Structural Role of Plasma Membrane Sterols in Osmotic Stress Tolerance of Yeast Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Large Unilamellar Vesicle Preparation
2.3. Stopped Flow Experiments
2.4. GUV Experiments
2.5. Yeast Strains and Growth Conditions
2.6. Deletion of LAM2
2.7. Growth Kinetics Analysis
2.8. Propidium Iodide Uptake upon Hypoosmotic Stress
3. Results
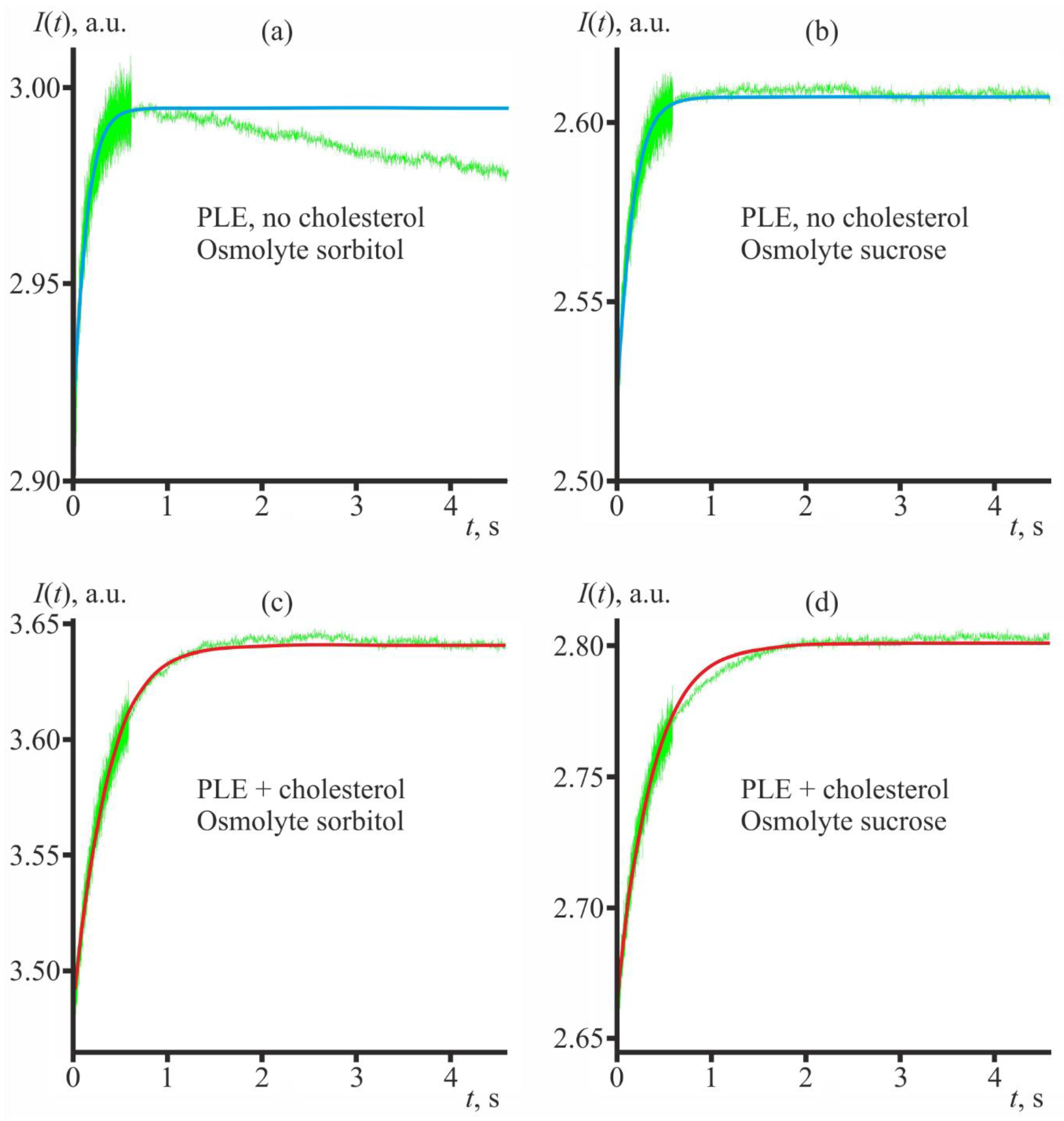
3.1. Lowering Sterol Content of Artificial Liposomes Increases Their Water Permeability
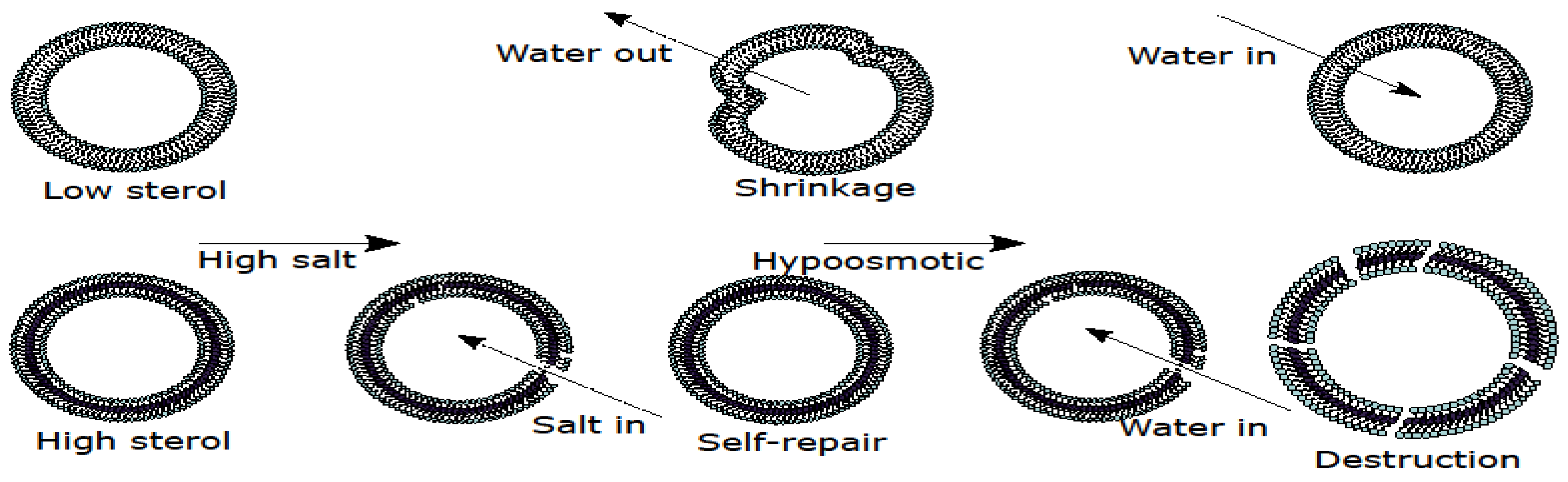
3.2. Sterol Prevents Shrinkage of Giant Unilamellar Vesicles upon Transient Hyperosmotic Stress
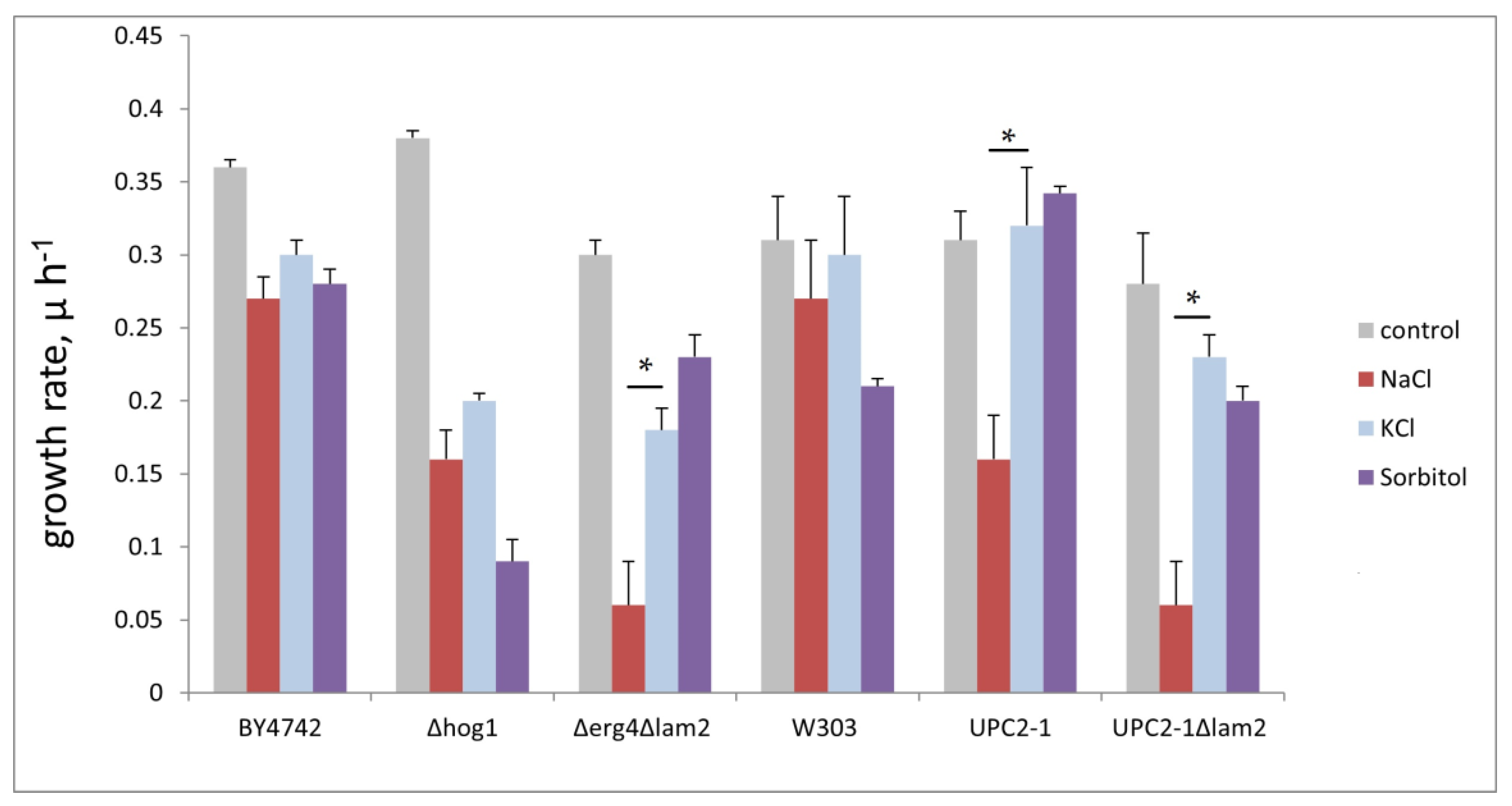
3.3. Yeast Mutants in Sterol Biosynthesis/Trafficking Are More Sensitive to Sodium Chloride than to Sorbitol
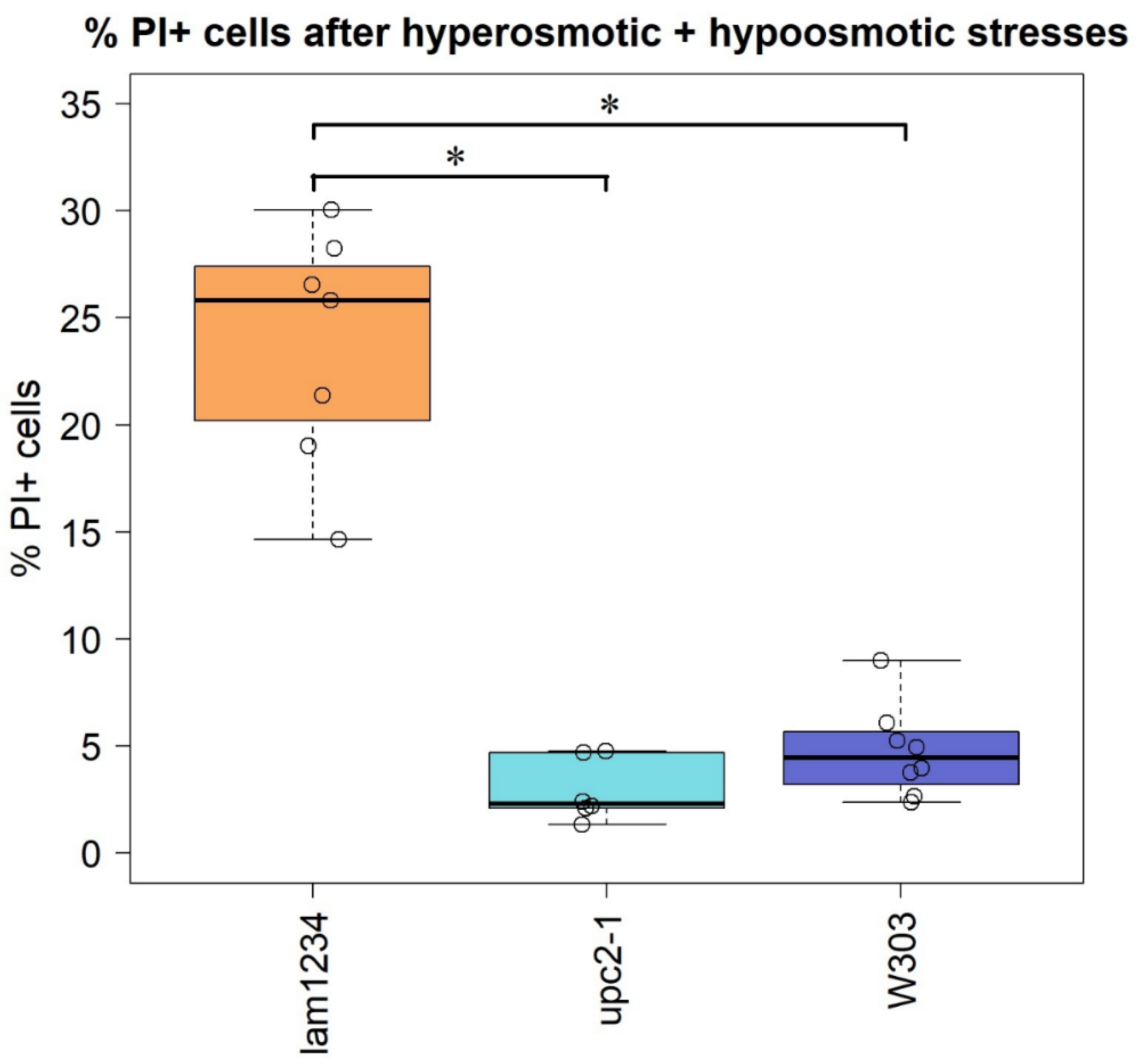
3.4. Yeast Cells Deficient in the Reverse Transport of Sterol from the PM Accumulate Propidium Iodide upon Hypoosmotic Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montañés, F.M.; Pascual-Ahuir, A.; Proft, M. Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors. Mol. Microbiol. 2011, 79, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Kodedová, M.; Sychrová, H. Changes in the sterol composition of the plasma membrane affect membrane potential, salt tolerance and the activity of multidrug resistance pumps in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0139306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S.; Beney, L.; Ferreira, T.; Gervais, P. Nature of sterols affects plasma membrane behavior and yeast survival during dehydration. Biochim. Biophys. Acta 2011, 1808, 1520–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudreault, F.; Tan, J.J.; Grygorczyk, R. Propidium uptake and ATP release in A549 cells share similar transport mechanisms. Biophys. J. 2022, 121, 1593–1609. [Google Scholar] [CrossRef]
