The State-of-the-Art Functionalized Nanomaterials for Carbon Dioxide Separation Membrane
Abstract
:1. Introduction
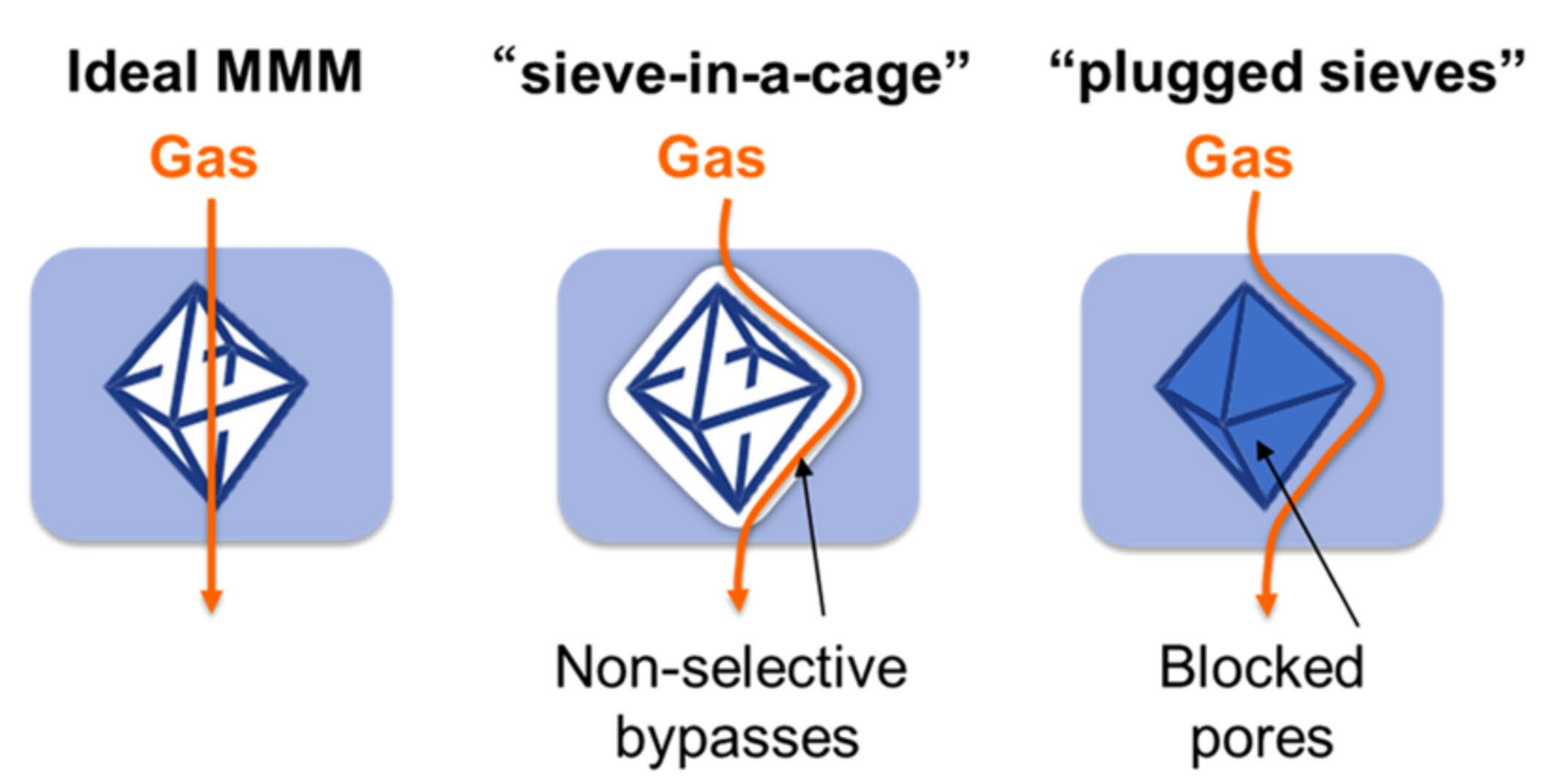
2. The Roles of Nanofiller in Gas Separation Membrane
3. Functionalization of Nanofiller
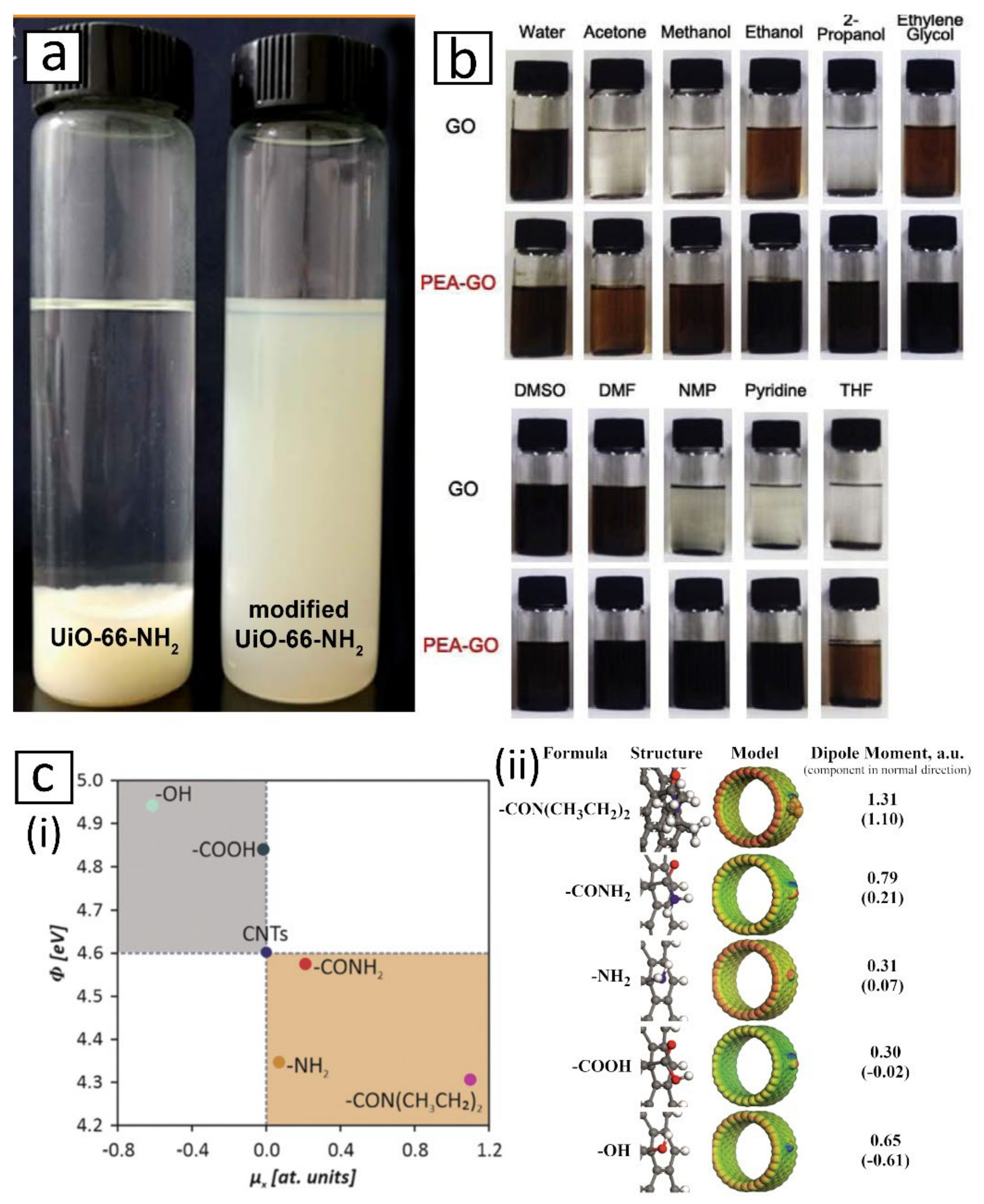
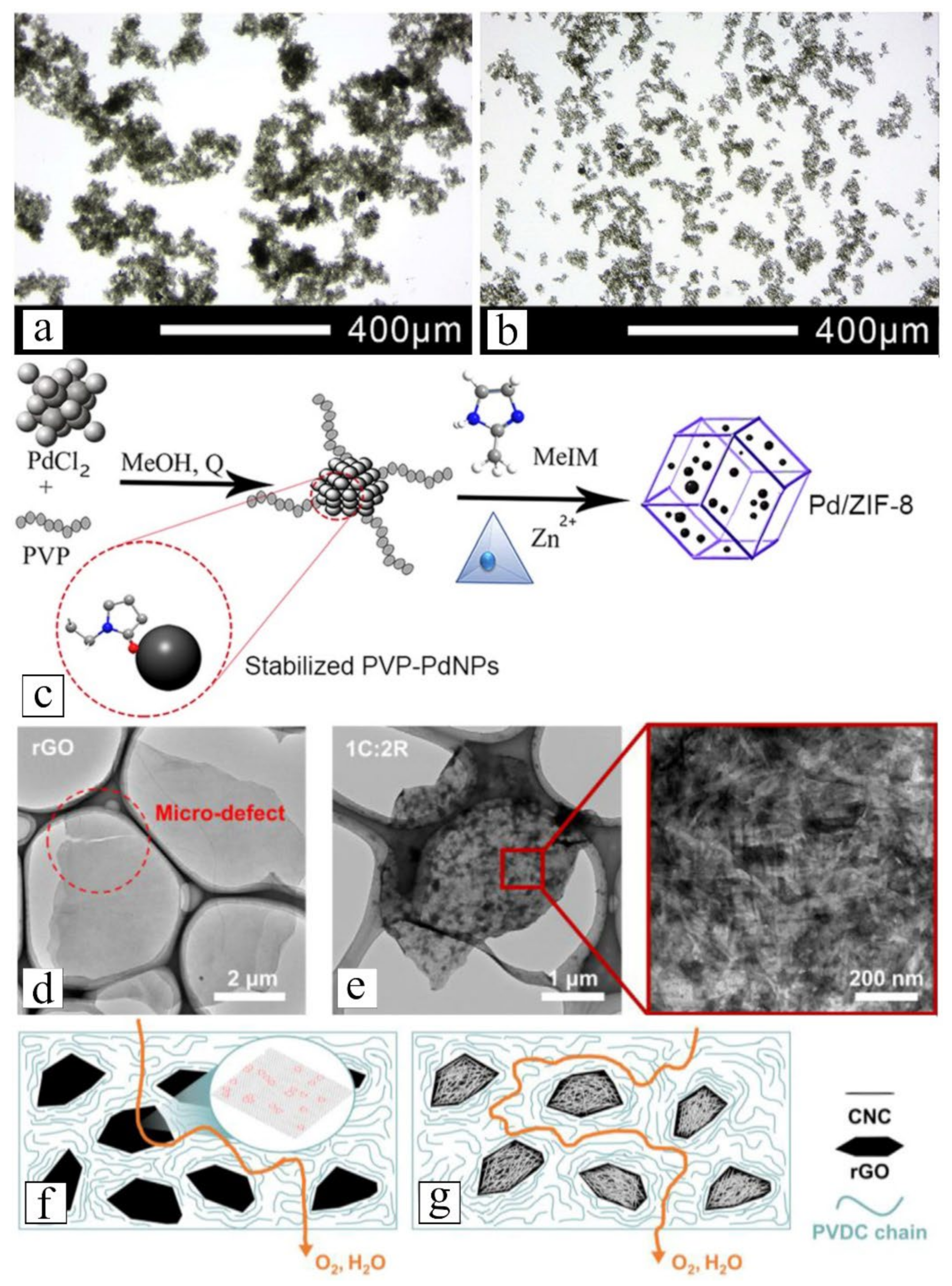
3.1. Compatibilizing Nanofiller with Solvent and Polymer Matrix
- observing the settling rate of nanofiller in a suspension:
- inspecting the suspension density and homogeneity via optical or electron microscopy:
- determining the surface charge of the nanofiller via zeta potential (ζ) analysis:
- suitable for highly charged nanomaterials and provides indicative information regarding suspension stability. Generally, nanomaterial suspension with a magnitude of ζ magnitude greater than 30 mV is considered stable, but the results may be affected by the material characteristics such as hydrophilicity, polarity, geometry and chemical groups. Hence, additional supporting characterizations are required [81,153,172,173].
- approximating the Hansen and/or Hildebrand solubility parameters of suspension:
- fairly accurate prediction of the suspension stability and provides information on dispersion mechanisms in terms of dispersive force, dipole interaction and hydrogen bonding parameters. Other molecular interactions such as electrostatic force, metallic interaction and ionic bond are not accounted for [173,174,175,176,177].
