Development of a Membrane Module Prototype for Oxygen Separation in Industrial Applications
Abstract
:1. Introduction
- Membrane manufacturing and scale up;
- Membrane characterization and testing;
- Membrane module design and joining.
2. Materials and Methods
2.1. Manufacturing and Scale Up
- Asymmetric LSCF tapes obtained by sequentially casting the thin dense membrane layer and, after drying, the porous support directly on top of it (see Figure S2).
- Single-layered LSCF tapes to be used as porous interlayers in the final membrane component, obtained by casting the same slurry used for the support layer.
2.2. Characterization Techniques and Testing
2.3. Module Design and Joining
2.3.1. Mechanical Design of Ceramic Component and Metallic Case
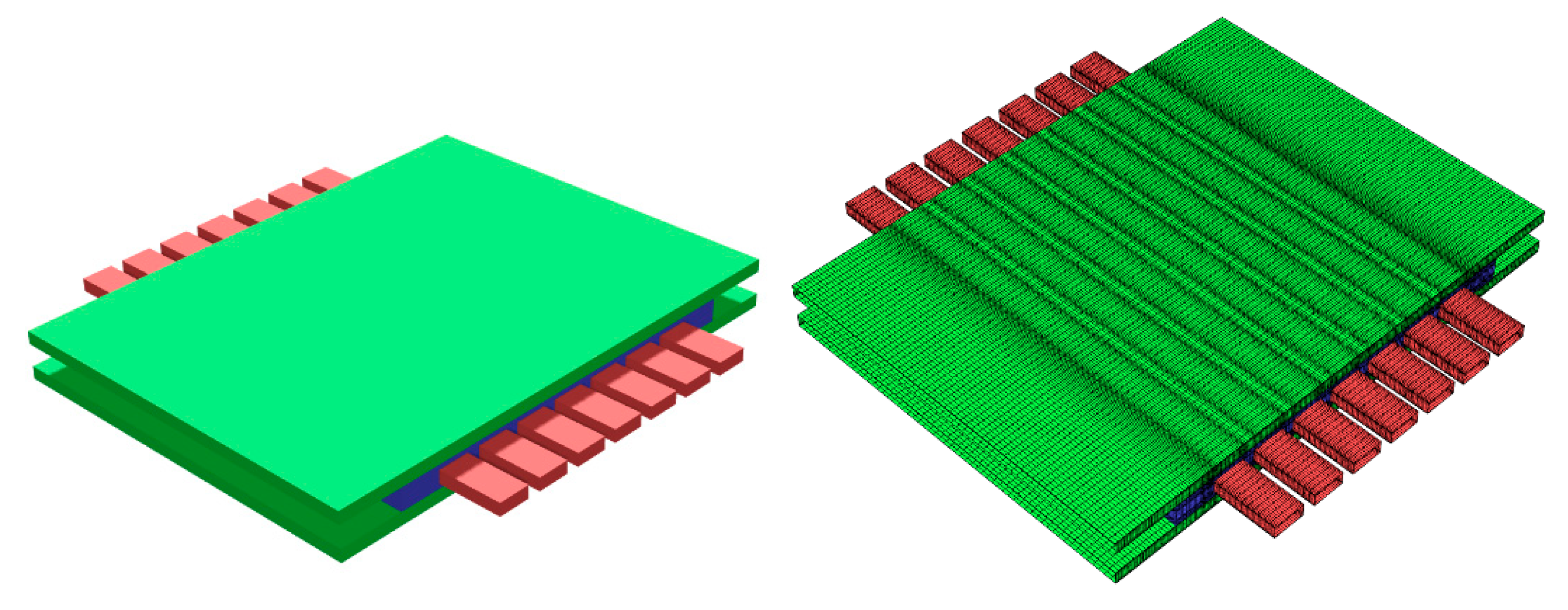
2.3.2. CFD
- Mass balance;
- Momentum balance in RANS formulation;
- Transport equation of turbulence quantities (k-omega SST model);
- Energy balance;
- Transport equation of chemical species.
- Inlet: imposed velocity, temperature and chemical species concentration;
- Outlet: imposed static pressure;
- Walls: no-slip condition and null mass flux through the boundary, imposed temperature;