- Rawicz, W.; Smith, B.A.; McIntosh, T.J.; Simon, S.A.; Evans, E. Elasticity, strength, and water permeability of bilayers that contain raft microdomain-forming lipids. Biophys. J. 2008, 94, 4725–4736. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Stenhammar, J.; Wennerström, H.; Sparr, E. Vesicles balance osmotic stress with bending energy that can be released to form daughter vesicles. J. Phys. Chem. Lett. 2022, 13, 498–507. [Google Scholar] [CrossRef]
- Zong, W.; Li, Q.; Zhang, X.; Han, X. Deformation of giant unilamellar vesicles under osmotic stress. Colloids Surf. B Biointerfaces 2018, 172, 459–463. [Google Scholar] [CrossRef]
- Meleard, P.; Gerbeaud, C.; Pott, T.; Fernandez-Puente, L.; Bivas, I.; Mitov, M.D.; Dufourcq, J.; Bothorel, P. Bending elasticities of model membranes: Influences of temperature and sterol content. Biophys. J. 1997, 72, 2616–2629. [Google Scholar] [CrossRef] [Green Version]
- Hannesschlaeger, C.; Horner, A.; Pohl, P. Intrinsic membrane permeability to small molecules. Chem. Rev. 2019, 119, 5922–5953. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Tabas, I. Role of cholesterol and lipid organization in disease. Nature 2005, 438, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Akimov, S.A.; Volynsky, P.E.; Galimzyanov, T.R.; Kuzmin, P.I.; Pavlov, K.V.; Batishchev, O.V. Pore formation in lipid membrane I: Continuous reversible trajectory from intact bilayer through hydrophobic defect to transversal pore. Sci. Rep. 2017, 7, 12152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, E.; Rawicz, W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 1990, 64, 2094. [Google Scholar] [CrossRef] [PubMed]