- evaluate changes in chemical properties:
- visually observe nanofiller–membrane matrix interfaces:
- can be conducted using field emission scanning electron microscopy (FESEM) or transmission electron microscopy (TEM). This analysis focuses on a small sample area; hence, scanning of multiple random spots is recommended to obtain reliable and accurate generalization of the nanofiller–polymer interface morphologies [135,155,197,198,199]
- evaluate changes in membrane separation behavior
3.2. Tuning Nanofiller Pore Size
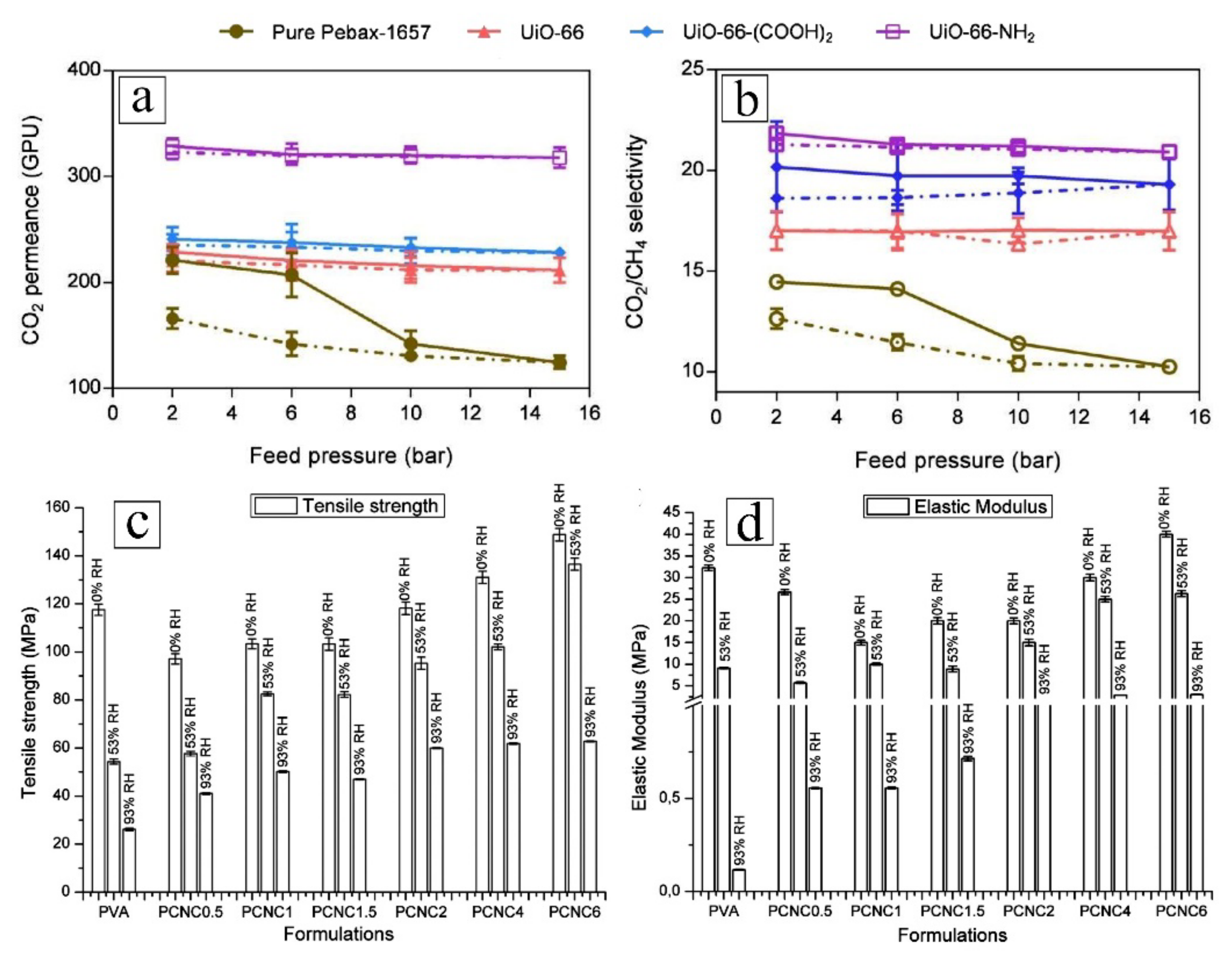
3.3. Enhancing Gas Separation Performance
3.3.1. Functional Groups with Gas-Specific Selectivity
| Base Polymer | Filler (Loading) | Modification | Test Conditions | Ref | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | APTMS-SiO2 (3 wt.%) | co-condensation of APTMS with hydrolyzed TEOS (SiO2 precursor) | 6 bar, 24 °C pure gas | - | 20 | 340 * | 38 | 29 | 98 * | 57 * | [248] |
| crosslinked PEO | 2-amBzIM-ZIF-7 (30 wt.%) | ligand substitution of BzIM by 2-amBzIM (70% substitution) | 5 bar, 35 °C CO2:CH4 = 50:50 mol. ratio | 213 | - | 10 * | - | 56 | - | 167 * | [56] |
| PEBAX-1657 | APTES-silica (15 wt.%) | grafting with acid hydrolyzed APTES | 10 bar, 25 °C, pure gas | 174 | - | 36 * 26 ϯ | - | 40.2 | - | 71 * 18 ϯ | [32] |
| PEBAX-1657 | UiO-66-NH2 (1.5 wt.%) | substitution of 1,4-dicarboxybenzene to 2-amino-1,4-dicarboxybenzene | 7 bar, 25 °C pure gas | 393 | - | 61 * 7 ϯ | 40 | 88 * 27 ϯ | [111] | ||
| PEBAX-1657 | UiO-66-NH2 (50 wt.%) | substitution with amine containing ligand | 2 bar, 25 °C CO2:CH4 = 20:80 mol. ratioCO2:N2 = 20:80 mol. ratio | - | 338 | 106 * 50 ϯ | 57 | 21 | 43 * 27 ϯ | 67 * 24 ϯ | [126] |
| XTR-PI | Am-BN (1 wt.%) | ball-mill with urea | -bar, 25 °C pure gas | 21 | - | −89 * | - | 69 | - | 212 * | [124] |
| CNF/MCE | UiO-66-NH2 | carboxylation of CNF and replacing UiO-66 ligand with ATA | 2 bar, 25 °C pure gas | 139 | - | 1886 * 178 ϯ | 46 | - | 667 * 64 ϯ | - | [204] |
| PEBAX-1657/PEG | TEPA-TNT (3 wt.%) | coating TNT with TEPA | 5 bar, 35 °C pure gas | 168 | - | 68 * | - | 16 | - | 12 * | [90] |
| PIM | UiO-66-NH2 (10 wt.%) | amine containing ligand | 1 bar, 35 °C pure gas | - | 7460 | 73 * | 26 | - | 37 * | - | [82] |
| PA | PEI-BN | coating with PEI | 3 bar, 25 °C pure gas | - | 47 | 37 * 5 ϯ | 47 | - | 20 * 21 ϯ | - | [93] |
| PEBAX-1657/PVC | PEBAX/SiO2 | priming with host matrix polymer | 1 bar, 25 °C pure gas | - | 29 | 63 * | 76 | - | 36 * | - | [249] |
| PVA | MPEG-TiO2 (3 wt.%) | grafting of MPEG via radical polymerization | 10 bar, 35 °C pure gas | 5.4 | - | 476 * | 49 | 6.1 | 31 * | 26 * | [250] |
| PMMA | MPEG-TiO2 (5 wt.%) | grafting of MPEG via radical polymerization | 10 bar, 35 °C pure gas | 32 | - | 1081 * | 57 | 4.2 | 55 * | 19 * | [151] |
| PDMS | PEO-Si | nucleophilic addition of epoxy group of GOTMS (Si-precursor) with amine group of Jeffamine ED-2003 | 2 bar, 25 °C pure gas | - | 3636 | 21 * | 28.2 | - | 103 * | - | [95] |
| PSF | GOTMS-SiO2 (20 wt.%) | adsorption | 10 bar, 30 °C pure gas | 13 | - | 75 * | 46 | 36 | 42 * | 25 * | [251] |
| PEBAX-1074 | OA-ZnO (8 wt.%) | esterification | 2 bar, 25 °C pure gas | 152 | - | 38 * | 62 | 14 | 24 * | 22 * | [84] |
| PEBEX-1074 | OMWCNT (2.5 wt.%) | acid oxidation | 2 bar, 25 °C pure gas | 134 | - | 106 * | - | 21 | - | 17 * | [252] |
| PMMA-co-MA-PEG/PC | OGNR (3 wt.%) | HNO3 treated GNR | 0.7 bar, 27 °C pure gas | 140 | - | 20 * | 42 | - | 107 * | - | [125] |
| PIM | OH-pDCX (5 wt.%) | hydroxylation via Friedel-Crafts reaction | 2 bar, 25 °C pure gas | 8510 | - | 14 * | 28 | 22 | 22 * | 24 * | [135] |
| PMP | hydrolyzed TNT (2 wt.%) | treatment of TNT with strong base | 2 bar, 25 °C pure gas | 269 | - | 445 * | 224 | 70 | 155 * | 224 * | [232] |
| PEBAX-1074 | [Bmim][PF6]/SiO2 (8 wt.%) | coating of IL on SiO2 (10:1 wt./wt.) | 2 bar, 25 °C pure gas | 154 | - | 47 * | - | 19 | - | 3 * | [39] |
| PSF | [Bmim][BF4]@KIT-6 (100% selective layer) | immobilization of [Bmim][BF4] by KIT-6 (1:0.2 w/w) | 2 bar, r.t., pure gas | - | 51.6 | 204 * | 5.4 | 4.8 | 7 * | 0 * | [243] |
| 6FDA-ODA | [Bmim][Tf2N]@UiO-66-PEI (15 wt.%) | post-synthetic modification with PEI and IL | 1 bar, 35 °C CO2:CH4 = 50:50 mol. ratio | 26 | - | 152 * | - | 60 | - | 66 * | [99] |
| PEO | [Bmim][BF4]/ZnO (0.8 wt.%) | coating ZnO with [Bmim][BF4] (1:2 w/w) | r.t. pure gas | - | 36 | 225 * | 30 | - | 357 * | - | [97] |
| Matrimid-5218 | [Co(tetra-aza)]2+-NaY (15wt.%) | ion exchange and complex formation | 2 bar, 35 °C CO2:CH4 = 10:90 mol. ratio | 19 | - | 127 * 8 ϯ | - | 112 | - | 207 * 158 ϯ | [206] |
| PEBAX-1657 | CuZnIF (0.5 wt.%) | inclusion of second metal to ZIF and PEBAX priming | 6 bar, 30 °C pure gas | 148 | - | 48 * | 162 | 46 | 51 * | 42 * | [83] |
| PEBAX-1657 | PSS-HNT (0.1 wt%) | grafting | 3 bar, 25 °C pure gas | - | 10 | 74 * 0 ϯ | 245 | - | 457 * 32 ϯ | - | [146] |
| PLA | LCNF (6.5 wt.%) | grafting of CNF | 0.4 bar, 37 °C pure gas | 0.6 | - | 58 * | 21 | - | 22 * | - | [200] |
| PEBAX-1657/PES | Nf/TiO2 0.075:3 filler:polymer wt. ratio | coating TiO2 with Nf (1:0.045 w/w) | 2.5 bar, pure gas | = 1671 | = 2928 | [253] | |||||
| 6FDA-TP | ZIF-90 (40 wt.%) | condensation polymerization of 6FDA with TP | 9.8 bar, 35 °C pure gas | 45 | - | 125 * | 20 | 36 | 0 * | 2.7 * | [254] |
| Polyactive | m-ZnTCPP (used as gutter layer) | Surfactant assisted synthesis in absence of pyrazine | 3.5 bar, 35 °C CO2:N2 = 10:90 mol. ratio | - | 2160 | - | 31 | - | - | - | [210] |
| PVA/PSF | PCNF (1 wt.%) | phosphorylation of CNF with diammonium hydrogen phosphate | 5 bar, CO2:CH4 = 40:60 mol. ratio | - | 78 | 200 * | - | 45 | - | 55 * | [37] |