- Membrane: permeability modeled with sub-routine (UDF);
- Porous support: porous region with isotropic permeability of 10−11 m2.
2.3.3. Joining
3. Results and Discussion
3.1. Manufacturing and Scale-Up
3.1.1. Tape Casting
3.1.2. Half-Components Fabrication
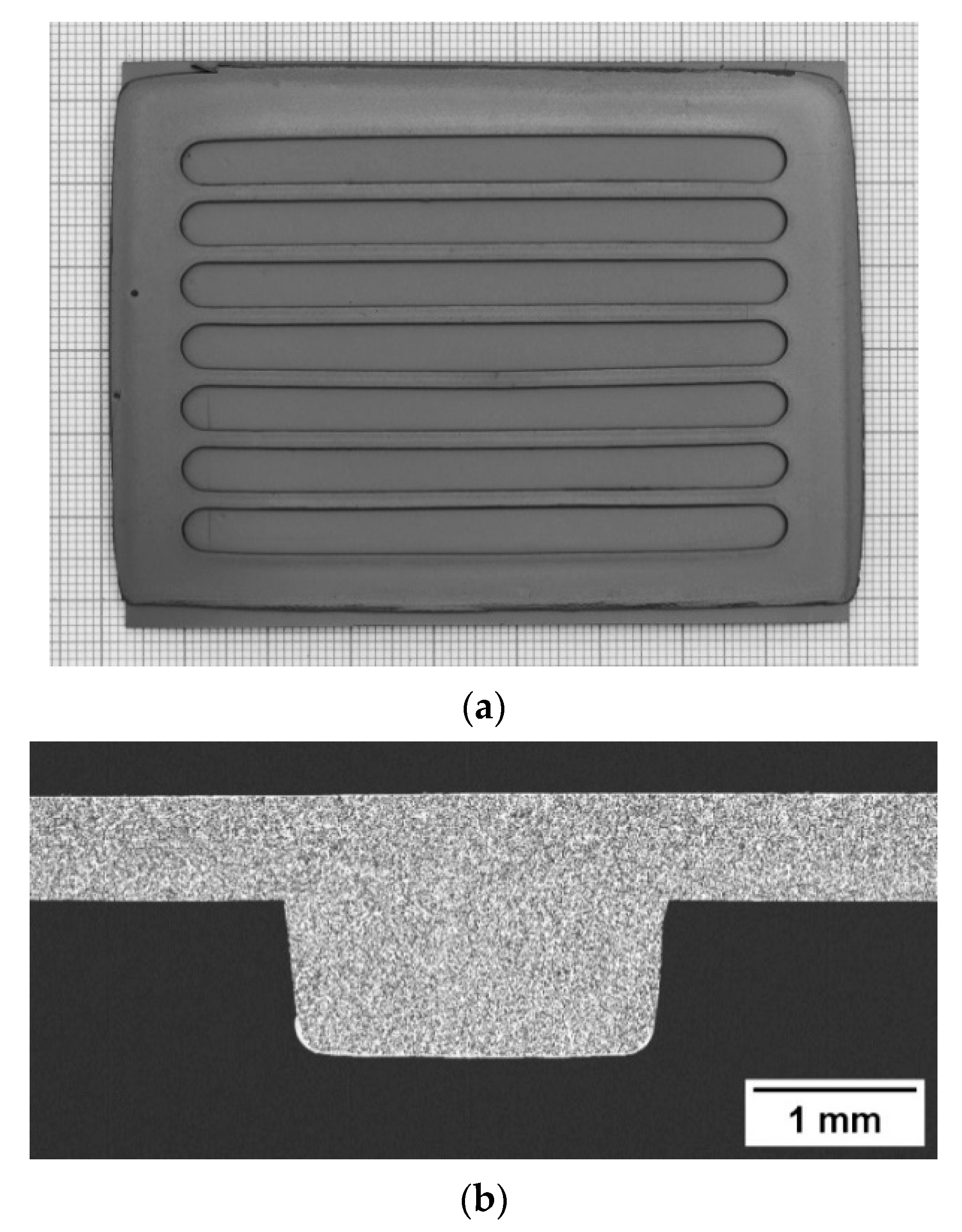
3.1.3. Membrane Component Assembling and Finishing
3.1.4. Microstructure of the Membrane Components
3.2. Characterization
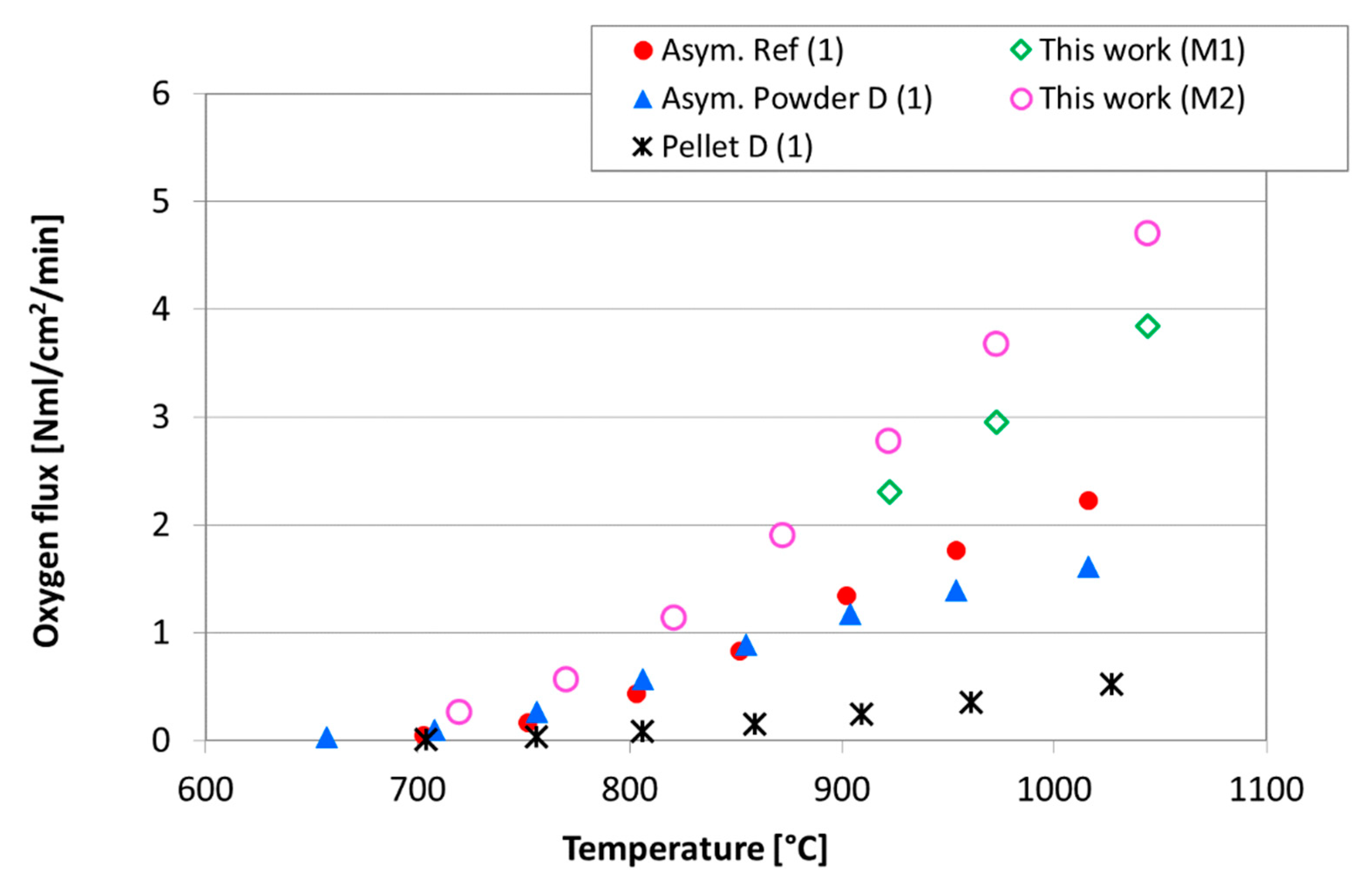
3.2.1. Permeation Tests
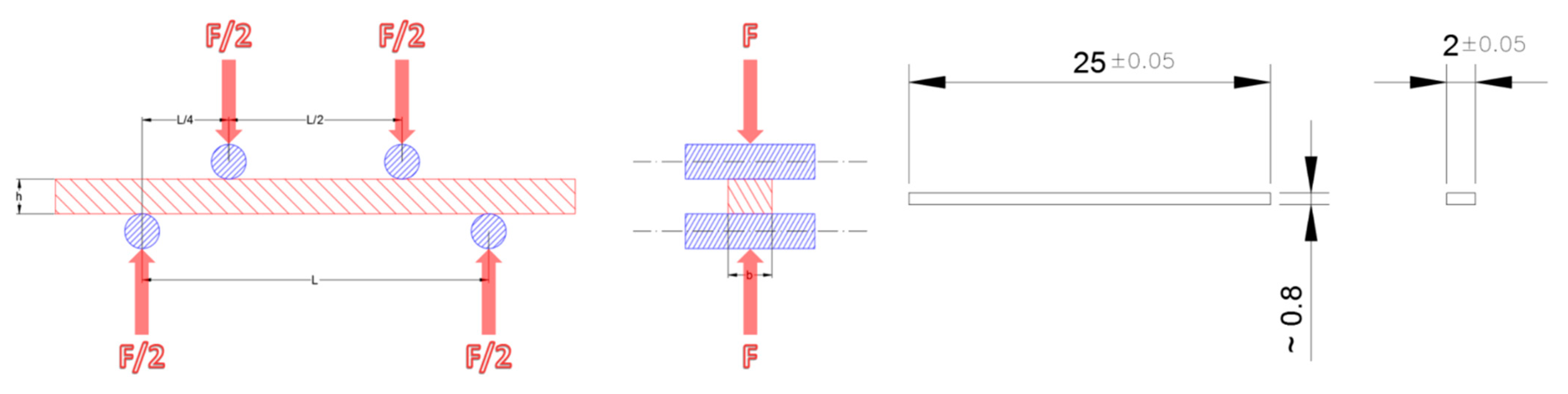
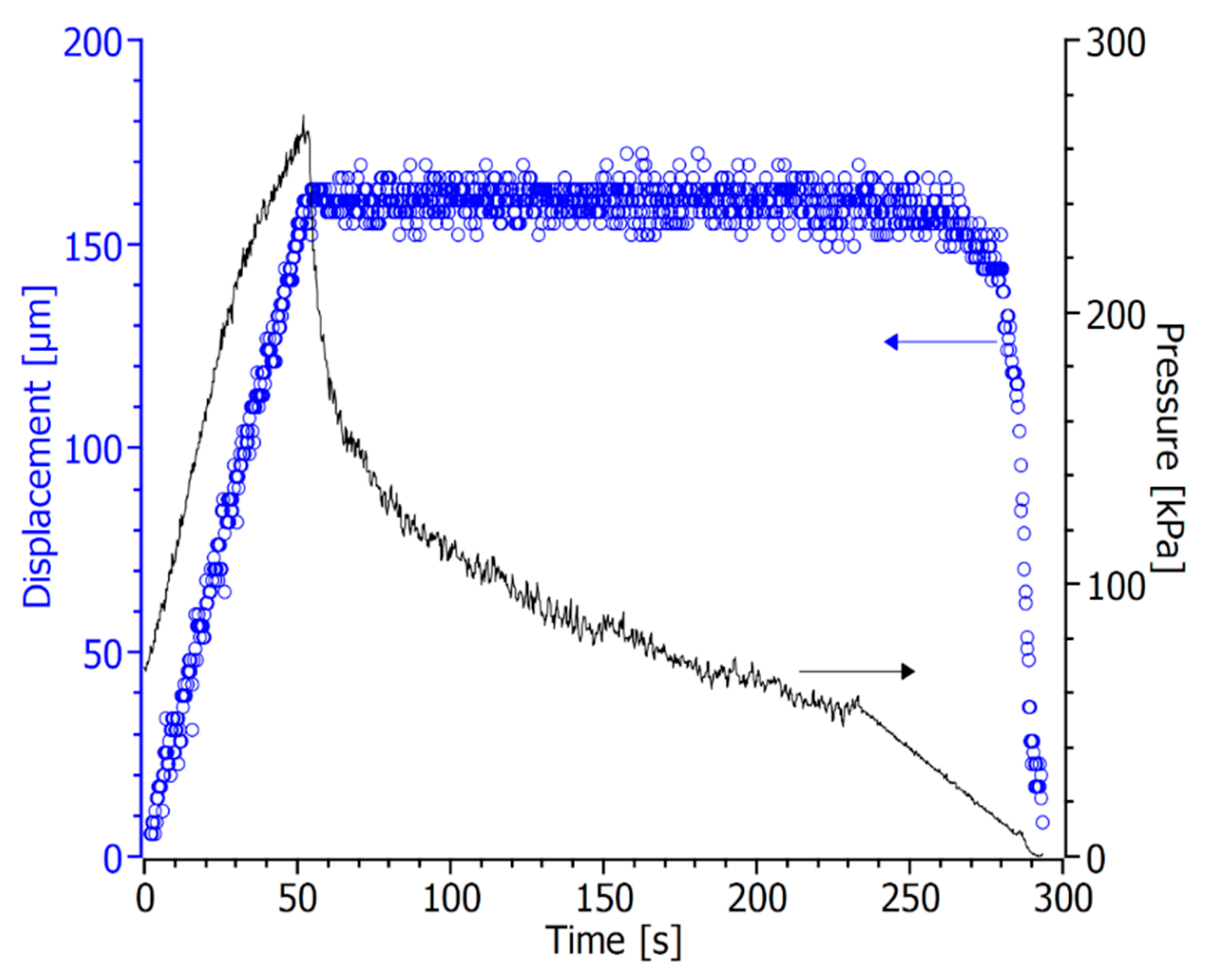
3.2.2. Mechanical Characterization
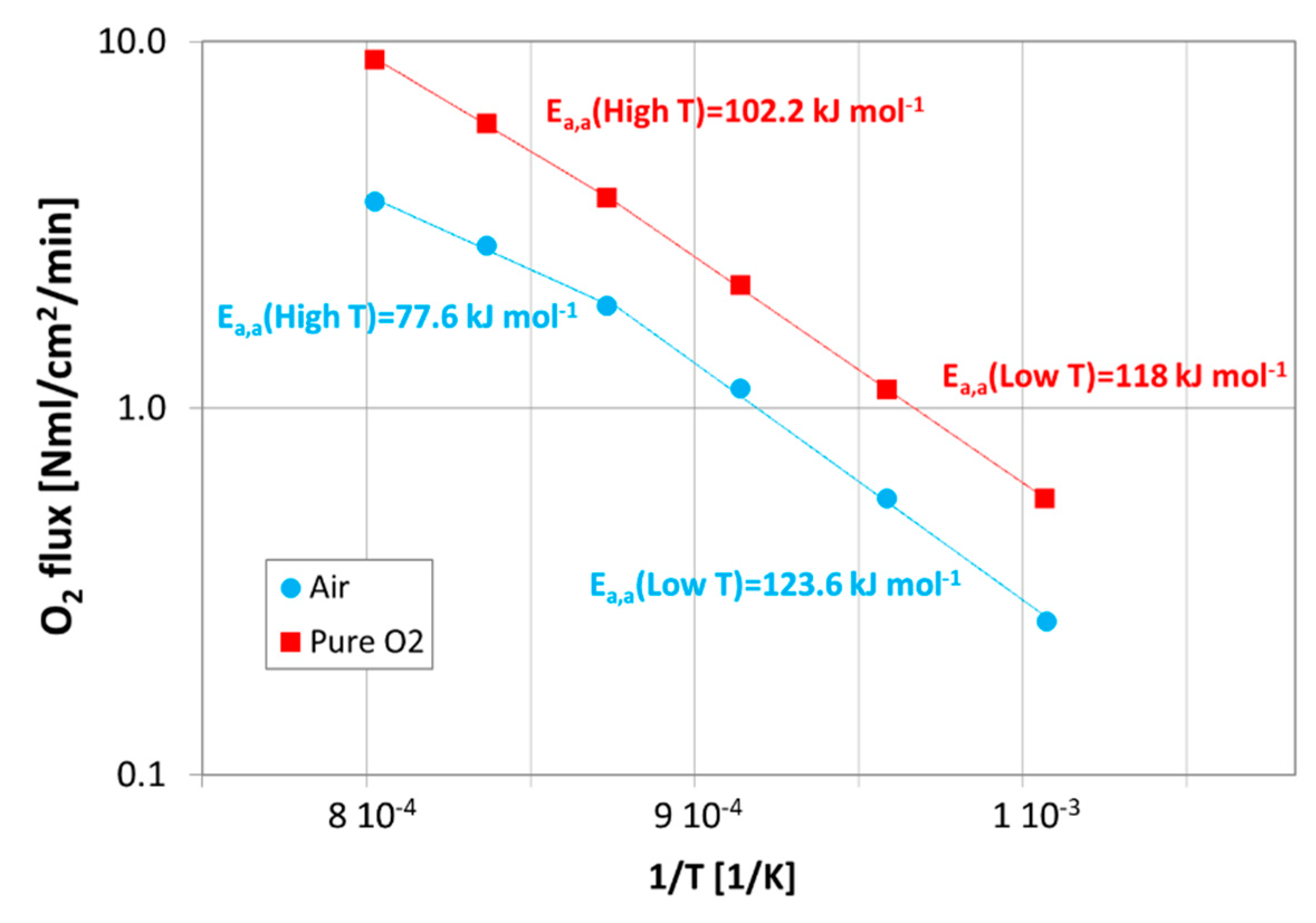
3.2.3. Thermal Expansion Characterization
3.3. Module Design and Joining
3.3.1. Mechanical Design of Ceramic Component and Metallic Case
3.3.2. CFD
- Flux of O2 permeated through the membrane J(O2)M;
- Permeation process efficiency, calculated as the ratio between permeated and feed oxygen mass flow rate;
- Oxygen molar fraction at the outlet of sweep channels;
- Oxygen molar fraction at the outlet of feed channels;
- Total pressure drop of the sweep and feed channels.