- Wachlmayr, J.; Hannesschlaeger, C.; Speletz, A.; Barta, T.; Eckerstorfer, A.; Siligan, C.; Horner, A. Scattering versus fluorescence self-quenching: More than a question of faith for the quantification of water flux in large unilamellar vesicles? Nanoscale Adv. 2022, 4, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Groulx, N.; Boudreault, F.; Orlov, S.N.; Grygorczyk, R. Membrane reserves and hypotonic cell swelling. J. Membr. Biol. 2006, 214, 43–56. [Google Scholar] [CrossRef]
- Berger, N.; Sachse, A.; Bender, J.; Schubert, R.; Brandl, M. Filter extrusion of liposomes using different devices: Comparison of liposome size, encapsulation efficiency, and process characteristics. Int. J. Pharmaceut. 2001, 223, 55–68. [Google Scholar] [CrossRef]
- Hannesschläger, C.; Barta, T.; Siligan, C.; Horner, A. Quantification of water flux in vesicular systems. Sci. Rep. 2018, 8, 8516. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Murase, O.; Sugishita, K.I.; Yoneyama, S.; Akada, K.Y.; Ueha, M.; Nakamura, A.; Kobayashi, S. Optical characterization of liposomes by right angle light scattering and turbidity measurement. Biochim. Biophys. Acta 2000, 1467, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Horner, A.; Zocher, F.; Preiner, J.; Ollinger, N.; Siligan, C.; Akimov, S.A.; Pohl, P. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci. Adv. 2015, 1, e1400083. [Google Scholar] [CrossRef] [Green Version]
- Vitkova, V.; Antonova, K.; Popkirov, G.; Mitov, M.D.; Ermakov, Y.A.; Bivas, I. Electrical resistivity of the liquid phase of vesicular suspensions prepared by different methods. J. Phys. Conf. Ser. 2010, 253, 012059. [Google Scholar] [CrossRef]