3.3.2. Nanofiller Hybridization
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications. In Canadian Consulting Engineer; John Wiley & Sons, Ltd.: Chichester, UK, 2004; pp. 1–538. [Google Scholar]
- Wang, M.; Zhao, J.; Wang, X.; Liu, A.; Gleason, K.K. Recent progress on submicron gas-selective polymeric membranes. J. Mater. Chem. A 2017, 5, 8860–8886. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane. In Sea Water Demineralization by Means of an Osmotic Membrane; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1963; Volume 38, pp. 117–132. [Google Scholar]
- Asatekin, A.; Mayes, A.M. Polymer Filtration Membranes. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–30. [Google Scholar]
- Cadotte, J.E.; Petersen, R.J.; Larson, R.E.; Erickson, E.E. A new thin-film composite seawater reverse osmosis membrane. Desalination 1980, 32, 25–31. [Google Scholar] [CrossRef]
- Cadotte, J.E. Continued evaluation of in situ-formed condensation polymers for reverse osmosis membranes. In U. S. NTIS, PB Rep.; MRI, North Star Division: Minnepolis, MN, USA, 1976; pp. 1–160. [Google Scholar]
- Wittbecker, E.L.; Morgan, P.W. Interfacial polycondensation. I. J. Polym. Sci. 1959, 40, 289–297. [Google Scholar] [CrossRef]
- Vakharia, V.; Salim, W.; Wu, D.; Han, Y.; Chen, Y.; Zhao, L.; Ho, W.S.W. Scale-up of amine-containing thin-film composite membranes for CO2 capture from flue gas. J. Memb. Sci. 2018, 555, 379–387. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Paul Chen, J.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hägg, M.B.; Gregersen, Ø.W. Decoupling the effect of membrane thickness and CNC concentration in PVA based nanocomposite membranes for CO2/CH4 separation. Sep. Purif. Technol. 2018, 204, 220–225. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. High speed spin coating in fabrication of Pebax 1657 based mixed matrix membrane filled with ultra-porous ZIF-8 particles for CO2/CH4 separation. Korean J. Chem. Eng. 2017, 34, 440–453. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Y.; Yu, S.; Lawless, D.; Feng, X. Thin film composite nanofiltration membranes assembled layer-by-layer via interfacial polymerization from polyethylenimine and trimesoyl chloride. J. Memb. Sci. 2014, 472, 141–153. [Google Scholar] [CrossRef]
- Fadhillah, F.; Zaidi, S.M.J.; Khan, Z.; Khaled, M.M.; Rahman, F.; Hammond, P.T. Development of polyelectrolyte multilayer thin film composite membrane for water desalination application. Desalination 2013, 318, 19–24. [Google Scholar] [CrossRef]
- Kim, J.; Fu, Q.; Scofield, J.M.P.; Kentish, S.E.; Qiao, G.G. Ultra-thin film composite mixed matrix membranes incorporating iron(III)-dopamine nanoparticles for CO2 separation. Nanoscale 2016, 8, 8312–8323. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Sevgili, E.; Karaman, M.; Lau, W.J.; Ismail, A.F. Hydroxypropyl methacrylate thin film coating on polyvinylidene fluoride hollow fiber membranes via initiated chemical vapor deposition. Eur. Polym. J. 2020, 122, 109360. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, A.; Zhang, C.; Wang, Y. Growing covalent organic frameworks on porous substrates for molecule-sieving membranes with pores tunable from ultra- to nanofiltration. J. Memb. Sci. 2019, 576, 116–122. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Lu, X.; Nejati, S.; Jaramillo, H.; Elimelech, M. In situ surface functionalization of reverse osmosis membranes with biocidal copper nanoparticles. Desalination 2016, 388, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pedram, S.; Mortaheb, H.R.; Fakhouri, H.; Arefi-Khonsari, F. Polytetrafluoroethylene sputtered PES membranes for membrane distillation: Influence of RF magnetron sputtering conditions. Plasma Chem. Plasma Process. 2017, 37, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the advancement of porous biopolymer scaffold: Tissue engineering application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Du, C.; Liang, Y.; Liu, M. Preparation and barrier properties of nanocellulose/layered double hydroxide composite film. BioResources 2018, 13, 1055–1064. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Kim, P.J.; Kim, K.; Qi, Z.; Wang, H.; Pol, V.G. Engineered heat dissipation and current distribution boron nitride-graphene layer coated on polypropylene separator for high performance lithium metal battery. J. Colloid Interface Sci. 2021, 583, 362–370. [Google Scholar] [CrossRef]
- Wang, Y.; Seo, B.; Wang, B.; Zamel, N.; Jiao, K.; Adroher, X.C. Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy AI 2020, 1, 100014. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tan, C.; Tian, X. Enhancement of CO2 biofixation and bioenergy generation using a novel airlift type photosynthetic microbial fuel cell. Bioresour. Technol. 2019, 272, 501–509. [Google Scholar] [CrossRef]
- Amirkhani, F.; Riasat, H.; Asghari, M.; Harami, H.R.; Asghari, M. CO2/CH4 mixed gas separation using poly(ether-b-amide)-ZnO nanocomposite membranes: Experimental and molecular dynamics study. Polym. Test. 2020, 86, 106464. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Carbon nanotubes as potential candidate for separation of H2-CO2 gas pairs. Int. J. Hydrog. Energy 2017, 42, 29283–29299. [Google Scholar] [CrossRef]
- Janakiram, S.; Ahmadi, M.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of nanocomposite membranes containing 0D to 2D nanofillers for CO2 separation: A review. Membranes 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Brunetti, A.; Drioli, E.; Barbieri, G. H2 separation From H2/N2 and H2/CO mixtures with co-polyimide hollow fiber module. Sep. Sci. Technol. 2011, 46, 1–13. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Samarasinghe, S.A.S.C.; Chuah, C.Y.; Li, W.; Sethunga, G.S.M.D.P.; Wang, R.; Bae, T.-H. Incorporation of CoIII acetylacetonate and SNW-1 nanoparticles to tailor O2/N2 separation performance of mixed-matrix membrane. Sep. Purif. Technol. 2019, 223, 133–141. [Google Scholar] [CrossRef]
