study on Autocatalytic Decomposition of Dimethyl Sulfoxide (DMSO). Org. Process Res. Dev. 2020, 24, 1614–1620. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.G.; Wu, N.F.; Fan, M.M.; Shen, X.L.; Chen, M.T.; Jiang, A.M.; Lai, L.S. Effect of hsian-tsao gum (HG) content upon rheological properties of film-forming solutions (FFS) and physical properties of soy protein/hsian-tsao gum films. Food Hydrocoll. 2015, 50, 211–218. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodriguez-Rodriguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Mutlu, B.; Farhan, M.; Kucuk, I. T-Shaped Microfluidic Junction Processing of Porous Alginate-Based Films and Their Characteristics. Polymers 2019, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, S.; Wang, J.; Li, Y.; Zhu, L.; Xu, M.; Wang, C. Hydrophilic SPEEK/PES composite membrane for pervaporation desalination. Sep. Purif. Technol. 2020, 250, 117265. [Google Scholar] [CrossRef]
- Hwang, L.-L.; Chen, J.-C.; Wey, M.-Y. The properties and filtration efficiency of activated carbon polymer composite membranes for the removal of humic acid. Desalination 2013, 313, 166–175. [Google Scholar] [CrossRef]
- Nallamolu, S.; Jayanti, V.R.; Chitneni, M.; Khoon, L.Y.; Sood, S.; Riadi, Y.; Kesharwani, P. Fabrication of thermodynamically stable self-microemulsifying drug delivery system of resveratrol with enhanced solubility and chemical stability. Appl. Energy 2022, 43, 1766–1777. [Google Scholar] [CrossRef]
- Steffy, N.; Parthiban, V.; Sahu, A. Uncovering Nafion-multiwalled carbon nanotube hybrid membrane for prospective polymer electrolyte membrane fuel cell under low humidity. J. Membr. Sci. 2018, 563, 65–74. [Google Scholar]