- Moghal, M.; Rahman, M.; Shuma, M.L.; Islam, M.; Yamazaki, M. A Single GUV method for revealing the action of cell-penetrating peptides in biomembranes. In Cell Penetrating Peptides; Humana: New York, NY, USA, 2022; pp. 167–179. [Google Scholar] [CrossRef]
- Sherman, F. Getting started with yeast. Methods Enzymol. 2004, 350, 3–41. [Google Scholar] [CrossRef]
- Sokolov, S.S.; Vorobeva, M.A.; Smirnova, A.I.; Smirnova, E.A.; Trushina, N.I.; Galkina, K.V.; Severin, F.F.; Knorre, D.A. LAM genes contribute to environmental stress tolerance but sensibilize yeast cells to azoles. Front. Microbiol. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Véronneau, S.; Dow, S.; Lucau-Danila, A.; Anderson, K.; André, B.; et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.S.; Galkina, K.V.; Litvinova, E.A.; Knorre, D.A.; Severin, F.F. The role of LAM genes in the pheromone-induced cell death of S. cerevisiae yeast. Biochemistry 2020, 85, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Saparov, S.M.; Antonenko, Y.N. The size of the unstirred layer as a function of the solute diffusion coefficient. Biophys. J. 1998, 75, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Claessens, M.M.A.E.; Leermakers, F.A.M.; Hoekstra, F.A.; Cohen Stuart, M.A. Osmotic shrinkage and reswelling of giant vesicles composed of DOPG and cholesterols. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 890–895. [Google Scholar] [CrossRef] [Green Version]
- De Nadal, E.; Posas, F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022, 22, foac013. [Google Scholar] [CrossRef]