- Harrigan, D.J.; Lawrence, J.A.; Reid, H.W.; Rivers, J.B.; O’Brien, J.T.; Sharber, S.A.; Sundell, B.J. Tunable sour gas separations: Simultaneous H2S and CO2 removal from natural gas via crosslinked telechelic poly(ethylene glycol) membranes. J. Memb. Sci. 2020, 602, 117947. [Google Scholar] [CrossRef]
- Norahim, N.; Faungnawakij, K.; Quitain, A.T.; Klaysom, C. Composite membranes of graphene oxide for CO2/CH4 separation. J. Chem. Technol. Biotechnol. 2019, 94, 2783–2791. [Google Scholar] [CrossRef]
- Isanejad, M.; Mohammadi, T. Effect of amine modification on morphology and performance of poly (ether-block-amide)/fumed silica nanocomposite membranes for CO2/CH4 separation. Mater. Chem. Phys. 2018, 205, 303–314. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Plasticization and swelling in polymeric membranes in CO2 removal from natural gas. Chem. Eng. Technol. 2016, 39, 1604–1616. [Google Scholar] [CrossRef]
- Hidalgo, D.; Sanz-Bedate, S.; Martín-Marroquín, J.M.; Castro, J.; Antolín, G. Selective separation of CH4 and CO2 using membrane contactors. Renew. Energy 2020, 150, 935–942. [Google Scholar] [CrossRef]
- Mahdavi, H.R.; Azizi, N.; Mohammadi, T. Performance evaluation of a synthesized and characterized Pebax1657/PEG1000/γ-Al2O3 membrane for CO2/CH4 separation using response surface methodology. J. Polym. Res. 2017, 24, 67. [Google Scholar] [CrossRef]
- Ghadimi, A.; Norouzbahari, S.; Vatanpour, V.; Mohammadi, F. An investigation on gas transport properties of cross-linked poly(ethylene glycol diacrylate) (XLPEGDA) and XLPEGDA/TiO2 Membranes with a focus on CO2 separation. Energy Fuels 2018, 32, 5418–5432. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hagg, M.B.; Gregersen, Ø.W.; Hussain, A. Phosphorylated nanocellulose fibrils/PVA nanocomposite membranes for biogas upgrading at higher pressure. Sep. Sci. Technol. 2020, 55, 1524–1534. [Google Scholar] [CrossRef]
- Zhang, X.M.; Tu, Z.H.; Li, H.; Li, L.; Wu, Y.T.; Hu, X.B. Supported protic-ionic-liquid membranes with facilitated transport mechanism for the selective separation of CO2. J. Memb. Sci. 2017, 527, 60–67. [Google Scholar] [CrossRef]
- Mahdavi, H.R.; Azizi, N.; Arzani, M.; Mohammadi, T. Improved CO2/CH4 separation using a nanocomposite ionic liquid gel membrane. J. Nat. Gas Sci. Eng. 2017, 46, 275–288. [Google Scholar] [CrossRef]
- Zeynali, R.; Ghasemzadeh, K.; Sarand, A.B.; Kheiri, F.; Basile, A. Experimental study on graphene-based nanocomposite membrane for hydrogen purification: Effect of temperature and pressure. Catal. Today 2019, 330, 16–23. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Hilmioglu, N.D. Enhanced hydrogen purification by graphene—poly(dimethyl siloxane) membrane. Int. J. Hydrog. Energy 2020, 45, 3549–3557. [Google Scholar] [CrossRef]
- Kanehashi, S.; Aguiar, A.; Lu, H.T.; Chen, G.Q.; Kentish, S.E. Effects of industrial gas impurities on the performance of mixed matrix membranes. J. Memb. Sci. 2018, 549, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Dolejš, P.; Poštulka, V.; Sedláková, Z.; Jandová, V.; Vejražka, J.; Esposito, E.; Jansen, J.C.; Izák, P. Simultaneous hydrogen sulphide and carbon dioxide removal from biogas by water-swollen reverse osmosis membrane. Sep. Purif. Technol. 2014, 131, 108–116. [Google Scholar] [CrossRef]
- Choi, W.; Ingole, P.G.; Park, J.S.; Lee, D.W.; Kim, J.H.; Lee, H.K. H2/CO mixture gas separation using composite hollow fiber membranes prepared by interfacial polymerization method. Chem. Eng. Res. Des. 2015, 102, 297–306. [Google Scholar] [CrossRef]
- Pian, C.; Shen, J.; Liu, G.; Liu, Z.; Jin, W. Ceramic hollow fiber-supported PDMS composite membranes for oxygen enrichment from air. Asia-Pac. J. Chem. Eng. 2016, 11, 460–466. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Solís, C.; Toldra-Reig, F.; Balaguer, M.; Somacescu, S.; Garcia-Fayos, J.; Palafox, E.; Serra, J.M. Mixed ionic–electronic conduction in NiFe2O4–Ce0.8Gd0.2O2−δ nanocomposite thin Films for oxygen separation. ChemSusChem 2018, 11, 2818–2827. [Google Scholar] [CrossRef]
- Chong, K.; Lai, S.; Lau, W.; Thiam, H.; Ismail, A.; Roslan, R. Preparation, characterization, and performance evaluation of polysulfone hollow fiber membrane with PEBAX or PDMS coating for oxygen enhancement process. Polymers 2018, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Chong, K.C.; Lai, S.O.; Thiam, H.S.; Teoh, H.C.; Heng, S.L. Recent progress of oxygen/nitrogen separation using membrane technology. J. Eng. Sci. Technol. 2016, 11, 1016–1030. [Google Scholar]
- Belaissaoui, B.; Le Moullec, Y.; Hagi, H.; Favre, E. Energy efficiency of oxygen enriched air production technologies: Cryogeny vs membranes. Energy Procedia 2014, 63, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Ge, L.; Liu, S.; Rudolph, V.; Zhu, Z. Mixed-matrix membranes with metal-organic framework-decorated CNT fillers for efficient CO2 separation. ACS Appl. Mater. Interfaces 2015, 7, 14750–14757. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Ionic liquid-modified Pebax® 1657 membrane filled by ZIF-8 particles for separation of CO2 from CH4, N2 and H2. J. Memb. Sci. 2017, 524, 652–662. [Google Scholar] [CrossRef]
- Razzaz, Z.; Rodrigue, D. Hollow fiber porous nanocomposite membranes produced via continuous extrusion: Morphology and gas transport properties. Materials 2018, 11, 2311. [Google Scholar] [CrossRef] [Green Version]