- Xia, Z.; Wang, S.; Jiang, L.; Sun, H.; Qi, F.; Jin, J.; Sun, G. Rational design of a highly efficient Pt/graphene–Nafion® composite fuel cell electrode architecture. J. Mater. Chem. A 2015, 3, 1641–1648. [Google Scholar] [CrossRef]
- Branchi, M.; Sgambetterra, M.; Pettiti, I.; Panero, S.; Navarra, M.A. Functionalized Al2O3 particles as additives in proton-conducting polymer electrolyte membranes for fuel cell applications. Int. J. Hydrogen Energy 2015, 40, 14757–14767. [Google Scholar] [CrossRef]
- Tang, H.; Pan, M.; Wang, X.; Ruan, Y. Fabrication and characterization of PFSI/ePTFE composite proton exchange membranes of polymer electrolyte fuel cells. Electrochim. Acta 2007, 52, 5304–5311. [Google Scholar] [CrossRef]
- Doglia, S.M.; Ami, D.; Natalello, A.; Gatti-Lafranconi, P.; Lotti, M. Fourier transform infrared spectroscopy analysis of the conformational quality of recombinant proteins within inclusion bodies. Biotechnol. J. 2008, 3, 193–201. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Patience and Jamal Chaouki, Experimental methods in chemical engineering: Thermogravimetric analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Q.; Li, W.; Ma, Y.; Zhao, X.; Zhang, C. Measurement of free water in foods by secondary derivative thermogravimetry. CyTA-J. Food 2018, 16, 438–443. [Google Scholar] [CrossRef]
- Pereira, L.H.; Catelani, T.A.; Costa, É.D.; Garcia, J.S.; Trevisan, M.G. Coffee adulterant quantification by derivative thermogravimetry and chemometrics analysis. J. Therm. Anal. Calorim. 2022, 147, 7353–7362. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)X] hybrid membranes for vanadium redox flow batteries. Solid State Ion. 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Huang, W.; Liaw, B.; Chang, M.; Han, Y.; Huang, P. High glass-transition temperature and organosoluble novel arylene ether polymers. Macromolecules 2007, 40, 8649–8657. [Google Scholar] [CrossRef]