3.3.3. Joining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission, Official Website 2012. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/2012_energy_roadmap_2050_en_0.pdf (accessed on 17 October 2021).
- Ministry of Economic Development 2017. Available online: https://www.mise.gov.it/images/stories/documenti/BROCHURE_ENG_SEN.PDF (accessed on 17 October 2021).
- Schulze-Küppers, F.; Drago, F.; Ferravante, L.; Herzog, S.; Baumann, S.; Pinacci, P.; Meulenberg, W.A. Design and fabrication of large-sized planar oxygen transport membrane components for direct integration in oxy-combustion processes. Sep. Purif. Technol. 2019, 220, 89–101. [Google Scholar] [CrossRef]
- Fedeli, P.; Drago, F.; Schulze-Küppers, F.; Baumann, S. Asymmetric LSCF Membranes Utilizing Commercial Powders. Materials 2020, 13, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, H.; Beggel, F.; Habermehl, M.; Persigehl, B.; Kneer, R.; Modigell, M.; Jeschke, P. Oxyfuel coal combustion by efficient integration of oxygen transport membranes. Int. J. Greenh. Gas Control 2011, 5, 7–15. [Google Scholar] [CrossRef]
- Engels, S.; Beggel, F.; Modigell, M.; Stadler, H. Simulation of a membrane unit for oxyfuel power plants under consideration of realistic BSCF membrane properties. J. Membr. Sci. 2010, 359, 93–101. [Google Scholar] [CrossRef]
- Engels, S.; Markus, T.; Modigell, M.; Singheiser, L. Oxygen permeation and stability investigations on MIEC membrane materials under operating conditions for power plant processes. J. Membr. Sci. 2011, 370, 58–69. [Google Scholar] [CrossRef]
- Kneer, R.; Toporov, D.; Förster, M.; Christ, D.; Broeckmann, C.; Pfaff, E.; Zwick, M.; Engels, S.; Modigell, M. OXYCOAL-AC: Towards an integrated coal-fired power plant process with ion transportmembrane-based oxygen supply. Energy Environ. Sci. 2009, 3, 198–207. [Google Scholar] [CrossRef]
- Castillo, R. Thermodynamic analysis of a hard coal oxyfuel power plant with high temperature three-end membrane for air separation. Appl. Energy 2011, 88, 1480–1493. [Google Scholar] [CrossRef]
- Castillo, R. Thermodynamic evaluation of membrane based oxyfuel power plants with 700 °C technology. Energy Procedia 2011, 4, 1026–1034. [Google Scholar] [CrossRef] [Green Version]
- Pfaff, I.; Kather, A. Comparative thermodynamic analysis and integration issues of CCS steam power plants based on oxy-combustion with cryogenic or membrane based air separation. Energy Procedia 2009, 1, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, A.; Chiesa, P.; Drago, F.; Mastropasqua, L. Integration of oxygen transport membranes in glass melting furnaces. Energy Procedia 2018, 148, 599–606. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Drago, F.; Chiesa, P.; Giuffrida, A. Oxygen Transport Membranes for Efficient Glass Melting. Membranes 2020, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Meulenberg, W.; Buchkremer, H. Manufacturing strategies for asymmetric ceramic membranes for efficient separation of oxygen from air. J. Eur. Ceram. Soc. 2013, 33, 1251–1261. [Google Scholar] [CrossRef]
- Hu, Q.; Pirou, S.; Engelbrecht, K.; Kriegel, R.; Pippardt, U.; Kiesel, L.; Sun, Q.; Kiebach, R. Testing of high performance asymmetric tubular BSCF membranes under pressurized operation—A proof-of-concept study on a 7 tube module. J. Membr. Sci. 2022, 644, 120176. [Google Scholar] [CrossRef]
- Tennant, J.; Maley, S.; Bennett, D. Development of Ion Transport Membrane (ITM) Oxygen Technology for Integration in IGCC and Other Advanced Power Generation Systems; FC26-98FT40343; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2013. [Google Scholar]
- Escolástico, S.; Schulze-Küppers, F.; Baumann, S.; Haas-Santo, K.; Dittmeyer, R. Development and Proof of Concept of a Compact Metallic Reactor for MIEC Ceramic Membranes. Membranes 2022, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Fontaine, M.-L.; Li, Z.; Polfus, J.M.; Larring, Y.; Denonville, C.; Nonnet, E.; Stevenson, A.; Henriksen, P.P.; Bredesen, R. Asymmetric tubular CaTi0.6Fe0.15Mn0.25O3- membranes: Membrane architecture and long-term stability. J. Membr. Sci. 2018, 548, 372–379. [Google Scholar] [CrossRef]
- Rosen, L.; Degenstein, N.; Shah, M.; Wilson, J.; Kelly, S.; Peck, J.; Christie, M. Development of oxygen transport membranes for coal-based power generation. Energy Procedia 2011, 4, 750–755. [Google Scholar] [CrossRef] [Green Version]
- Lemes-Rachadel, P.; Garcia, G.S.; Machado, R.A.F.; Hotza, D.; Da Costa, J.C.D. Current developments of mixed conducting membranes on porous substrates. Mater. Res. 2013, 17, 242–249. [Google Scholar] [CrossRef] [Green Version]
- ISO/DIS 18754:2019; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Density and Apparent Porosity. ISO: Geneva, Switzerland, 2019.
- Klande, T.; Ravkina, O.; Feldhoff, A. Effect of microstructure on oxygen permeation of Ba0.5Sr0.5Co0.8Fe0.2O3−δ and SrCo0.8Fe0.2O3−δ membranes. J. Eur. Ceram. Soc. 2010, 33, 1129–1136. [Google Scholar] [CrossRef]
- Schlehuber, D.; Wessel, E.; Singheiser, L.; Markus, T. Long-term operation of a La0.58Sr0.4Co0.2Fe0.8O3−δ-membrane for oxygen separation. J. Membr. Sci. 2010, 351, 16–20. [Google Scholar] [CrossRef]
- ASTM. C1161—; Standard Test Method for Flexural Strength of Advanced Ceramics at Ambient Temperature. ASTM: West Conshohocken, PA, USA, 2018.
- EN, 843-2—; Advanced Technical Ceramics—Mechanical Properties of Monolithic Ceramics at Room Temperature—Part 2: Determination of Young’s Modulus, Shear Modulus and Poisson’s Ratio. UNI: Milan, Italy, 2006.
- ASTM, C1211—; Standard Test Method for Flexural Strength of Advanced Ceramics at Elevated Temperatures. ASTM: West Conshohocken, PA, USA, 2018.