- Waqas Anjum, M.; de Clippel, F.; Didden, J.; Laeeq Khan, A.; Couck, S.; Baron, G.V.; Denayer, J.F.M.; Sels, B.F.; Vankelecom, I.F.J. Polyimide mixed matrix membranes for CO2 separations using carbon-silica nanocomposite fillers. J. Memb. Sci. 2015, 495, 121–129. [Google Scholar] [CrossRef]
- Patel, A.K.; Acharya, N.K. Thermally rearranged (TR) HAB-6FDA nanocomposite membranes for hydrogen separation. Int. J. Hydrog. Energy 2020, 45, 18685–18692. [Google Scholar] [CrossRef]
- Xiang, L.; Sheng, L.; Wang, C.; Zhang, L.; Pan, Y.; Li, Y. Amino-functionalized ZIF-7 nanocrystals: Improved intrinsic separation ability and interfacial compatibility in mixed-matrix membranes for CO2/CH4 separation. Adv. Mater. 2017, 29, 1606999. [Google Scholar] [CrossRef]
- Liu, M.; Gurr, P.A.; Fu, Q.; Webley, P.A.; Qiao, G.G. Two-dimensional nanosheet-based gas separation membranes. J. Mater. Chem. A 2018, 6, 23169–23196. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van Der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Shirke, Y.M.; Abou-Elanwar, A.M.; Choi, W.K.; Lee, H.; Hong, S.U.; Lee, H.K.; Jeon, J.D. Influence of nitrogen/phosphorus-doped carbon dots on polyamide thin film membranes for water vapor/N2 mixture gas separation. RSC Adv. 2019, 9, 32121–32129. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Saqib, S.; Rafiq, S.; Chawla, M.; Saeed, M.; Muhammad, N.; Khurram, S.; Majeed, K.; Khan, A.L.; Ghauri, M.; Jamil, F.; et al. Facile CO2 separation in composite membranes. Chem. Eng. Technol. 2019, 42, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.A.; Gupta, V.K. Applications of nanomaterial-polymer membranes for oil and gas separation. In Nanomaterial and Polymer Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 251–265. [Google Scholar]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- He, X. A review of material development in the field of carbon capture and the application of membrane-based processes in power plants and energy-intensive industries. Energy Sustain. Soc. 2018, 8, 34. [Google Scholar] [CrossRef]
- Goh, P.S.; Wong, K.C.; Ismail, A.F. Nanocomposite membranes for liquid and gas separations from the perspective of nanostructure dimensions. Membranes 2020, 10, 297. [Google Scholar] [CrossRef]
- Rafiq, S.; Man, Z.; Ahmad, F.; Maitra, S. Silica-polymer nanocomposite membranes for gas separation—A review, part 1. InterCeram Int. Ceram. Rev. 2010, 59, 341–349. [Google Scholar]
- Ismail, N.M.; Ismail, A.F.; Mustafa, A.; Zulhairun, A.K.; Aziz, F.; Bolong, N.; Razali, A.R. Polymer clay nanocomposites for gas separation: A review. Environ. Contam. Rev. 2019, 2, 01–05. [Google Scholar] [CrossRef]
- Zhang, Z.; Ibrahim, M.H.; El-Naas, M.H.; Cai, J. Zeolites nanocomposite membrane applications in CO2 capture. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 916–921. [Google Scholar]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Nejad, M.N.; Asghari, M.; Afsari, M. Investigation of carbon nanotubes in mixed matrix membranes for gas separation: A review. ChemBioEng Rev. 2016, 3, 276–298. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Carbon-based nanocomposite membrane for acidic gas separation. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Ismail, A.F., Goh, P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 233–260. [Google Scholar]
- Ahmad, A.L.; Jawad, Z.A.; Low, S.C.; Zein, S.H.S. Prospect of mixed matrix membrane towards CO2 separation. J. Membr. Sci. Technol. 2012, 02, 2–3. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Thin film nanocomposite: The next generation selective membrane for CO2 removal. J. Mater. Chem. A 2016, 4, 15726–15748. [Google Scholar] [CrossRef]
- Lamouroux, E.; Fort, Y. An overview of nanocomposite nanofillers and their functionalization. In Spectroscopy of Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 15–64. [Google Scholar]
- Roy, N.; Sengupta, R.; Bhowmick, A.K. Modifications of carbon for polymer composites and nanocomposites. Prog. Polym. Sci. 2012, 37, 781–819. [Google Scholar] [CrossRef]
- Rosyadah Ahmad, N.N.; Mukhtar, H.; Mohshim, D.F.; Nasir, R.; Man, Z. Surface modification in inorganic filler of mixed matrix membrane for enhancing the gas separation performance. Rev. Chem. Eng. 2016, 32, 181–200. [Google Scholar]
- Goh, P.S.; Wong, K.C.; Yogarathinam, L.T.; Ismail, A.F.; Abdullah, M.S.; Ng, B.C. Surface modifications of nanofillers for carbon dioxide separation nanocomposite membrane. Symmetry 2020, 12, 1102. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Mashhadikhan, S.; Sanaeepur, H.; Moghadassi, A.; Matsuura, T.; Ramakrishna, S. Substantial breakthroughs on function-led design of advanced materials used in mixed matrix membranes (MMMs): A new horizon for efficient CO2 separation. Prog. Mater. Sci. 2019, 102, 222–295. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Taniguchi, T.; Ismail, A.F.; Zahri, K. The role of geometrically different carbon-based fillers on the formation and gas separation performance of nanocomposite membrane. Carbon 2019, 149, 33–44. [Google Scholar] [CrossRef]
- Liu, M.; Nothling, M.D.; Webley, P.A.; Jin, J.; Fu, Q.; Qiao, G.G. High-throughput CO2 capture using PIM-1@MOF based thin film composite membranes. Chem. Eng. J. 2020, 396, 125328. [Google Scholar] [CrossRef]
- Kardani, R.; Asghari, M.; Hamedani, N.F.; Afsari, M. Mesoporous copper zinc bimetallic imidazolate MOF as nanofiller to improve gas separation performance of PEBA-based membranes. J. Ind. Eng. Chem. 2020, 83, 100–110. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Synthesis of a PEBAX-1074/ZnO nanocomposite membrane with improved CO2 separation performance. J. Energy Chem. 2017, 26, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, A.; Navarchian, A.H.; Tangestaninejad, S. Mixed matrix membranes on the basis of Matrimid and palladium-zeolitic imidazolate framework for hydrogen separation. Iran. Polym. J. 2020, 29, 479–491. [Google Scholar] [CrossRef]