- Huang, W.Y.; Chang, M.; Han, Y.; Huang, P. Sterically encumbered poly (arylene ether) s containing spiro-annulated substituents: Synthesis and thermal properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5872–5884. [Google Scholar] [CrossRef]
- Lee, C.-C.; Huang, W.-Y. The influence of arylene ether positions and phenyl substituents on the physical properties of functional fluorinated polymers. Polym. J. 2011, 43, 180–185. [Google Scholar] [CrossRef][Green Version]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef]
- Huang, T.-S.; Hsieh, T.-L.; Lai, C.-C.; Wen, H.-Y.; Huang, W.-Y.; Chang, M.-Y. Highly Proton-Conducting Membranes Based on Poly(arylene ether)s with Densely Sulfonated and Partially Fluorinated Multiphenyl for Fuel Cell Applications. Membranes 2021, 11, 626. [Google Scholar] [CrossRef]
- Vickraman, P.; Ramamurthy, S. A study on the blending effect of PVDF in the ionic transport mechanism of plasticized PVC–LiBF4 polymer electrolyte. Mater. Lett. 2006, 60, 3431–3436. [Google Scholar] [CrossRef]
- Panwar, V.; Kumar, P.; Bansal, A.; Ray, S.S.; Jain, S.L. PEGylated magnetic nanoparticles (PEG@Fe3O4) as cost effective alternative for oxidative cyanation of tertiary amines via C-H activation. Appl. Catal. A Gen. 2015, 498, 25–31. [Google Scholar] [CrossRef]
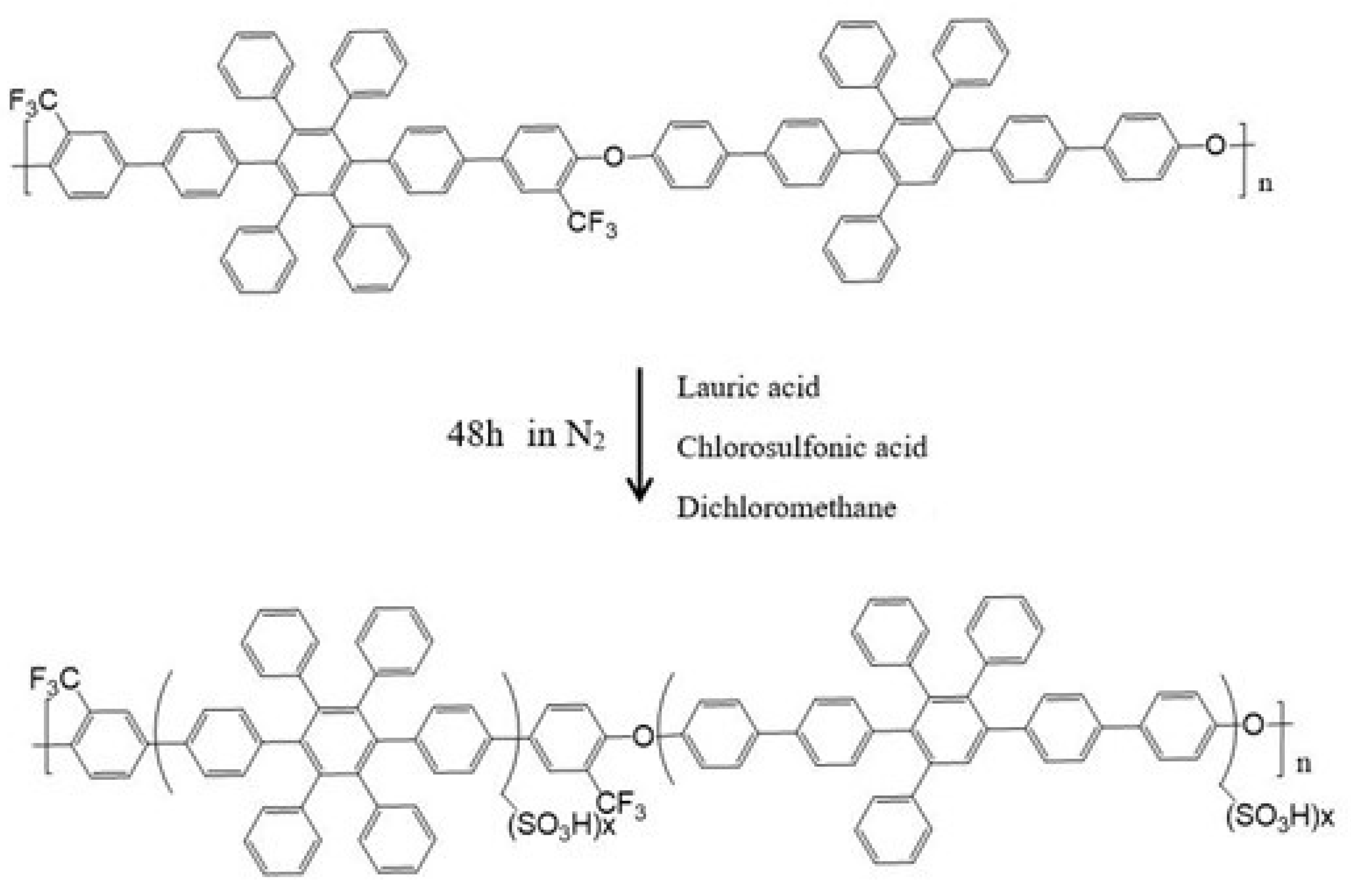
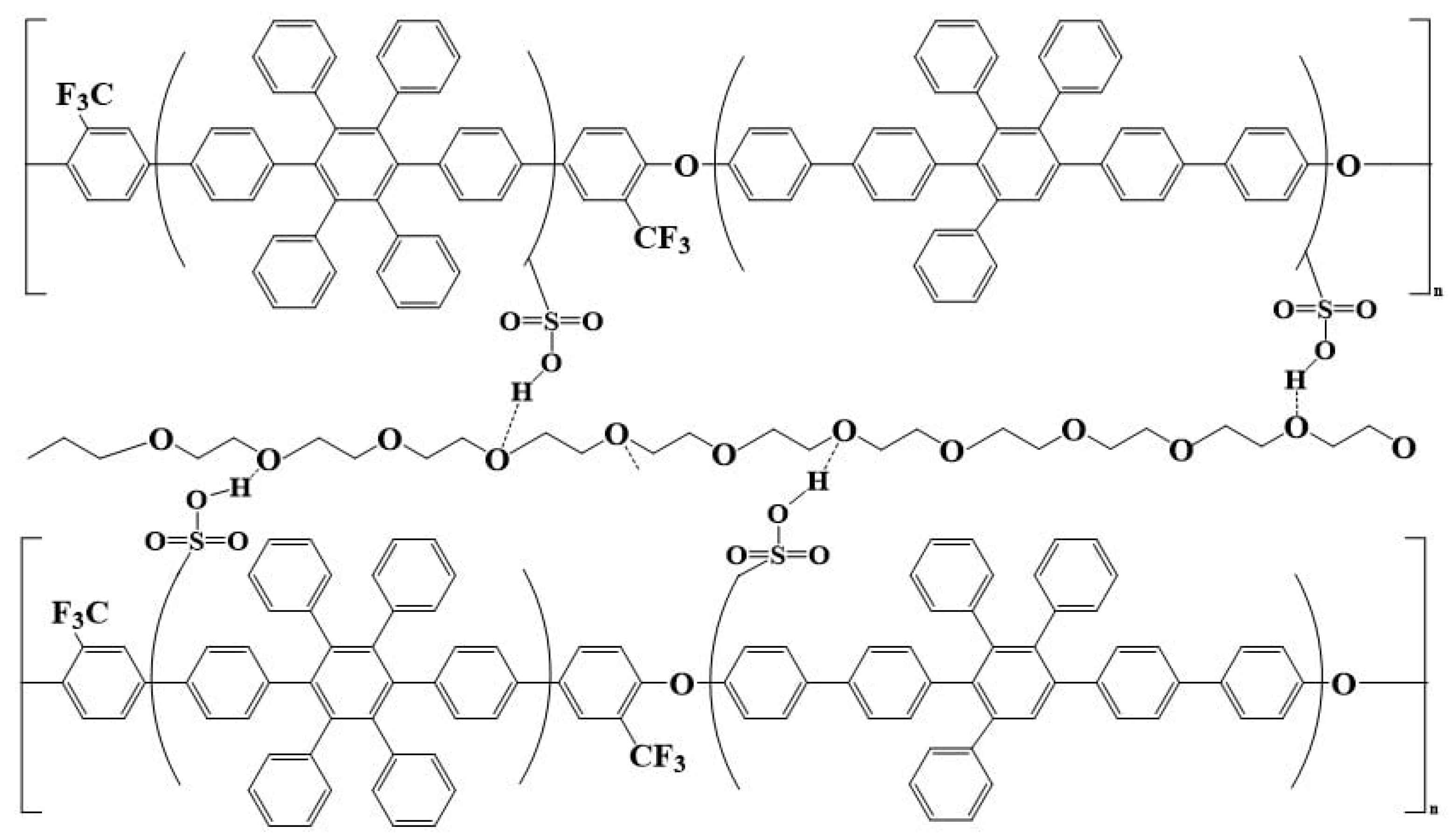
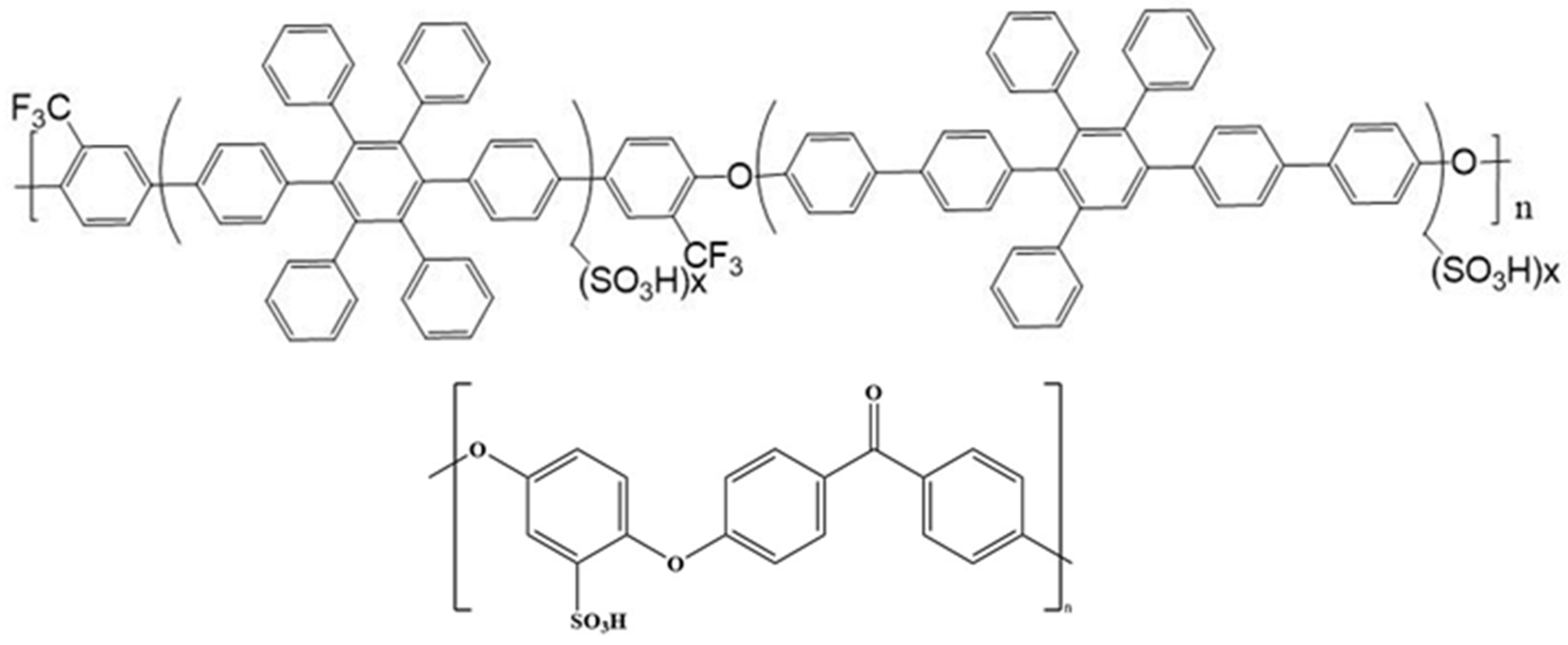









| Sulfonated Polymer | Solid Content of SA8 | Solid Content of PEG |
|---|---|---|
| 100%SA8 | 1.000 g | 0.000 g |
| 99%SA8 + 1%PEG | 0.990 g | 0.004 g |
| 95%SA8 + 5%PEG | 0.950 g | 0.020 g |