- Brisotto, M.; Cernuschi, F.; Drago, F.; Lenardi, C.; Rosa, P.; Meneghini, C.; Merlini, M.; Rinaldi, C. High temperature stability of Ba0.5Sr0.5Co0.8Fe0.2O3− and La0.6Sr0.4Co1Fe O3− oxygen separation perovskite membranes. J. Eur. Ceram. Soc. 2016, 36, 1679–1690. [Google Scholar] [CrossRef]
- Schulze-Küppers, F.; Baumann, S.; Tietz, F.; Bouwmeester, H.; Meulenberg, W. Towards the fabrication of La0.98−xSrxCo0.2Fe0.8O3−δ perovskite-type oxygen transport membranes. J. Eur. Ceram. Soc. 2014, 34, 3741–3748. [Google Scholar] [CrossRef]
- Wang, B.; Zydorczak, B.; Wu, Z.-T.; Li, K. Stabilities of La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen separation membranes—Effects of kinetic demixing/decomposition and impurity segregation. J. Membr. Sci. 2009, 344, 101–106. [Google Scholar] [CrossRef]
- Zou, Y.; Schulze-Küppers, F.; Malzbender, J. Creep behavior of porous La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen transport membrane supports. Ceram. Int. 2015, 41, 4064–4069. [Google Scholar] [CrossRef]
- Mistler, R.E.; Twiname, E.R. Tape Casting Theory and Practice; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Jabbari, M.; Bulatova, R.; Tok, A.; Bahl, C.; Mitsoulis, E.; Hattel, J. Ceramic tape casting: A review of current methods and trends with emphasis on rheological behaviour and flow analysis. Mater. Sci. Eng. B 2012, 212, 39–61. [Google Scholar] [CrossRef]
- Khoong, L.; Tan, Y.; Lam, Y.C. Overview on fabrication of three-dimensional structures in multi-layer ceramic substrate. J. Eur. Ceram. Soc. 2010, 30, 1973–1987. [Google Scholar] [CrossRef]
- Niehoff, P.; Schulze-Küppers, F.; Baumann, S.; Meulenberg, W.A.; Guillon, O.; Vaßen, R. Fabrication of laboratory-scale planar oxygen separation membrane modules. ACerS Bull. 2015, 94, 28–31. [Google Scholar]
- Schulze-Küppers, F.; Unije, U.V.; Blank, H.; Balaguer, M.; Baumann, S.; Mücke, R.; Meulenberg, W.A. Comparison of freeze-dried and tape-cast support microstructure on high-flux oxygen transport membrane performance. J. Membr. Sci. 2018, 564, 218–226. [Google Scholar] [CrossRef]