- Compañ, V.; Del Castillo, L.F.; Hernández, S.I.; Mar López-González, M.; Riande, E. Crystallinity effect on the gas transport in semicrystalline coextruded films based on linear low density polyethylene. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 634–642. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Li, X.; Deng, M. Gas separation properties of poly(amide-6-b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J. Memb. Sci. 2014, 467, 41–47. [Google Scholar] [CrossRef]
- Acharya, N.K. Temperature-dependent gas transport and its correlation with kinetic diameter in polymer nanocomposite membrane. Bull. Mater. Sci. 2017, 40, 537–543. [Google Scholar] [CrossRef]
- Ogbole, E.O.; Lou, J.; Ilias, S.; Desmane, V. Influence of surface-treated SiO2 on the transport behavior of O2 and N2 through polydimethylsiloxane nanocomposite membrane. Sep. Purif. Technol. 2017, 175, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Noroozi, Z.; Bakhtiari, O. Preparation of amino functionalized titanium oxide nanotubes and their incorporation within Pebax/PEG blended matrix for CO2/CH4 separation. Chem. Eng. Res. Des. 2019, 152, 149–164. [Google Scholar] [CrossRef]
- Khdary, N.H.; Abdelsalam, M.E. Polymer-silica nanocomposite membranes for CO2 capturing. Arab. J. Chem. 2020, 13, 557–567. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Liu, D.; Yang, C.; Liu, Y.; Ruoff, R.S.; Lei, W. Functionalized boron nitride membranes with ultrafast solvent transport performance for molecular separation. Nat. Commun. 2018, 9, 1902. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Suzaimi, N.D.; Ng, Z.C.; Ismail, A.F.; Jiang, X.; Hu, X.; Taniguchi, T. Tailoring the CO2-selectivity of interfacial polymerized thin film nanocomposite membrane via the barrier effect of functionalized boron nitride. J. Colloid Interface Sci. 2021, 603, 810–821. [Google Scholar] [CrossRef]
- Jackson, G.L.; Lin, X.M.; Austin, J.; Wen, J.; Jaeger, H.M. Ultrathin porous hydrocarbon membranes templated by nanoparticle assemblies. Nano Lett. 2021, 21, 166–174. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, M.; Guan, K.; Zhao, J.; Liu, G.; Jin, W. High-performance CO2 capture through polymer-based ultrathin membranes. Adv. Funct. Mater. 2019, 29, 1900735. [Google Scholar] [CrossRef]
- Zhang, L.; Xin, Q.; Lou, L.; Li, X.; Zhang, L.; Wang, S.; Li, Y.; Zhang, Y.; Wu, H.; Jiang, Z. Mixed matrix membrane contactor containing core-shell hierarchical Cu@4A filler for efficient SO2 capture. J. Hazard. Mater. 2019, 376, 160–169. [Google Scholar] [CrossRef]
- Rhyu, S.Y.; Cho, Y.; Kang, S.W. Nanocomposite membranes consisting of poly(ethylene oxide)/ionic liquid/ZnO for CO2 separation. J. Ind. Eng. Chem. 2020, 85, 75–80. [Google Scholar] [CrossRef]
- Kong, C.; Du, H.; Chen, L.; Chen, B. Nanoscale MOF/organosilica membranes on tubular ceramic substrates for highly selective gas separation. Energy Environ. Sci. 2017, 10, 1812–1819. [Google Scholar] [CrossRef]
- Liu, B.; Li, D.; Yao, J.; Sun, H. Improved CO2 separation performance and interfacial affinity of mixed matrix membrane by incorporating UiO-66-PEI@[bmim][Tf2N] particles. Sep. Purif. Technol. 2020, 239, 116519. [Google Scholar] [CrossRef]
- Vainrot, N.; Li, M.; Isloor, A.M.; Eisen, M.S. New preparation methods for pore formation on polysulfone membranes. Membranes 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Li, W.; Yang, S.; Wei, Y.; Zhang, Z.; Li, Y. Layered double hydroxides (LDHs) as novel macropore-templates: The importance of porous structures for forward osmosis desalination. J. Memb. Sci. 2019, 585, 175–183. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, C.R.; Luechinger, N.A.; Lamprou, A.; Rossier, M.; Grass, R.N.; Stark, W.J. Soluble nanoparticles as removable pore templates for the preparation of polymer ultrafiltration membranes. J. Memb. Sci. 2012, 387–388, 76–82. [Google Scholar] [CrossRef]
- Lee, J.; Tang, C.Y.; Huo, F. Fabrication of Porous Matrix Membrane as Green Template for Water Treatment. Sci. Rep. 2014, 4, 3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roilo, D.; Maestri, C.A.; Scarpa, M.; Bettotti, P.; Checchetto, R. Gas barrier and optical properties of cellulose nanofiber coatings with dispersed TiO2 nanoparticles. Surf. Coat. Technol. 2018, 343, 131–137. [Google Scholar] [CrossRef]
- Shirvani, H.; Maghami, S.; Isfahani, A.P.; Sadeghi, M. Influence of blend composition and silica nanoparticles on the morphology and gas separation performance of PU/PVA blend membranes. Membranes 2019, 9, 82. [Google Scholar] [CrossRef] [Green Version]
- Motedayen, A.A.; Rezaeigolestani, M.; Guillaume, C.; Guillard, V.; Gontard, N. Gas barrier enhancement of uncharged apolar polymeric films by self-assembling stratified nano-composite films. RSC Adv. 2019, 9, 10938–10947. [Google Scholar] [CrossRef] [Green Version]
- Roilo, D.; Patil, P.N.; Brusa, R.S.; Miotello, A.; Aghion, S.; Ferragut, R.; Checchetto, R. Polymer rigidification in graphene based nanocomposites: Gas barrier effects and free volume reduction. Polymer 2017, 121, 17–25. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Goh, K.; Wang, R.; Bae, T.H. High-performance nanocomposite membranes realized by efficient molecular sieving with CuBDC nanosheets. Chem. Commun. 2017, 53, 4254–4257. [Google Scholar] [CrossRef]