- Brewster, J.L.; Gustin, M.C. Hog1: 20 years of discovery and impact. Sci. Signal. 2014, 7, re7. [Google Scholar] [CrossRef]
- Hohmann, S. An integrated view on a eukaryotic osmoregulation system. Curr. Genetics 2015, 61, 373–382. [Google Scholar] [CrossRef]
- Sarthy, A.V.; Schopp, C.; Idler, K.B. Cloning and sequence determination of the gene encoding sorbitol dehydrogenase from Saccharomyces cerevisiae. Gene 1994, 140, 121–126. [Google Scholar] [CrossRef]
- Kristan, K.; Rižner, T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. Mol. Biol. 2012, 129, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, M.M.F. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant. 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell. Physiol. 2009, 219, 251–258. [Google Scholar] [CrossRef]
- Zelazny, A.M.; Shaish, A.; Pick, U. Plasma Membrane Sterols Are Essential for Sensing Osmotic Changes in the Halotolerant Alga Dunaliella. Plant Physiol. 1995, 109, 1395–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatibzadeh, N.; Gupta, S.; Farrell, B.; Brownell, W.E.; Anvari, B. Effects of cholesterol on nano-mechanical properties of the living cell plasma membrane. Soft Matter 2012, 8, 8350–8360. [Google Scholar] [CrossRef]

| Strain | Genotype | Parental Strain | Reference |
|---|---|---|---|
| W303 | MATa ade2–101 his3–11 trp1–1 ura3–52 can1–100 leu2–3 | W303 | Laboratory of A. Hyman |
| UPC2-1 | MATa UPC2–1 ura3–1 his3–11,- 15 leu2–3,-112 trp1–1 | W303 | [23] |
| UPC2-1Δlam2 | MATa UPC2–1 ura3–1 his3–11,- 15 leu2–3,-112 trp1–1Δlam2::TRP1 | W303 | This study |
| Δlam1Δlam2Δlam3Δlam4 | MATa ade2–101 his3–11 trp1–1 ura3–52 can1–100 leu2–3 MATa ade2–101 his3–11 trp1–1 ura3– 52 can1–100 leu2–3Δlam3::kanMX4Δlam2::TRP1 | W303 | [23] |