- Baumann, S.; Serra, J.; Lobera, M.; Escolástico, S.; Schulze-Küppers, F.; Meulenberg, W.A. Ultrahigh oxygen permeation flux through supported Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. J. Membr. Sci. 2011, 377, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Serra, J.M.; Garcia-Fayos, J.; Baumann, S.; Schulze-Küppers, F.; Meulenberg, W. Oxygen permeation through tape-cast asymmetric all-La0.6Sr0.4Co0.2Fe0.8O3−δ membranes. J. Membr. Sci. 2013, 447, 297–305. [Google Scholar] [CrossRef]
- Lein, H.L.; Wiik, K.; Grande, T. Kinetic demixing and decomposition of oxygen permeable membranes. Solid State Ionics 2006, 177, 1587–1590. [Google Scholar] [CrossRef]
- Wang, B.; Zydorczak, B.; Poulidi, D.; Metcalfe, I.; Li, K. A further investigation of the kinetic demixing/decomposition of La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen separation membranes. J. Membr. Sci. 2011, 369, 526–535. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Yang, N.-T.; Zahid, S.; Kawi, S. Oxygen permeation and stability study of La0.6Sr0.4Co0.8Ga0.2O3−δ (LSCG) hollow fiber membrane with exposure to CO2, CH4 and He. J. Membr. Sci. 2013, 427, 240–249. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G. The catalytic activity of cobalt nanoparticles for low-temperature oxidation of carbon monoxide. Mater. Today Chem. 2019, 14, 100198. [Google Scholar] [CrossRef]
- Tan, X.; Liu, N.; Meng, B.; Sunarso, J.; Zhang, K.; Liu, S. Oxygen permeation behavior of La0.6Sr0.4Co0.8Fe0.2O3 hollow fibre membranes with highly concentrated CO2 exposure. J. Membr. Sci. 2012, 389, 216–222. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Garcia-Fayos, J.; Reina, T.R.; Reed, G.; Persoon, E.S.; Görtz, D.; Schroeder, M.; Millan, M.; Serra, J.M. Thermochemical stability of LaxSr1-xCoyFe1-yO3-δ and NiFe2O4-Ce0.8Tb0.2O2-δ under real conditions for its application in oxygen transport membranes for oxyfuel combustion. J. Membr. Sci. 2018, 562, 26–37. [Google Scholar] [CrossRef]
- Drago, F.; Pinacci, P. Performance and long term stability of asymmetric La0.6Sr0.4Co0.2Fe0.8O3-δ membranes in CO2-rich atmosphere. In Proceedings of the 15th International Conference on Inorganic Membranes, Book of Abstracts, Dresden, Germany, 18–22 June 2018. [Google Scholar]
- Drago, F.; Pinacci, P. Microstructural investigation of La0.6Sr0.4Co0.2Fe0.8O3-δ after long-term operation in the presence of CO2-rich atmosphere. In Proceedings of the Euromembrane 2018, Book of Abstracts, Valencia, Spain, 9–13 July 2018. [Google Scholar]
