Influence of Dialysis Membranes on Clinical Outcomes: From History to Innovation
Abstract
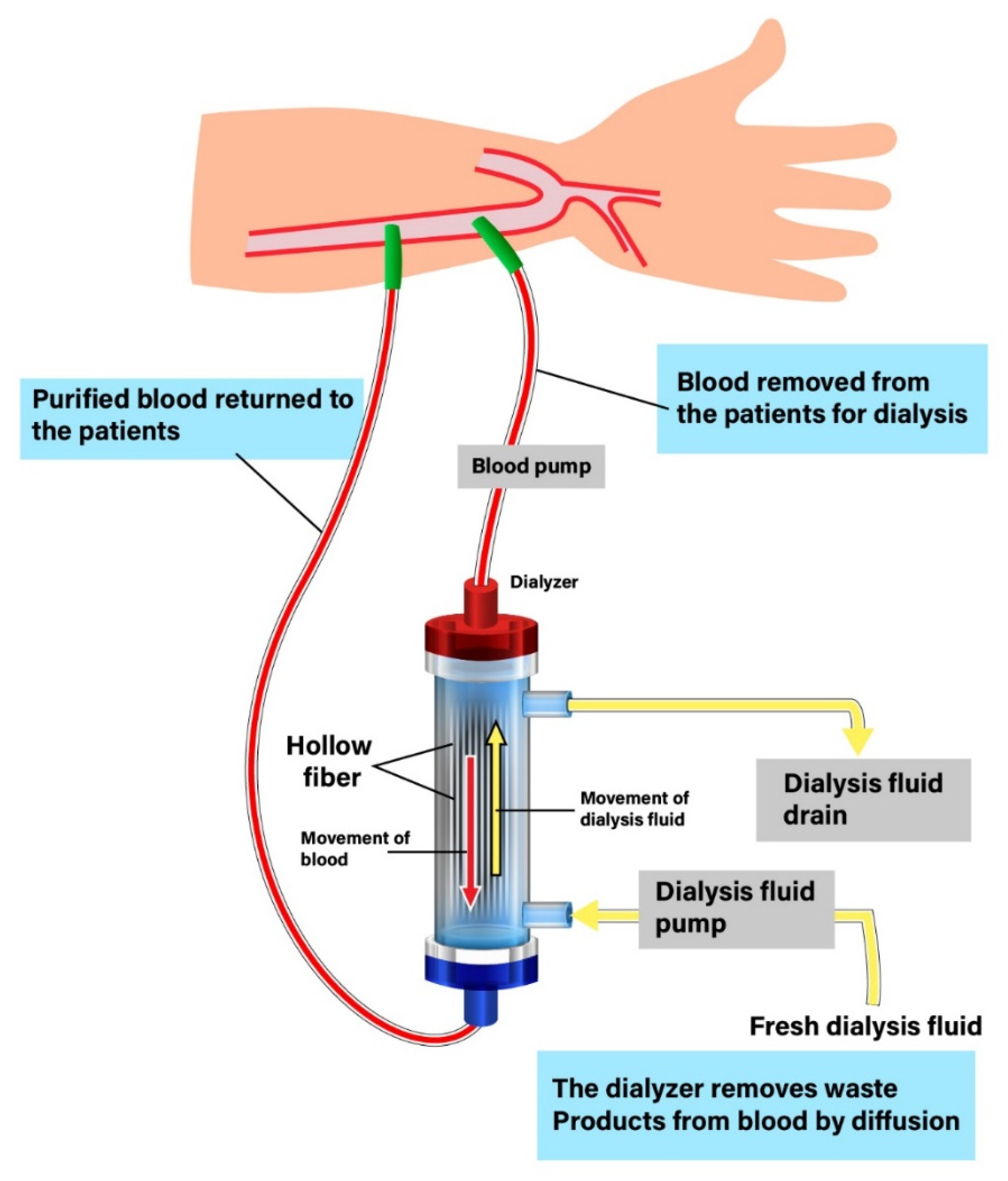
:1. Introduction and the History of Dialysis Membranes
2. Classification of Dialysis Membranes
3. Cellulose-Based Membranes
4. Synthetic Polymer Membranes
5. Morphological Difference in Cellulose-Based membranes and Synthetic Polymer Membranes
6. Innovation of Membranes
6.1. Medium Cutoff Membranes
6.2. Graphene Oxide Membranes
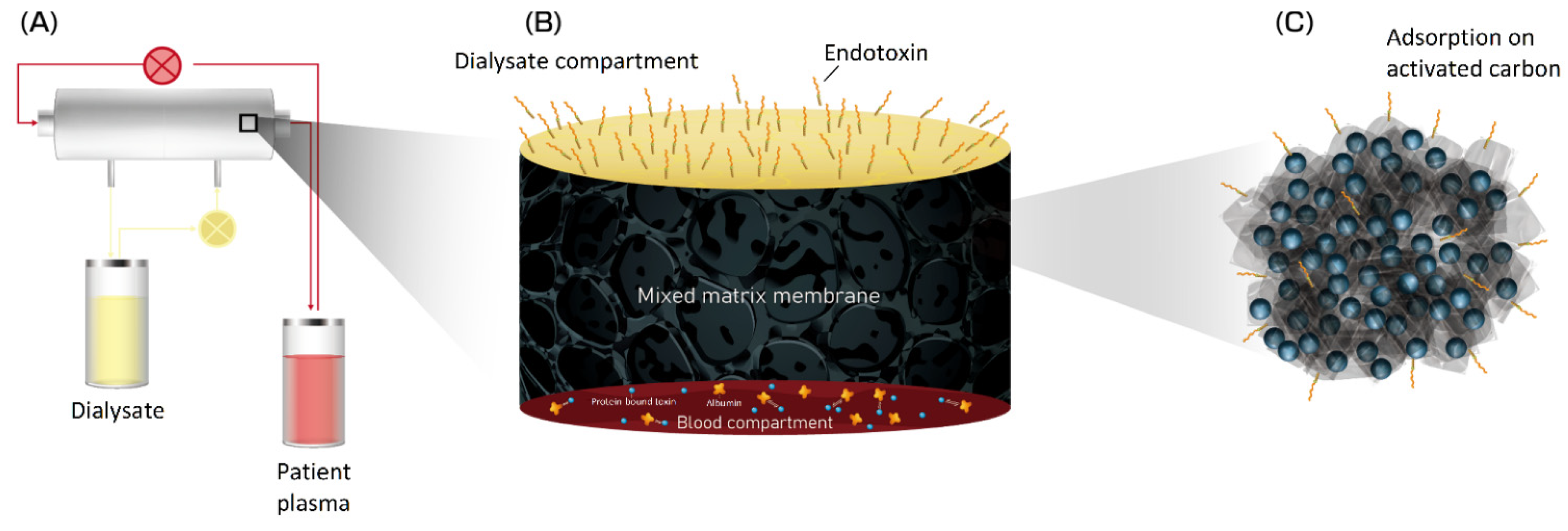
6.3. Mixed Matrix Membranes
6.4. Bioartificial Kidneys
6.5. Vitamin E-Modified Membranes
6.6. Lipoic Acid-Modified Membranes
6.7. Neutrophil Elastase Inhibitor-Modified Membranes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blowey, D.L.; Alon, U.S. Dialysis principles for primary health-care providers. Clin. Pediatr. Phila 2005, 44, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kolff, W.J.; Berk, H.T.; Welle, N.M.; van der Ley, A.J.; van Dijk, E.C.; van Noordwijk, J. The artificial kidney: A dialyser with a great area. 1944. J. Am. Soc. Nephrol. 1997, 8, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Kolff, W.J.; Watschinger, B.; Vertes, V. Results in patients treated with the coil kidney (disposable dialyzing unit). J. Am. Med. Assoc. 1956, 161, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Kiil, F. Development of a parallel-flow artificial kidney in plastics. Acta Chir. Scand. Suppl. 1960, 253, 142–150. [Google Scholar]
- Ronco, C.; Clark, W.R. Haemodialysis membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef]
- Twardowski, Z.J. History of hemodialyzers' designs. Hemodial. Int. 2008, 12, 173–210. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, F.; Yu, X.; Xue, L. Poly(Lactic Acid) Hemodialysis Membranes with Poly(Lactic Acid)-block-Poly(2-Hydroxyethyl Methacrylate) Copolymer As Additive: Preparation, Characterization, and Performance. ACS Appl. Mater. Interfaces 2015, 7, 17748–17755. [Google Scholar] [CrossRef]
- FDA; US Food and Drug Administration. Guidance for Thecontent of Premarket Notifications for Conventional and High Per-Meability Hemodialyzers; FDA: Silver Spring, MD, USA, 1998; pp. 4–7.