| BY4742 | MATalpha his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | BY4742 | Deletion collection [24] |
| Δhog1 | MATalpha his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 hog1::kanMX4 | BY4742 | Deletion collection [24] |
| Δerg4Δlam2 | MATalpha his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 erg4::kanMX4Δlam2::HIS3 | BY4742 | [25] |
| GUV Composition | 15 s from the Start of Application of the Hyperosmotic Solution | 30 s from the Start of Application of the Hyperosmotic Solution |
|---|---|---|
| DOPC | 12 ± 5% | 3 ± 2% |
| 70 mol.% DOPC + 30 mol./% Ergosterol | 5 ± 3% | 2 ± 1% |
| 70 mol.% DOPC + 30 mol./% Cholesterol | 4 ± 2% | 3 ± 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, S.S.; Popova, M.M.; Pohl, P.; Horner, A.; Akimov, S.A.; Kireeva, N.A.; Knorre, D.A.; Batishchev, O.V.; Severin, F.F. Structural Role of Plasma Membrane Sterols in Osmotic Stress Tolerance of Yeast Saccharomyces cerevisiae. Membranes 2022, 12, 1278. https://doi.org/10.3390/membranes12121278
Sokolov SS, Popova MM, Pohl P, Horner A, Akimov SA, Kireeva NA, Knorre DA, Batishchev OV, Severin FF. Structural Role of Plasma Membrane Sterols in Osmotic Stress Tolerance of Yeast Saccharomyces cerevisiae. Membranes. 2022; 12(12):1278. https://doi.org/10.3390/membranes12121278
Chicago/Turabian StyleSokolov, Svyatoslav S., Marina M. Popova, Peter Pohl, Andreas Horner, Sergey A. Akimov, Natalia A. Kireeva, Dmitry A. Knorre, Oleg V. Batishchev, and Fedor F. Severin. 2022. "Structural Role of Plasma Membrane Sterols in Osmotic Stress Tolerance of Yeast Saccharomyces cerevisiae" Membranes 12, no. 12: 1278. https://doi.org/10.3390/membranes12121278