- Lipinska-Chwalek, M.; Schulze-Küppers, F.; Malzbender, J. Strength and elastic modulus of lanthanum strontium cobalt ferrite membrane materials. Ceram. Int. 2015, 41, 1355–1360. [Google Scholar] [CrossRef]
- Araki, W.; Malzbender, J. Ferroelastic deformation of La0.58Sr0.4Co0.2Fe0.8O3−δ under uniaxial compressive loading. J. Eur. Ceram. Soc. 2013, 33, 805–812. [Google Scholar] [CrossRef]
- Pećanac, G.; Foghmoes, S.; Lipińska-Chwałek, M.; Baumann, S.; Beck, T.; Malzbender, J. Strength degradation and failure limits of dense and porous ceramic membrane materials. J. Eur. Ceram. Soc. 2013, 33, 2689–2698. [Google Scholar] [CrossRef]
- Zou, Y.; Araki, W.; Balaguer, M.; Malzbender, J. Elastic properties of freeze-cast La0.6Sr0.4Co0.2Fe0.8O3–δ. J. Eur. Ceram. Soc. 2016, 36, 1651–1657. [Google Scholar] [CrossRef]
- Islam, M.; Araki, W.; Arai, Y. Mechanical behavior of ferroelastic porous La0.6Sr0.4Co0.2Fe0.8O3− prepared with different pore formers. Ceram. Int. 2017, 43, 14989–14995. [Google Scholar] [CrossRef]
- Islam, N.; Araki, W.; Arai, Y. Mechanical properties of ferroelastic La0.6Sr0.4Co0.2Fe0.8O3−δ with various porosities and pore sizes. J. Mater. Sci. 2019, 54, 5256–5265. [Google Scholar] [CrossRef]
- Huang, B.; Malzbender, J.; Steinbrech, R.; Wessel, E.; Penkalla, H.; Singheiser, L. Mechanical aspects of ferro-elastic behavior and phase composition of La0.58Sr0.4Co0.2Fe0.8O3−δ. J. Membr. Sci. 2010, 349, 183–188. [Google Scholar] [CrossRef]
- Kimura, Y.; Kushi, T.; Hashimoto, S.-I.; Amezawa, K.; Kawada, T. Influences of Temperature and Oxygen Partial Pressure on Mechanical Properties of La0.6Sr0.4Co1−yFeyO3−δ. J. Am. Ceram. Soc. 2012, 95, 2608–2613. [Google Scholar] [CrossRef]
- Atkinson, A.; Ramos, T. Chemically-induced stresses in ceramic oxygen ion-conducting membranes. Solid State Ionics 2000, 129, 259–269. [Google Scholar] [CrossRef]
- Lein, H.; Wiik, K.; Grande, T. Thermal and chemical expansion of mixed conducting La0.5Sr0.5Fe1−xCoxO3−δ materials. Solid State Ionics 2006, 177, 1795–1798. [Google Scholar] [CrossRef]