- Locatelli, F. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 2003, 348, 1491–1494. [Google Scholar]
- Ward, R.A. Protein-leaking membranes for hemodialysis: A new class of membranes in search of an application? J. Am. Soc. Nephrol. 2005, 16, 2421–2430. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, A.; Daly, C.; Khan, I.; Vale, L.; Campbell, M.; Wallace, S.; Cody, J.; Donaldson, C.; Grant, A. Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease. Cochrane Database Syst. Rev. 2001. [Google Scholar] [CrossRef]
- Clark, W.R.; Hamburger, R.J.; Lysaght, M.J. Effect of membrane composition and structure on solute removal and biocompatibility in hemodialysis. Kidney Int. 1999, 56, 2005–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, R.M.; Hörl, W.H.; Kokot, K.; Heidland, A. Enhanced biocompatibility with a new cellulosic membrane: Cuprophan versus Hemophan. Blood Purif. 1987, 5, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Hoenich, N.A.; Woffindin, C.; Stamp, S.; Roberts, S.J.; Turnbull, J. Synthetically modified cellulose: An alternative to synthetic membranes for use in haemodialysis? Biomaterials 1997, 18, 1299–1303. [Google Scholar] [CrossRef]
- Subramanian, S.; Venkataraman, R.; Kellum, J.A. Influence of dialysis membranes on outcomes in acute renal failure: A meta-analysis. Kidney Int. 2002, 62, 1819–1823. [Google Scholar] [CrossRef] [Green Version]
- Drioli, E.; Giorno, L.; Fontananova, E. (Eds.) Comprehensive Membrane Science and Engineering 2.13—Progress in the Development of Membranes for Kidney-Replacement Therapy; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Janeca, A.; Rodrigues, F.S.C.; Gonçalves, M.C.; Faria, M. Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions. Membranes 2021, 11, 825. [Google Scholar] [CrossRef]
- Ismail, A.F.; Rahman, M.A.; Othman, M.H.; Matsuura, T. (Eds.) Membrane Separation Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ao, X.; Stenken, J.A. Water-soluble cyclodextrin polymers for enhanced relative recovery of hydrophobic analytes during microdialysis sampling. Analyst 2003, 128, 1143–1149. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Cheng, C.H. The effect of contaminated particle sphericity and size on membrane fouling in cross flow ultrafiltration. Environ. Technol 2018, 39, 203–220. [Google Scholar] [CrossRef]
- Dang, H.; Narbaitz, R.; Matsuura, T.; Khulbe, K. A Comparison of Commercial and Experimental Ultrafiltration Membranes via Surface Property Analysis and Fouling Tests. Water Qual. Res. J. Can. 2006, 41, 84–93. [Google Scholar] [CrossRef] [Green Version]
- A Clinical Update on Dialyzer Membranes State-of-the-Art Considerations for Optimal Care in Hemodialysis. Available online: https://www.semanticscholar.org/paper/A-Clinical-Update-on-Dialyzer-Membranes-for-Optimal/17673ccd07a90b45f3d68d178be3031ba2bff55a (accessed on 8 January 2021).