| 90%SA8 + 10%PEG | 0.900 g | 0.040 g |
| 50%SA8 + 50%PEG | 0.500 g | 0.200 g |
| 25%SA8 + 75%PEG | 0.250 g | 0.300 g |
| Sulfonated Polymer | Solid Content of SA8 | Solid Content of sPEEK |
|---|---|---|
| 100%SA8 | 1.00 g | 0.00 g |
| 90%SA8 + 10%SPEEK | 0.09 g | 0.04 g |
| 70%SA8 + 30% SPEEK | 0.70 g | 0.12 g |
| 50%SA8 + 50% SPEEK | 0.50 g | 0.20 g |
| 100%SPEEK | 0.00 g | 0.40 g |
| Sulfonated Polymer | Solid Content of SA8 | Solid Content of PEG |
|---|---|---|
| SA8(PEG) | 4.95 g | 0.02 g |
| Sulfonated Polymer | Weight of SA8(PEG) Solution | Weight of sPEEK Solution |
|---|---|---|
| 100%SA8(PEG) | 8.0 g | 0.0 g |
| 90%SA8(PEG) + 10%SPEEK | 7.2 g | 0.8 g |
| 70%SA8(PEG) + 30%SPEEK | 5.6 g | 2.4 g |
| 50%SA8(PEG) + 50%SPEEK | 4.0 g | 4.0 g |
| 100%SPEEK | 0.0 g | 8.0 g |
| Sulfonated Polymer | Anode Loading (mg/cm2) | Cathode Loading (mg/cm2) | Open Circuit Voltage (V) | Power Density (W/cm2) |
|---|---|---|---|---|
| SA8 | 0.2 | 0.4 | 0.958 | 0.92 |
| SA8 + 1%PEG | 0.2 | 0.4 | 0.962 | 1.02 |
| SA8 + 10%PEG | 0.2 | 0.4 | 0.885 | 1.18 |
| Nafion 211 | 0.2 | 0.4 | 0.955 | 0.90 |
| Sulfonated Polymer | Anode Loading (mg/cm2) | Cathode Loading (mg/cm2) | Open Circuit Voltage (V) | Power Density (W/cm2) |
|---|---|---|---|---|
| SA8 | 0.2 | 0.4 | 0.958 | 0.92 |
| SA8 + 10%SPEEK | 0.2 | 0.4 | 0.963 | 1.05 |
| SA8 + 30%SPEEK | 0.2 | 0.4 | 0.960 | 0.92 |
| Nafion 211 | 0.2 | 0.4 | 0.955 | 0.90 |
| Sulfonated Polymer | Anode Loading (mg/cm2) | Cathode Loading (mg/cm2) | Open Circuit Voltage (V) | Power Density (W/cm2) |
|---|---|---|---|---|
| SA8 + 10%SPEEK | 0.2 | 0.4 | 0.963 | 1.04 |
| SA8(PEG) + 10%SPEEK | 0.2 | 0.4 | 0.947 | 1.10 |
| SA8 + 30%SPEEK | 0.2 | 0.4 | 0.960 | 0.92 |
| SA8(PEG) + 30%SPEEK | 0.2 | 0.4 | 0.965 | 1.06 |