- Rice, R. Porosity of Ceramics; Marcel Dekker, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Plazaola, A.A.; Labella, A.C.; Liu, Y.; Porras, N.B.; Tanaka, D.A.P.; Annaland, M.V.S.; Gallucci, F. Mixed Ionic-Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review. Processes 2019, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Kiebach, R.; Engelbrecht, K.; Kwok, K.; Molin, S.; Søgaard, M.; Niehoff, P.; Schulze-Küppers, F.; Kriegel, R.; Kluge, J.; Hendriksen, P.V. Joining of ceramic Ba0.5Sr0.5Co0.8Fe0.2O3 membranes for oxygen production to high temperature alloys. J. Membr. Sci. 2016, 506, 11–21. [Google Scholar] [CrossRef]
- Raju, K.; Kim, S.; Song, K.-S.; Yu, J.H.; Yoon, D.-H. Joining of metal-ceramic using reactive air brazing for oxygen transport membrane applications. Mater. Des. 2016, 109, 233–241. [Google Scholar] [CrossRef]

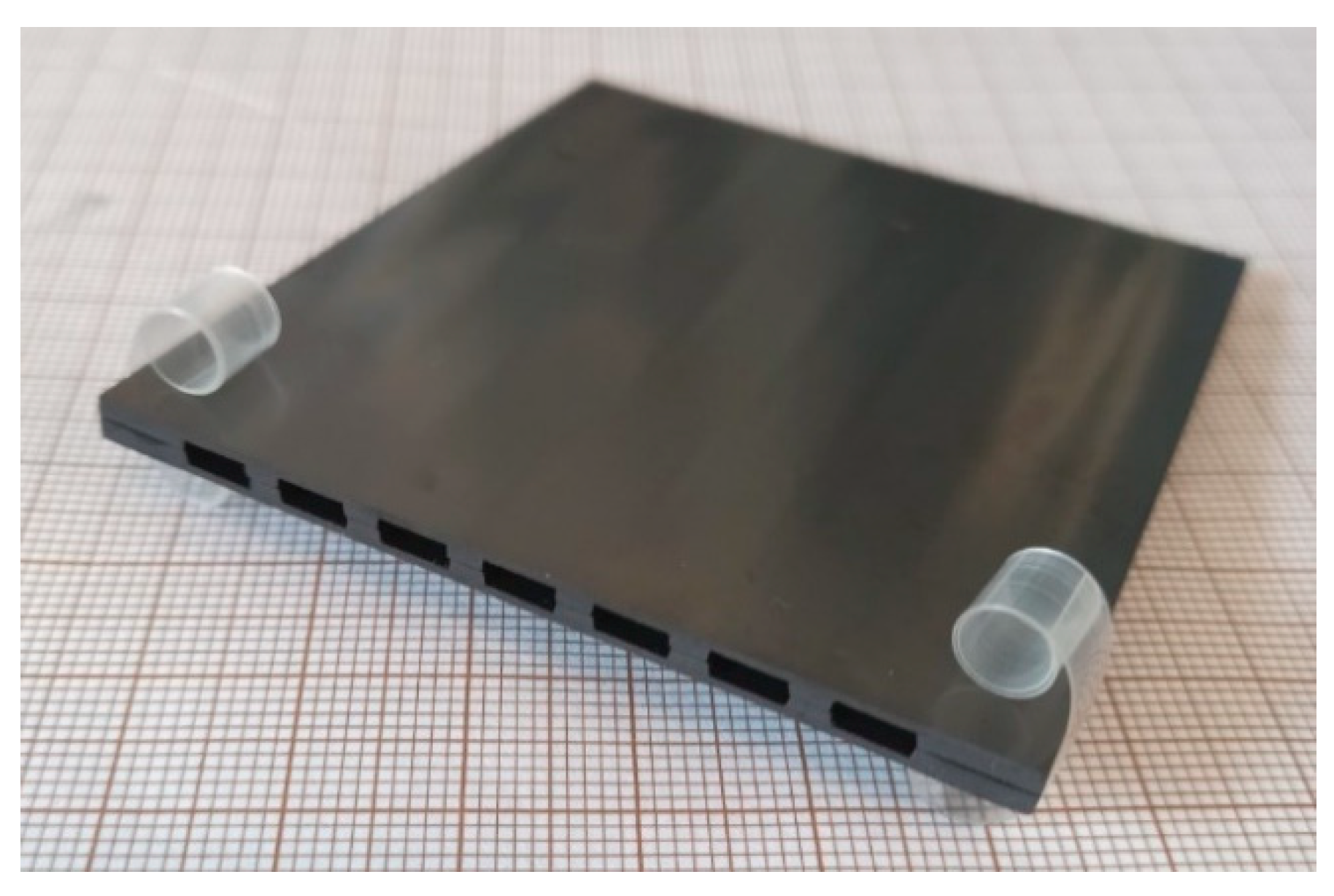

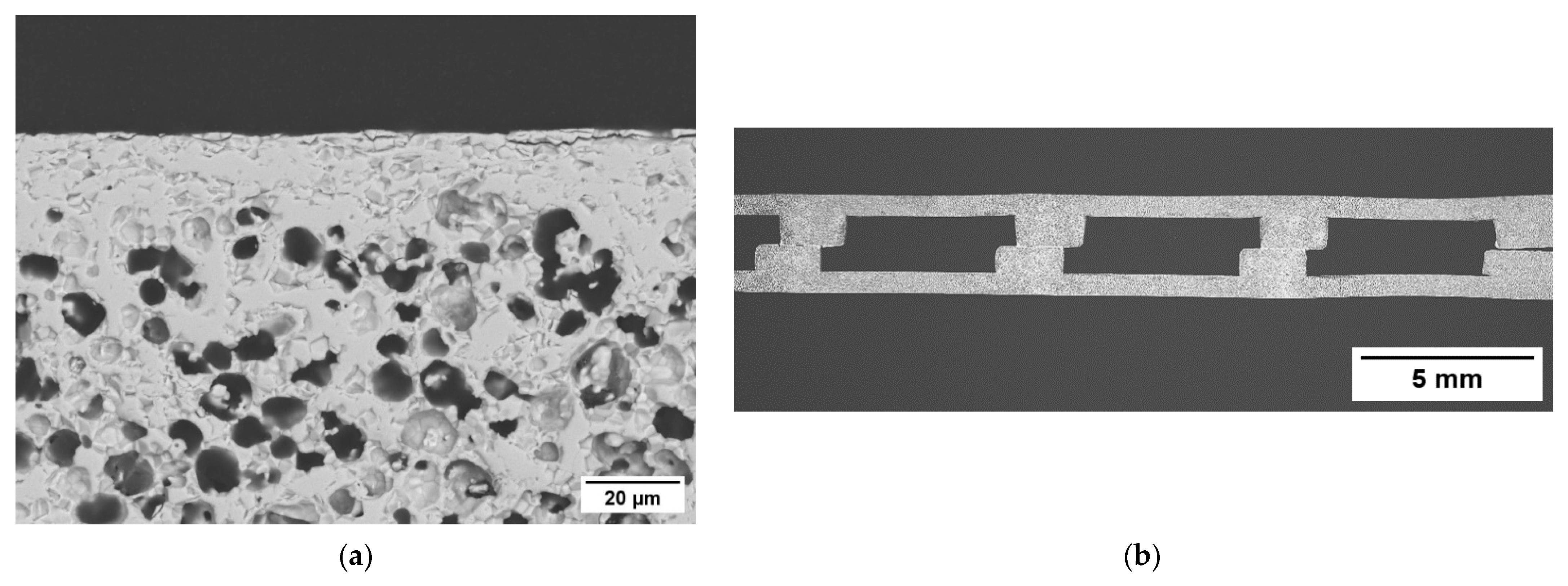
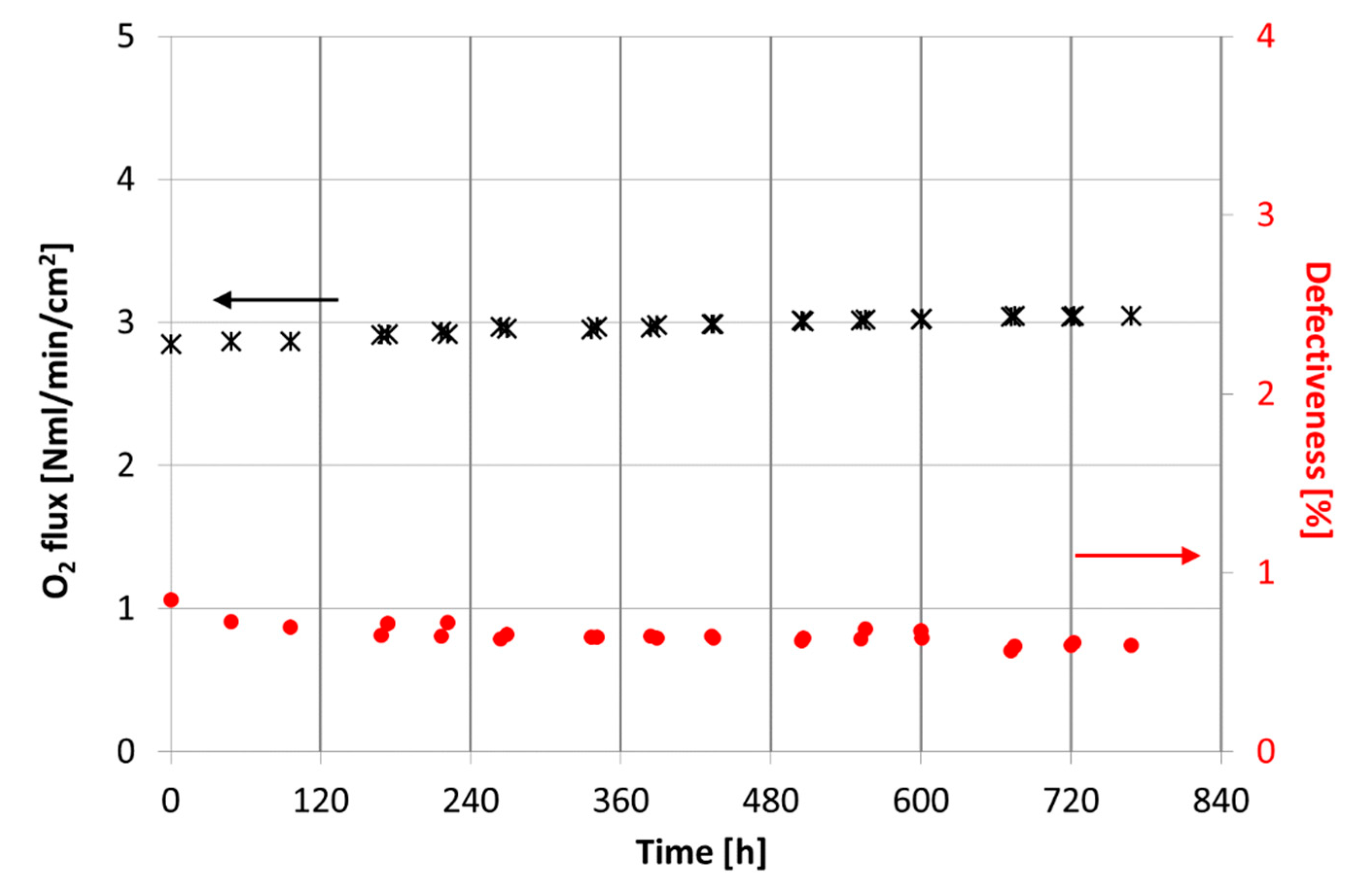
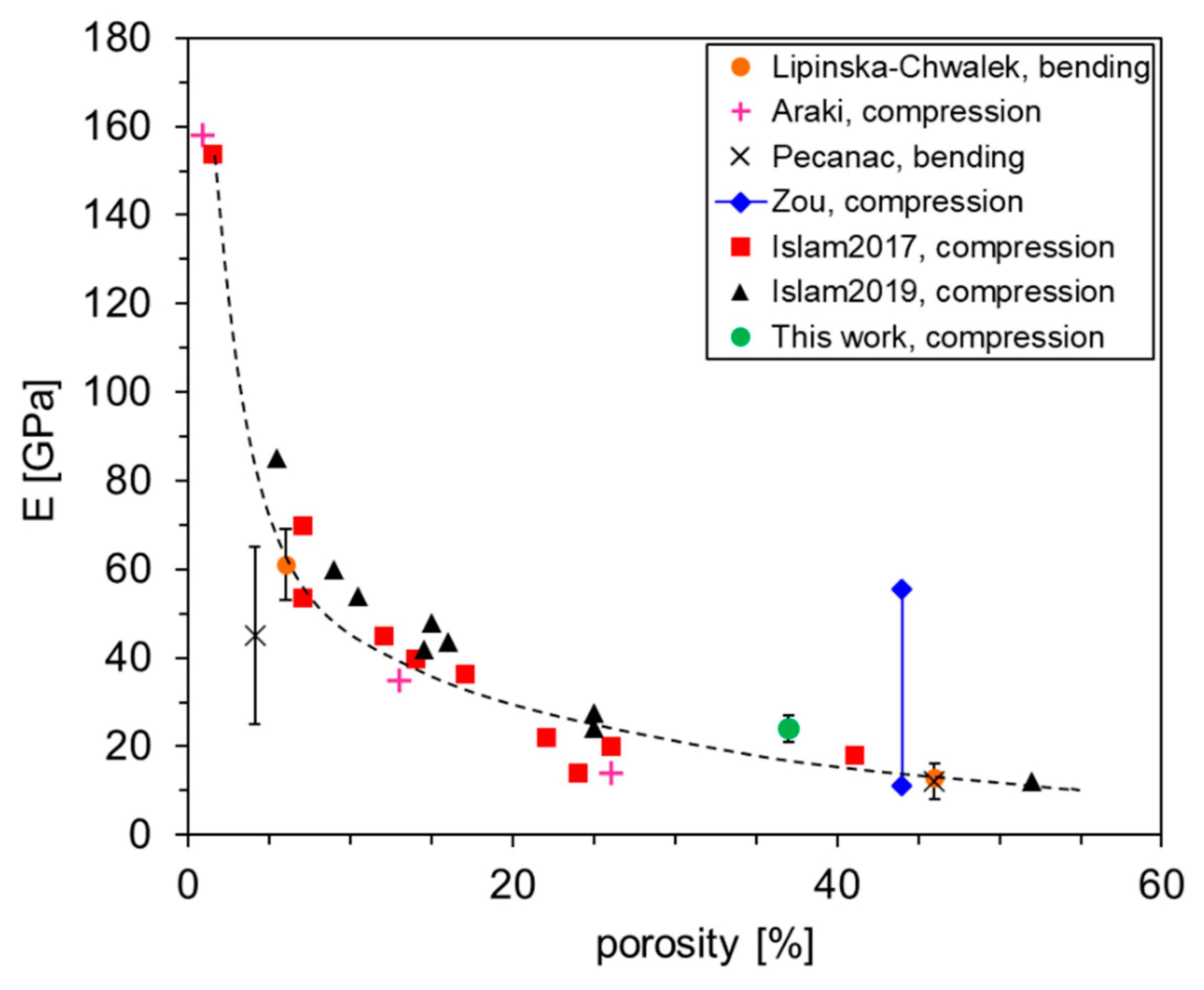
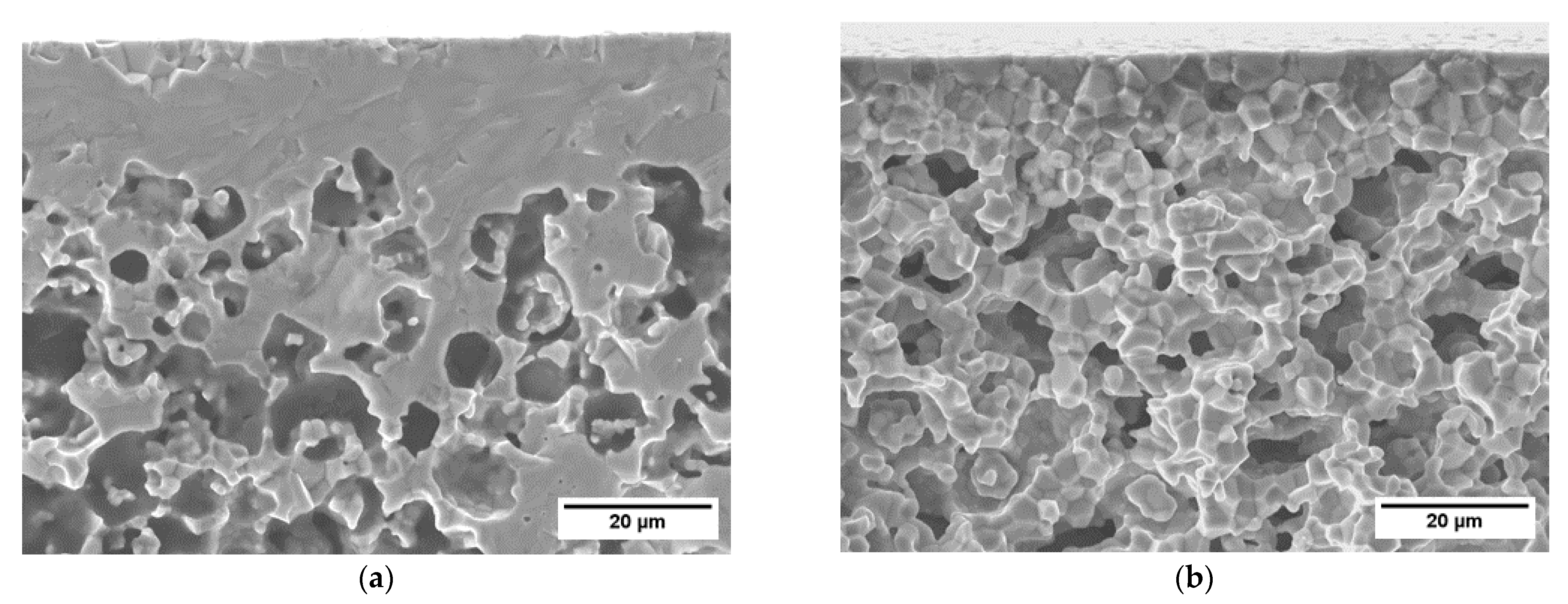
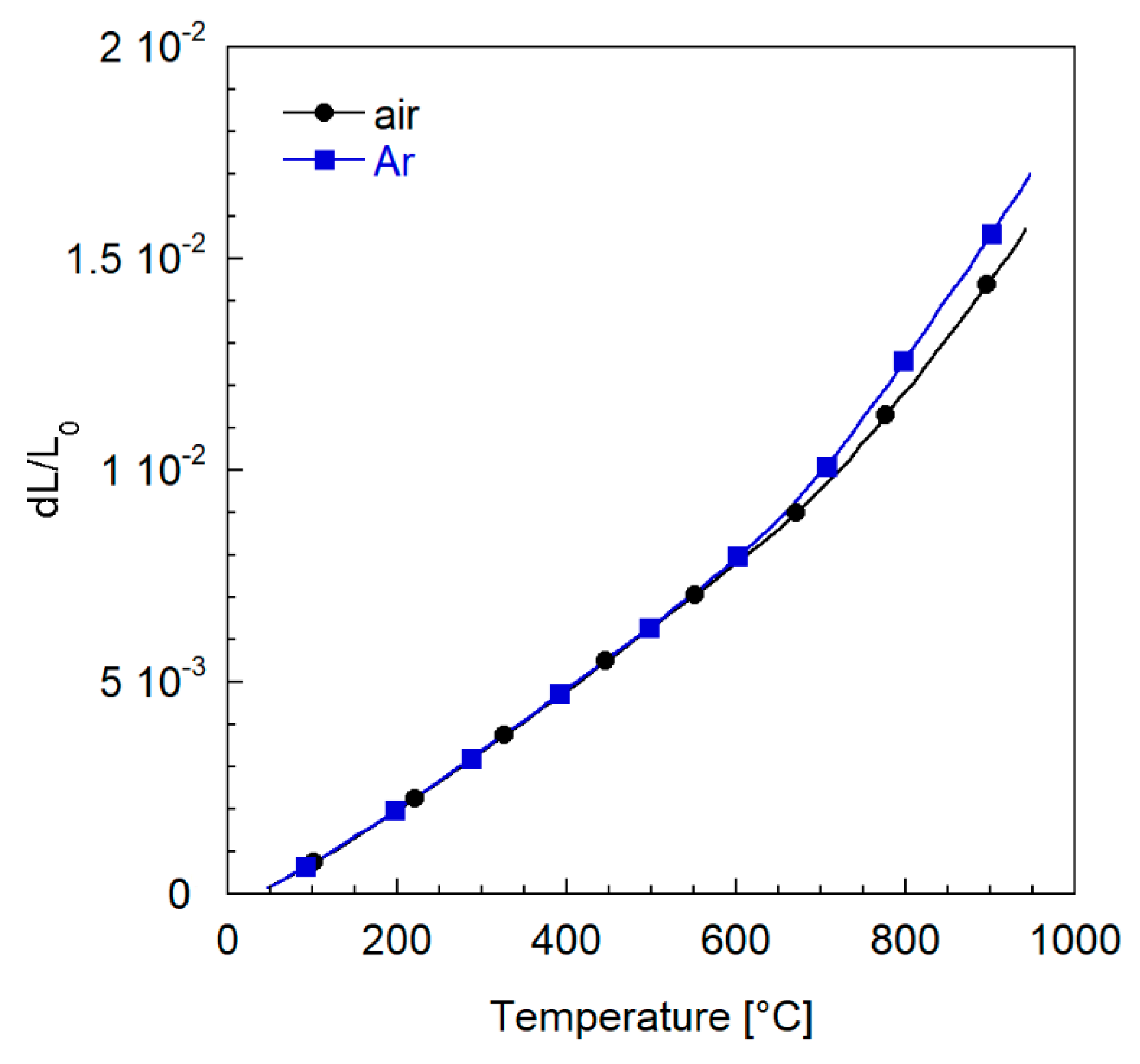
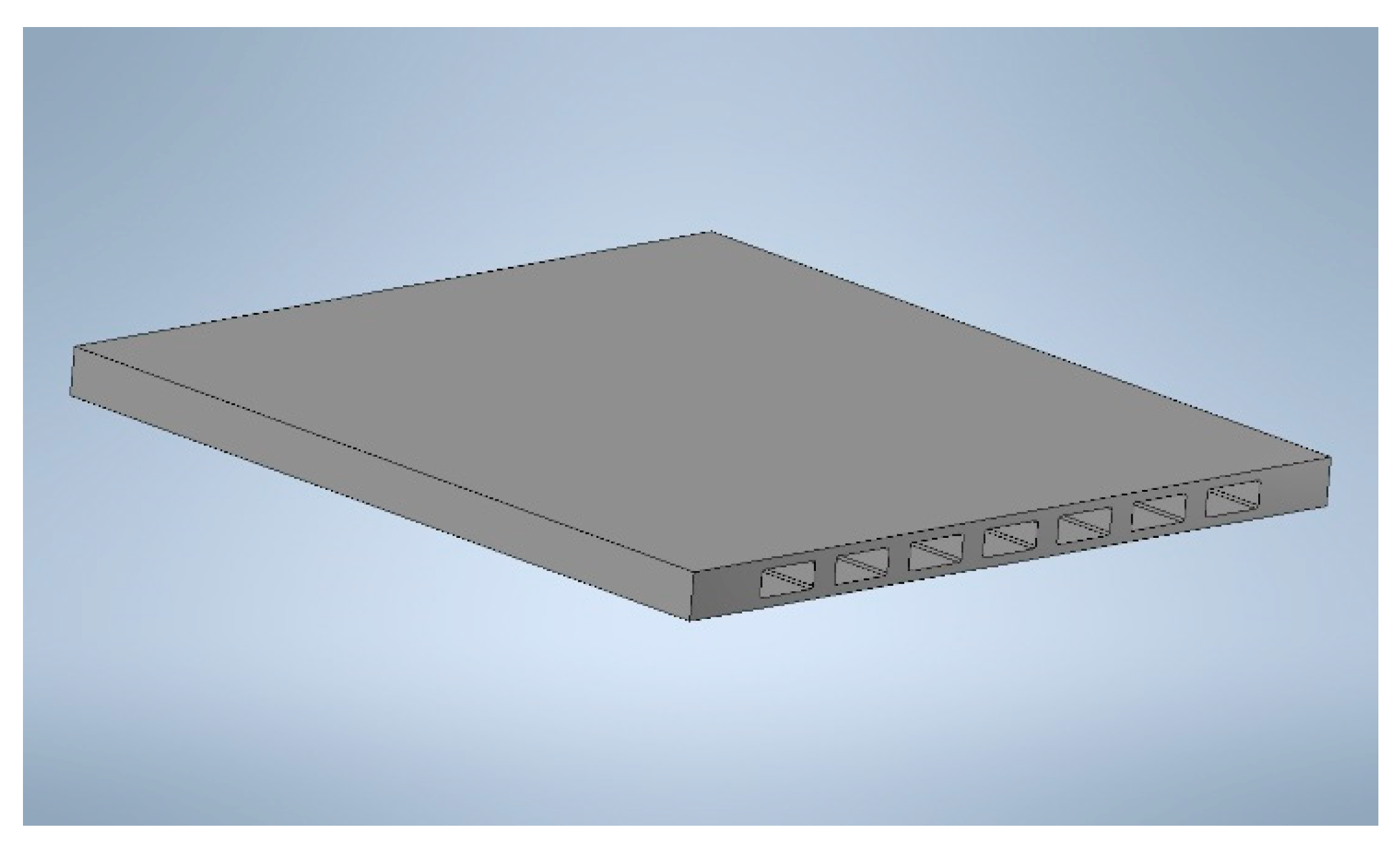
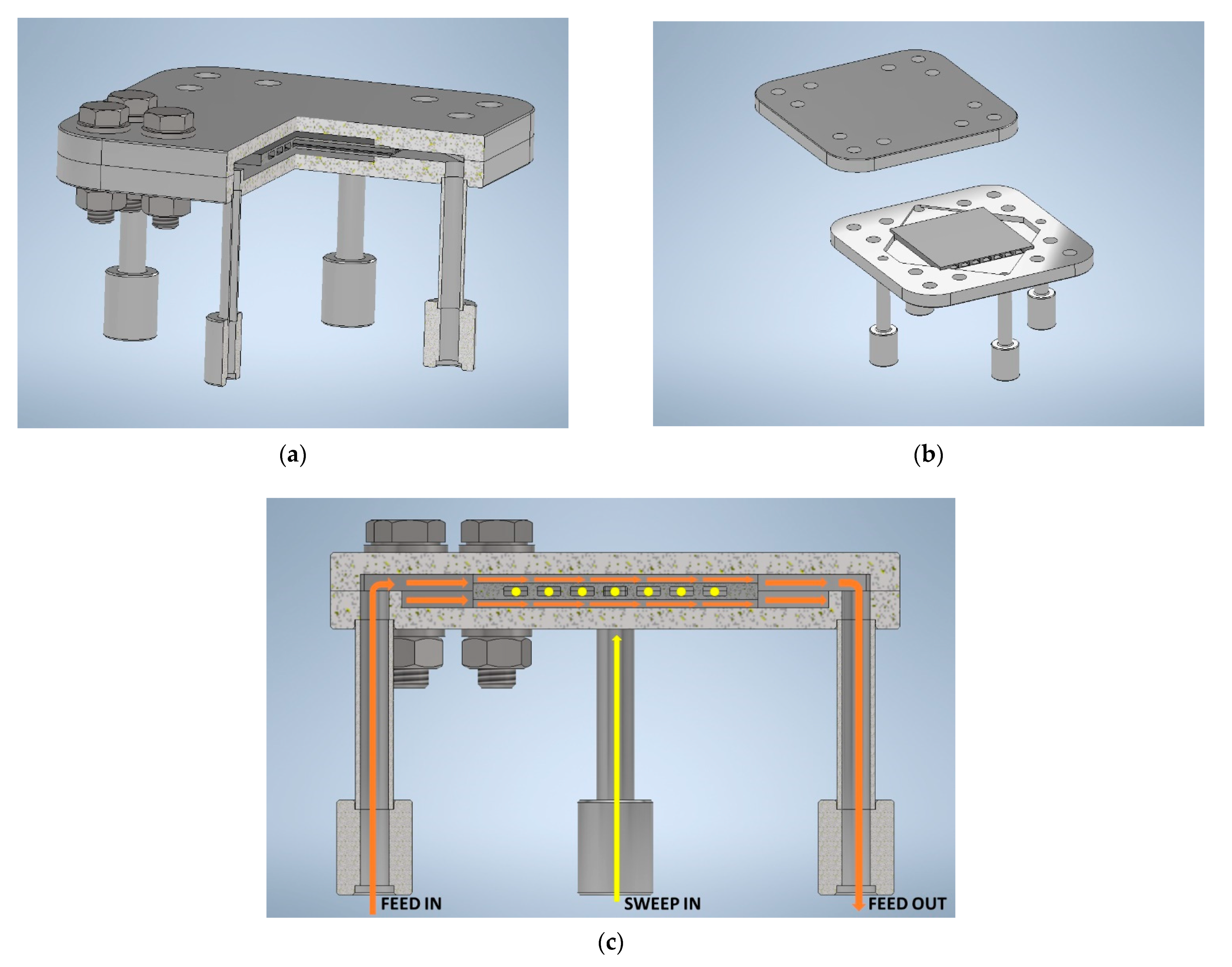
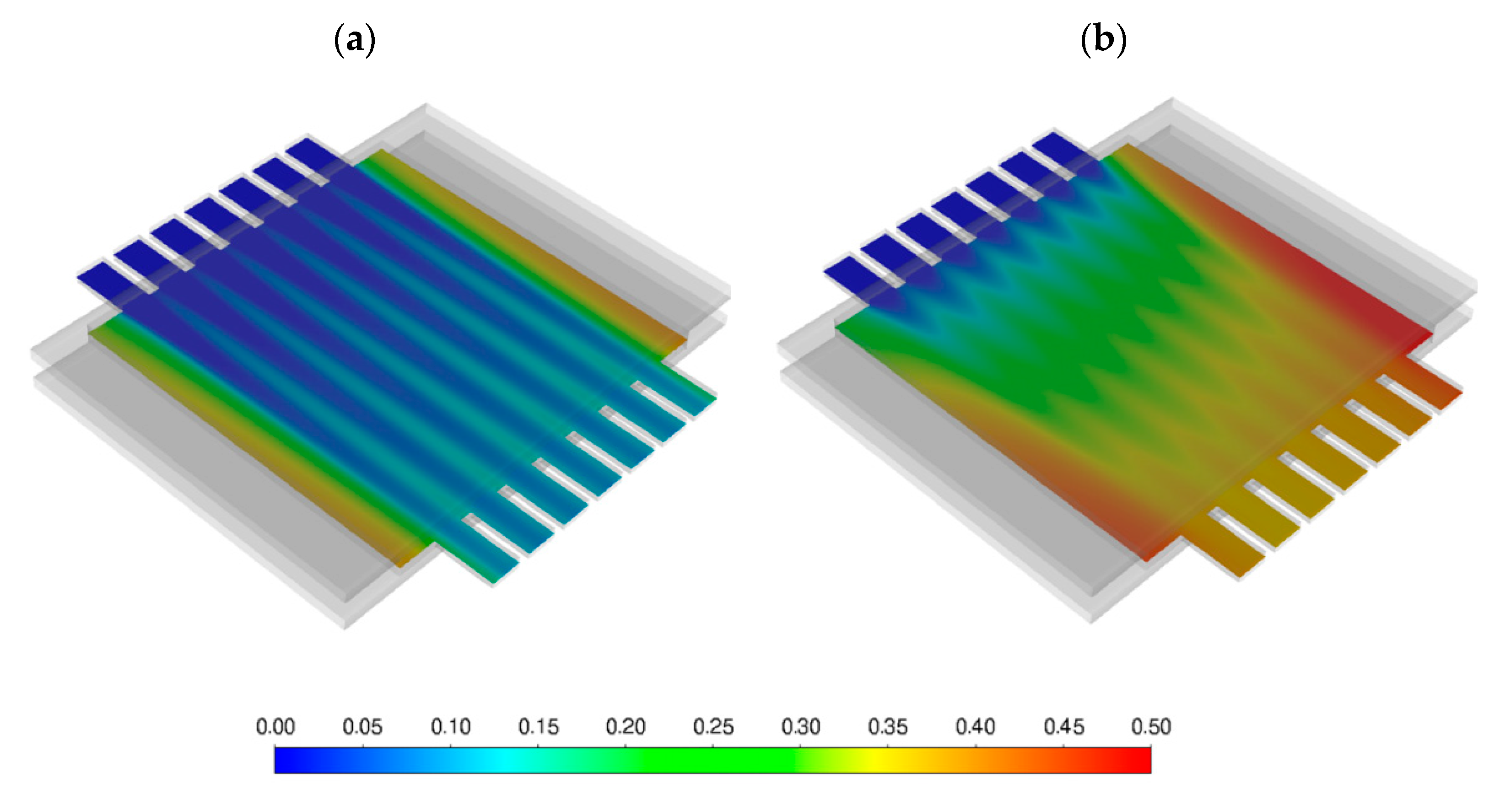
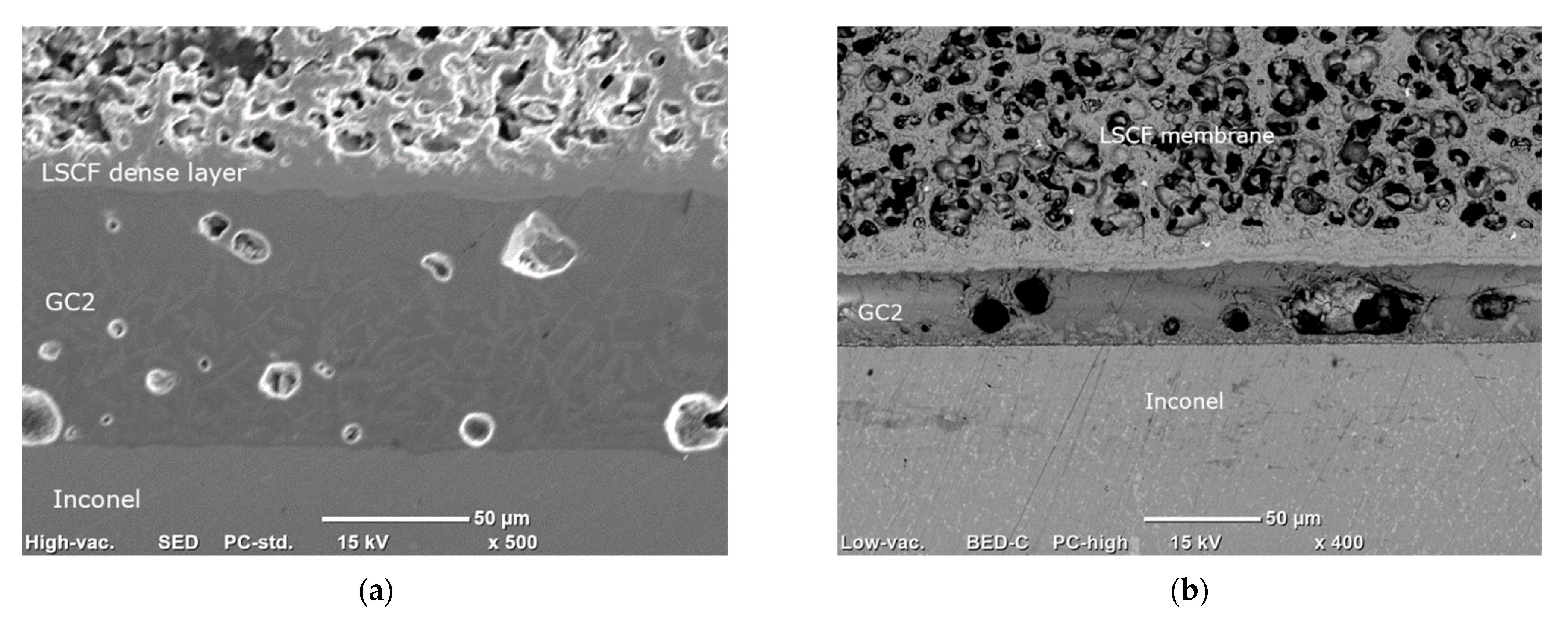
| Casted Layer | Doctor Blade Gap (µm) | Drying Time (h) | Thickness after Drying (µm) | |
|---|---|---|---|---|
| Tape i | Dense | 50 | 1 | 900 |
| (asymmetric) | Porous | 1900 | 12 | |
| Tape ii | Interlayer | 2700 | 24 | 1300 |
| Temperature | 70 °C |
| Plates displacement | 160 µm |
| Displacement rate | 3 µm × s−1 |
| Dwell time | 180 s |
| Load release time | 60 s |
| Ea,a in Air (kJ/mol) | Ea,a in Pure O2 (kJ/mol) | ||||
|---|---|---|---|---|---|
| Sample | Thickness [µm] | High T | Low T | High T | Low T |
| This work (M2) | 12 | 77.6 | 123.6 | 102.2 | 118 |
| Asymmetric LSCF [37] | 30 | 72 | 123 | 119 | 146 |
| Asymmetric D [4] | 20 | 44 | 153 | - | - |
| Asymmetric Ref [4] | 20 | 71 | 179 | - | - |
| Temperature | Dense Membrane Layer in… | # of Tested Specimens | E (GPa) | σf (MPa) |
|---|---|---|---|---|
| RT | Compression | 18 | 25 ± 3 | 36 ± 5 |
| Tension | 17 | 26 ± 2 | 34 ± 4 | |
| 950 °C | Compression | 25 | 59 ± 5 | 47 ± 2 |
| Tension | 25 | 57 ± 3 | 43 ± 1 |
| Atmosphere | Air | Ar |
|---|---|---|
| pO2 (atm) | 0.21 | 10−5 |