- Huang, L.; Ye, H.; Yu, T.; Zhang, X.; Zhang, Y.; Zhao, L.; Xin, Q.; Wang, S.; Ding, X.; Li, H. Similarly sized protein separation of charge-selective ethylene-vinyl alcohol copolymer membrane by grafting dimethylaminoethyl methacrylate. J. Appl. Polym. Sci. 2018, 135, 46374. [Google Scholar] [CrossRef]
- Kohlová, M.; Amorim, C.G.; Araújo, A.; Santos-Silva, A.; Solich, P.; Montenegro, M. The biocompatibility and bioactivity of hemodialysis membranes: Their impact in end-stage renal disease. J. Artif. Organs 2019, 22, 14–28. [Google Scholar] [CrossRef]
- Olczyk, P.; Małyszczak, A.; Kusztal, M. Dialysis membranes: A 2018 update. Polim. Med. 2018, 48, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastaldello, K.; Melot, C.; Kahn, R.J.; Vanherweghem, J.L.; Vincent, J.L.; Tielemans, C. Comparison of cellulose diacetate and polysulfone membranes in the outcome of acute renal failure. A prospective randomized study. Nephrol. Dial. Transplant. 2000, 15, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikvar, J.B.; Rus, R.R.; Kenda, R.B.; Bren, A.F.; Ponikvar, R.R. Low-flux versus high-flux synthetic dialysis membrane in acute renal failure: Prospective randomized study. Artif. Organs 2001, 25, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Levin, N.; Brendolan, A.; Nalesso, F.; Cruz, D.; Ocampo, C.; Kuang, D.; Bonello, M.; De Cal, M.; Corradi, V.; et al. Flow distribution analysis by helical scanning in polysulfone hemodialyzers: Effects of fiber structure and design on flow patterns and solute clearances. Hemodial. Int. 2006, 10, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Macleod, A.M.; Campbell, M.; Cody, J.D.; Daly, C.; Donaldson, C.; Grant, A.; Khan, I.; Rabindranath, K.S.; Vale, L.; Wallace, S. Cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Su, B.-H.; Shi, Y.; Fu, P.; Tao, Y.; Nie, S.; Zhao, C.-S. Clinical evaluation of polyethersulfone high-flux hemodialysis membrane compared to other membranes. J. Appl. Polym. Sci. 2012, 124, E91–E98. [Google Scholar] [CrossRef]
- Wenten, I.G.; Aryanti, P.T.P.; Khoiruddin, K.; Hakim, A.N.; Himma, N.F. Advances in Polysulfone-Based Membranes for Hemodialysis. J. Membr. Sci. Res. 2016, 2, 78–89. [Google Scholar] [CrossRef]
- Sakurai, A. Dialysis Membranes—Physicochemical Structures and Features, Updates in Hemodialysis; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Bowry, S.K. Dialysis membranes today. Int. J. Artif. Organs 2002, 25, 447–460. [Google Scholar] [CrossRef]
- Reis, T.; Anwar, S.; Neves, F.; Ronco, C. Disruptive technologies for hemodialysis: Medium and high cutoff membranes. Is the future now? J. Bras. Nefrol. 2021. [Google Scholar] [CrossRef]
- Belmouaz, M.; Bauwens, M.; Hauet, T.; Bossard, V.; Jamet, P.; Joly, F.; Chikhi, E.; Joffrion, S.; Gand, E.; Bridoux, F. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: A randomized clinical trial. Nephrol. Dial. Transplant. 2020, 35, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.J.; Park, S.; Islam, M.I.; Song, H.Y.; Lee, E.Y.; Gil, H.W. Long-term effect of medium cut-off dialyzer on middle uremic toxins and cell-free hemoglobin. PLoS ONE 2019, 14, e0220448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zickler, D.; Schindler, R.; Willy, K.; Martus, P.; Pawlak, M.; Storr, M.; Hulko, M.; Boehler, T.; Glomb, M.A.; Liehr, K.; et al. Medium Cut-Off (MCO) Membranes Reduce Inflammation in Chronic Dialysis Patients-A Randomized Controlled Clinical Trial. PLoS ONE 2017, 12, e0169024. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, J.C.; Bunch, A.; Ardila, F.; Zuñiga, E.; Vesga, J.I.; Rivera, A.; Sánchez, R.; Sanabria, R.M. Impact of Medium Cut-Off Dialyzers on Patient-Reported Outcomes: COREXH Registry. Blood Purif. 2021, 50, 110–118. [Google Scholar] [CrossRef]