| Nafion 211 | 0.2 | 0.4 | 0.955 | 0.90 |
| Sulfonated Polymer | Water Uptake (%) | Dimensional Swelling (L%) | Dimensional Swelling (W%) | Hydration Number (λ) |
|---|---|---|---|---|
| SA8(PEG) | 82 | 12.50 | 12.82 | 15.3 |
| SA8(PEG) + 10%SPEEK | 63.9 | 11.25 | 10.00 | 12.1 |
| SA8(PEG) + 30%SPEEK | 73.7 | 8.75 | 10.00 | 14.8 |
| SA8(PEG) + 50%SPEEK | 54 | 8.75 | 7.14 | 10.6 |
| SPEEK | 17.9 | 5.00 | 5.00 | 8.6 |
| Nafion 211 | 33.3 | 7.50 | 7.50 | 20.3 |
| Sulfonated Polymer | Hydrolytic Stability (%) | Oxidative Stability (%) |
|---|---|---|
| SA8 | 98.4% | 87.4% |
| SA8(PEG) | 99.1% | 92.7% |
| SA8(PEG) + 10%SPEEK | 99.3% | 95.5% |
| SA8(PEG) + 30%SPEEK | 98.7% | 94.5% |
| SA8(PEG) + 50%SPEEK | 98.5% | 96.8% |
| SPEEK | 100.0% | 57.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.-Y.; Wang, G.-H.; Chang, M.-Y.; Huang, W.-Y.; Hsieh, T.-L. Efficiency Analysis of Fuel Cell Components with Ionic Poly-Arylether Composite Membrane. Membranes 2022, 12, 1238. https://doi.org/10.3390/membranes12121238
Wen H-Y, Wang G-H, Chang M-Y, Huang W-Y, Hsieh T-L. Efficiency Analysis of Fuel Cell Components with Ionic Poly-Arylether Composite Membrane. Membranes. 2022; 12(12):1238. https://doi.org/10.3390/membranes12121238
Chicago/Turabian StyleWen, Hsin-Yi, Guang-Hsiang Wang, Mei-Ying Chang, Wen-Yao Huang, and Tung-Li Hsieh. 2022. "Efficiency Analysis of Fuel Cell Components with Ionic Poly-Arylether Composite Membrane" Membranes 12, no. 12: 1238. https://doi.org/10.3390/membranes12121238
APA StyleWen, H.-Y., Wang, G.-H., Chang, M.-Y., Huang, W.-Y., & Hsieh, T.-L. (2022). Efficiency Analysis of Fuel Cell Components with Ionic Poly-Arylether Composite Membrane. Membranes, 12(12), 1238. https://doi.org/10.3390/membranes12121238