| CTE 60–700 °C | 15.4 | 14.9 |
| CTE 700–950 °C | 26 | 28.4 |
| CTE 60–950 °C | 18 | 17.7 |
| CTE 60–1000 °C [28] | 18.4 | - |
| T = 950 °C; Pfeed = 400 kPa; Psweep = 107 kPa | |||
| Eact = 108.57253 kJ/mol | |||
| k0 = 5.5379·10−5 mol/s·cm | |||
| s = 0.00122 cm | |||
| Sweep velocity [m/s] | 1 | 0.5 | 0.1 |
| Sweep Flow Rate (m3/s) (7 channels) | 7·10−5 | 3.5·10−5 | 7·10−6 |
| Feed Flow Rate (m3/s) (1 channel) | 1.017·10−4 | 5.086·10−5 | 1.017·10−5 |
| Feed Velocity (m/s) | 0.8477 | 0.4239 | 0.0848 |
| Permeated O2 (kg/s) | 3.25·10−6 | 2.79·10−6 | 1.51·10−6 |
| Permeation Process Efficiency | 8.75% | 15.02% | 40.56% |
| %O2 | |||
| Out 1 | 14.659 | 22.292 | 43.865 |
| Out 2 | 12.126 | 18.875 | 39.994 |
| Out 3 | 12.072 | 18.712 | 38.727 |
| Out 4 | 12.033 | 18.614 | 37.982 |
| Out 5 | 11.996 | 18.516 | 37.327 |
| Out 6 | 11.967 | 18.469 | 37.070 |
| Out 7 | 14.260 | 21.389 | 39.079 |
| Out Feed | 17.224 | 16.450 | 13.179 |
| ΔPtot in-out (Pa) | |||
| Sweep | 5.04 | 2.59 | 0.62 |
| Feed | 3.61 | 1.75 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drago, F.; Fedeli, P.; Cavaliere, A.; Cammi, A.; Passoni, S.; Mereu, R.; De La Pierre, S.; Smeacetto, F.; Ferraris, M. Development of a Membrane Module Prototype for Oxygen Separation in Industrial Applications. Membranes 2022, 12, 167. https://doi.org/10.3390/membranes12020167
Drago F, Fedeli P, Cavaliere A, Cammi A, Passoni S, Mereu R, De La Pierre S, Smeacetto F, Ferraris M. Development of a Membrane Module Prototype for Oxygen Separation in Industrial Applications. Membranes. 2022; 12(2):167. https://doi.org/10.3390/membranes12020167
Chicago/Turabian StyleDrago, Francesca, Paolo Fedeli, Angelo Cavaliere, Andrea Cammi, Stefano Passoni, Riccardo Mereu, Stefano De La Pierre, Federico Smeacetto, and Monica Ferraris. 2022. "Development of a Membrane Module Prototype for Oxygen Separation in Industrial Applications" Membranes 12, no. 2: 167. https://doi.org/10.3390/membranes12020167
APA StyleDrago, F., Fedeli, P., Cavaliere, A., Cammi, A., Passoni, S., Mereu, R., De La Pierre, S., Smeacetto, F., & Ferraris, M. (2022). Development of a Membrane Module Prototype for Oxygen Separation in Industrial Applications. Membranes, 12(2), 167. https://doi.org/10.3390/membranes12020167