- Krishnasamy, R.; Hawley, C.M.; Jardine, M.J.; Roberts, M.A.; Cho, Y.; Wong, M.; Heath, A.; Nelson, C.L.; Sen, S.; Mount, P.F.; et al. A tRial Evaluating Mid Cut-Off Value Membrane Clearance of Albumin and Light Chains in HemoDialysis Patients: A Safety Device Study. Blood Purif. 2020, 49, 468–478. [Google Scholar] [CrossRef]
- Weiner, D.E.; Falzon, L.; Skoufos, L.; Bernardo, A.; Beck, W.; Xiao, M.; Tran, H. Efficacy and Safety of Expanded Hemodialysis with the Theranova 400 Dialyzer: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 1310–1319. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, M.J.; Jeon, J.; Lee, J.E.; Huh, W.; Choi, B.S.; Park, C.W.; Chin, H.J.; Kang, C.L.; Kim, D.K.; et al. Cardiovascular Risk Comparison between Expanded Hemodialysis Using Theranova and Online Hemodiafiltration (CARTOON): A Multicenter Randomized Controlled Trial. Sci. Rep. 2021, 11, 10807. [Google Scholar] [CrossRef]
- Basile, C.; Davenport, A.; Mitra, S.; Pal, A.; Stamatialis, D.; Chrysochou, C.; Kirmizis, D. Frontiers in hemodialysis: Innovations and technological advances. Artif. Organs 2021, 45, 175–182. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Graphene oxide-doping improves the biocompatibility and separation performance of polyethersulfone hollow fiber membranes for bioartificial kidney application. J. Colloid. Interface Sci. 2018, 514, 750–759. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Jang, D.; Idrobo, J.C.; Boutilier, M.S.H.; Wang, L.; Kong, J.; Karnik, R. Nanoporous Atomically Thin Graphene Membranes for Desalting and Dialysis Applications. Adv. Mater. 2017, 29, 1700277. [Google Scholar] [CrossRef]
- Geremia, I.; Pavlenko, D.; Maksymow, K.; Rüth, M.; Lemke, H.D.; Stamatialis, D. Ex vivo evaluation of the blood compatibility of mixed matrix haemodialysis membranes. Acta Biomater. 2020, 111, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, D.; van Geffen, E.; van Steenbergen, M.J.; Glorieux, G.; Vanholder, R.; Gerritsen, K.G.; Stamatialis, D. New low-flux mixed matrix membranes that offer superior removal of protein-bound toxins from human plasma. Sci. Rep. 2016, 6, 34429. [Google Scholar] [CrossRef] [PubMed]
- Geremia, I.; Bansal, R.; Stamatialis, D. In vitro assessment of mixed matrix hemodialysis membrane for achieving endotoxin-free dialysate combined with high removal of uremic toxins from human plasma. Acta Biomater. 2019, 90, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; De Napoli, I.E.; Fedecostante, M.; Schophuizen, C.M.; Chevtchik, N.V.; Wilmer, M.J.; van Asbeck, A.H.; Croes, H.J.; Pertijs, J.C.; Wetzels, J.F.; et al. Human proximal tubule epithelial cells cultured on hollow fibers: Living membranes that actively transport organic cations. Sci. Rep. 2015, 5, 16702. [Google Scholar] [CrossRef] [PubMed]
- Chevtchik, N.V.; Mihajlovic, M.; Fedecostante, M.; Bolhuis-Versteeg, L.; Sastre Toraño, J.; Masereeuw, R.; Stamatialis, D. A bioartificial kidney device with polarized secretion of immune modulators. J. Tissue Eng. Regen. Med. 2018, 12, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Tumlin, J.; Wali, R.; Williams, W.; Murray, P.; Tolwani, A.J.; Vinnikova, A.K.; Szerlip, H.M.; Ye, J.; Paganini, E.P.; Dworkin, L.; et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J. Am. Soc. Nephrol. 2008, 19, 1034–1040. [Google Scholar] [CrossRef]
- Sepe, V.; Gregorini, M.; Rampino, T.; Esposito, P.; Coppo, R.; Galli, F.; Libetta, C. Vitamin e-loaded membrane dialyzers reduce hemodialysis inflammaging. BMC Nephrol. 2019, 20, 412. [Google Scholar] [CrossRef]
- Locatelli, F.; Andrulli, S.; Viganò, S.M.; Concetti, M.; Urbini, S.; Giacchino, F.; Broccoli, R.; Aucella, F.; Cossu, M.; Conti, P.; et al. Evaluation of the Impact of a New Synthetic Vitamin E-Bonded Membrane on the Hypo-Responsiveness to the Erythropoietin Therapy in Hemodialysis Patients: A Multicenter Study. Blood Purif. 2017, 43, 338–345. [Google Scholar] [CrossRef]
- Djuric, P.; Suvakov, S.; Simic, T.; Markovic, D.; Jerotic, D.; Jankovic, A.; Bulatovic, A.; Tosic Dragovic, J.; Damjanovic, T.; Marinkovic, J.; et al. Vitamin E-Bonded Membranes Do Not Influence Markers of Oxidative Stress in Hemodialysis Patients with Homozygous Glutathione Transferase M1 Gene Deletion. Toxins 2020, 12, 352. [Google Scholar] [CrossRef]
- D'Arrigo, G.; Baggetta, R.; Tripepi, G.; Galli, F.; Bolignano, D. Effects of Vitamin E-Coated versus Conventional Membranes in Chronic Hemodialysis Patients: A Systematic Review and Meta-Analysis. Blood Purif. 2017, 43, 101–122. [Google Scholar] [CrossRef]
- Tarng, D.C.; Huang, T.P.; Liu, T.Y.; Chen, H.W.; Sung, Y.J.; Wei, Y.H. Effect of vitamin E-bonded membrane on the 8-hydroxy 2′-deoxyguanosine level in leukocyte DNA of hemodialysis patients. Kidney Int. 2000, 58, 790–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.S.; Hassan, Z.A.; Chalmin, F.; Vido, S.; Berrada, M.; Verhelst, D.; Donnadieu, P.; Moranne, O.; Esnault, V.L. Vitamin E-Coated and Heparin-Coated Dialyzer Membranes for Heparin-Free Hemodialysis: A Multicenter, Randomized, Crossover Trial. Am. J. Kidney Dis. 2016, 68, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Mahlicli, F.Y.; Altinkaya, S.A. Immobilization of alpha lipoic acid onto polysulfone membranes to suppress hemodialysis induced oxidative stress. J. Membr. Sci. 2014, 449, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Kohlová, M.; Rocha, S.; Gomes Amorim, C.; de Nova Araújo, A.; Santos-Silva, A.; Solich, P.; Branco da Silva Montenegro, M.C. Doping Polysulfone Membrane with Alpha-Tocopherol and Alpha-Lipoic Acid for Suppressing Oxidative Stress Induced by Hemodialysis Treatment. Macromol. Biosci. 2020, 20, e2000046. [Google Scholar] [CrossRef] [PubMed]
- Kohira, S.; Oka, N.; Inoue, N.; Itatani, K.; Hanayama, N.; Kitamura, T.; Fujii, M.; Takeda, A.; Oshima, H.; Tojo, K.; et al. Effect of the neutrophil elastase inhibitor sivelestat on perioperative inflammatory response after pediatric heart surgery with cardiopulmonary bypass: A prospective randomized study. Artif. Organs 2013, 37, 1027–1033. [Google Scholar] [CrossRef]
- Stockley, R.; De Soyza, A.; Gunawardena, K.; Perrett, J.; Forsman-Semb, K.; Entwistle, N.; Snell, N. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir. Med. 2013, 107, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Grano, V.; Tasco, G.; Casadio, R.; Diano, N.; Portaccio, M.; Rossi, S.; Bencivenga, U.; Compiani, M.; De Maio, A.; Mita, D.G. Reduction of active elastase concentration by means of immobilized inhibitors: A novel therapeutic approach. Biotechnol. Prog. 2004, 20, 968–974. [Google Scholar] [CrossRef]

| MWRO(Da) | MWCO(Da) | Water Permeability (mL/h/mmHg/m2) | Sieving Coefficient | Pore Radius (nm) | ||
|---|---|---|---|---|---|---|
| β2m | Albumin | |||||
| Low-flux | 2000–3000 | 15,000 | 10–20 | - | <0.010 | 2.0–3.0 |
| High-flux | 4000–10,000 | 15,000–16,000 | 200–400 | 0.7–0.8 | <0.010 | 3.5–5.5 |
| Medium cut-off | 10,000–13,000 | 60,000–100,000 | 600–850 | 1 | 0.008 | 5.0 |
| High cut-off | 15,000–20,000 | 200,000–300,000 | 1100 | 1 | 0.200 | 8.0–12.0 |
| Type of Membrane | Designation | Advantages | Disadvantage |
|---|---|---|---|
| Unmodified cellulose | Cuprophan® |
|
|
| Modified cellulose | Cellulose acetate (CA) |
|
|
| Hemophan® |
|
| |
| Synthetically modified cellulose (SMC) |
|
| |
| Synthetic | Polycarbonate (PC) |
|
|
| Polysulfone (PSU) |
|
| |
| Polyamide (PAM) |
|
| |
| Polyethersulfone (PES) |
|
| |
| Polyacrylonitrile (PAN) |
|
| |
| Polymethyl methacrylate (PMMA) |
|
| |
| Polyester polymer alloy (PEPA) |
|
| |
| Ethylene-vinyl alcohol copolymer (EVAL) |
|
|
| Type of Membrane | Designation | MWCO (kDa) | Pore Size | Ref. |
|---|---|---|---|---|
| Unmodified cellulose | Cuprophan® | 10 kDa | 1.72 nm | [16] |
| Modified cellulose | Cellulose acetate (CA) | 17.6–18.6 kDa | 84 nm | [17] |
| Hemophan® | 2 kDa | 22 nm | [18] | |
| Synthetic | Polycarbonate (PC) | 20 kDa | 10–600nm | [19] |
| Polysulfone (PSU) | 60,000 kDa | 5–11 nm | [20] | |
| Polyamide (PAM) | 1000 kDa | - | [20] | |
| Polyethersulfone (PES) | 1–500 kDa | 5.12–6.33 nm | [21] | |
| Polyacrylonitrile (PAN) | 100 kDa | 5.4 nm | [21] | |
| Polymethyl methacrylate (PMMA) | 55–130 kDa | 3.5–5.5 nm | [22] | |
| Polyester polymer alloy (PEPA) | 55–130 kDa | 50–500 nm | [22] | |
| Ethylene-vinyl alcohol copolymer (EVAL) | 500 kDa | 0.1–0.2 mm | [23] |
| Cellulose-Based (Cuprophan) | Synthetic (Polysulfone) |
|---|---|
 |  |
| Natural polymer | Synthetic polymer |
| Homogeneous | Asymmetry |
| Hydrophilic (hydrogel) | Hydrophobic structure |
| Low hydraulic permeability | High hydraulic permeability |
| Low sieving properties | High sieving properties |
| Prevalent use in hemodialysis | Exclusively used for hemofiltration |
| Membrane Type | MWCO (kDa) | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Medium cutoff membranes | 60–100 |
|
| RCTs: [35,37,39,40,41] Observational study: [36,38] |
| Graphene oxide membranes | 1–3 |
|
| In vitro study: [43,44] |
| Mixed-matrix membranes | 47 |
|
| In vitro study: [45,46,47] |
| Bioartificial kidneys | 10–30 |
|
| RCTs: [50] In vitro study: [48,49] |
| Vitamin E-modified membranes | 10–300 |
|
| RCTs: [51,52,53,55,56] Meta-analysis: [54] |
| Lipoic acid-modified membranes | 10 |
|
| In vitro study: [57,58] |
| Neutrophil elastase inhibitor modified membranes | 2 |
|
| In vitro study: [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-A.; Ou, S.-M.; Lin, C.-C. Influence of Dialysis Membranes on Clinical Outcomes: From History to Innovation. Membranes 2022, 12, 152. https://doi.org/10.3390/membranes12020152
Chen Y-A, Ou S-M, Lin C-C. Influence of Dialysis Membranes on Clinical Outcomes: From History to Innovation. Membranes. 2022; 12(2):152. https://doi.org/10.3390/membranes12020152
Chicago/Turabian StyleChen, Yee-An, Shuo-Ming Ou, and Chih-Ching Lin. 2022. "Influence of Dialysis Membranes on Clinical Outcomes: From History to Innovation" Membranes 12, no. 2: 152. https://doi.org/10.3390/membranes12020152
APA StyleChen, Y.-A., Ou, S.-M., & Lin, C.-C. (2022). Influence of Dialysis Membranes on Clinical Outcomes: From History to Innovation. Membranes, 12(2), 152. https://doi.org/10.3390/membranes12020152