- Mozafari, M.; Abedini, R.; Rahimpour, A. Zr-MOFs-incorporated thin film nanocomposite Pebax 1657 membranes dip-coated on polymethylpentyne layer for efficient separation of CO2/CH4. J. Mater. Chem. A 2018, 6, 12380–12392. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Wong, K.C.; Zulhairun, A.K.; Ismail, A.F. Enhancing desalination performance of thin film composite membrane through layer by layer assembly of oppositely charged titania nanosheet. Desalination 2020, 476, 114167. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, H.; Jin, M.L.; Ellison, C.J. Impermeable gas barrier coating by facilitated diffusion of ethylenediamine through graphene oxide liquid crystals. Carbon 2019, 148, 28–35. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Bandyopadhyay, P.; Lan, Q.; Xie, H.; Liu, G.; Liu, X.; Cheng, X.; Kim, N.H.; Lee, J.H. Two-dimensional materials modified layered double hydroxides: A series of fillers for improving gas barrier and permselectivity of poly(vinyl alcohol). Compos. Part B Eng. 2021, 207, 108568. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Y.; Zhou, T.; Wang, Q.; Li, Y. Recent advances in layered double hydroxides (LDHs) as two-dimensional membrane materials for gas and liquid separations. J. Memb. Sci. 2018, 567, 89–103. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, K.; Hou, D.; Liu, X.; Li, G.; Sang, Y.; Liu, H.; Li, L.; Chen, S. Three-dimensional hierarchical frameworks based on MoS2 nanosheets self-Assembled on graphene oxide for efficient electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2014, 6, 21534–21540. [Google Scholar] [CrossRef]
- Hou, Y.; Wen, Z.; Cui, S.; Feng, X.; Chen, J. Strongly coupled ternary hybrid aerogels of N-deficient porous graphitic-C3N4 nanosheets/N-doped graphene/NiFe-layered double hydroxide for solar-driven photoelectrochemical water oxidation. Nano Lett. 2016, 16, 2268–2277. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, H.; Zhang, Y.Y.; Ding, X.; Liu, J. Thin film composite membranes functionalized with montmorillonite and hydrotalcite nanosheets for CO2/N2 separation. Sep. Purif. Technol. 2017, 189, 128–137. [Google Scholar] [CrossRef]
- Ang, E.H.; Velioğlu, S.; Chew, J.W. Tunable affinity separation enables ultrafast solvent permeation through layered double hydroxide membranes. J. Memb. Sci. 2019, 591. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Ji, Y.; Liu, Q.; Cheng, L.; Guan, K.; Zhang, M.; Liu, G.; Xiong, J.; Yang, J.; et al. 2D MXene nanofilms with tunable gas transport channels. Adv. Funct. Mater. 2018, 28, 1801511. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D.; Ma, J.; Tong, M.; Zhang, W.; Huang, H.; Yang, Q.; Zhong, C. A GO-assisted method for the preparation of ultrathin covalent organic framework membranes for gas separation. J. Mater. Chem. A 2016, 4, 13444–13449. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, H. Effect of ZnO nanoparticles on the mechanical, barrier and optical properties of thermoplastic cationic starch/montmorillonite biodegradable films. Int. J. Biol. Macromol. 2019, 124, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Shang, Y.; Zhu, X.; Jiang, Z.; Zhou, C.; Han, J.; Zhang, H. Non-covalent modification of boron nitride nanoparticle-reinforced PEEK composite: Thermally conductive, interfacial, and mechanical properties. Polymer 2020, 203, 122763. [Google Scholar] [CrossRef]
- Wang, Y.; Low, Z.; Kim, S.; Zhang, H.; Chen, X.; Hou, J.; Seong, J.G.; Lee, Y.M.; Simon, G.P.; Davies, C.H.J.; et al. Functionalized boron nitride nanosheets: A thermally rearranged polymer nanocomposite membrane for hydrogen separation. Angew. Chem. 2018, 130, 16288–16293. [Google Scholar] [CrossRef]
- Kausar, A. Poly(methyl methacrylate-co-methacrylic amide)-polyethylene glycol/polycarbonate and graphene nanoribbon-based nanocomposite membrane for gas separation. Int. J. Polym. Anal. Charact. 2018, 23, 450–462. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Zulkifli, M.Y.; Li, H.; Zhang, Y.; Liang, W.; D’Alessandro, D.M.; Chen, V. Surface functionalized UiO-66/Pebax-based ultrathin composite hollow fiber gas separation membranes. J. Mater. Chem. A 2018, 6, 918–931. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Budhi, Y.W.; Suganda, W.; Irawan, H.K.; Restiawaty, E.; Miyamoto, M.; Uemiya, S.; Nishiyama, N.; van Sint Annaland, M. Hydrogen separation from mixed gas (H2, N2) using Pd/Al2O3 membrane under forced unsteady state operations. Int. J. Hydrog. Energy 2020, 45, 9821–9835. [Google Scholar] [CrossRef]
- Pan, Z.; Chan, W.P.; Da Oh, W.; Veksha, A.; Giannis, A.; Tamilselvam, K.S.O.; Lei, J.; Binte Mohamed, D.K.; Wang, H.; Lisak, G.; et al. Regenerable Co-ZnO-based nanocomposites for high-temperature syngas desulfurization. Fuel Process. Technol. 2020, 201, 106344. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Manipulating the H2/CO ratio from dry reforming of simulated mixed waste plastics by the addition of steam. Fuel Process. Technol. 2017, 156, 331–338. [Google Scholar] [CrossRef]
- Wang, H.; Paul, D.R.; Chung, T.S. Surface modification of polyimide membranes by diethylenetriamine (DETA) vapor for H2 purification and moisture effect on gas permeation. J. Memb. Sci. 2013, 430, 223–233. [Google Scholar] [CrossRef]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of graphene and graphene oxide nanoplatelets on the gas permselectivity and aging behavior of poly(trimethylsilyl propyne) (PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Hou, R.; Smith, S.J.D.D.; Wood, C.D.; Mulder, R.J.; Lau, C.H.; Wang, H.; Hill, M.R. Solvation effects on the permeation and aging performance of PIM-1-based MMMs for gas separation. ACS Appl. Mater. Interfaces 2019, 11, 6502–6511. [Google Scholar] [CrossRef]
- Xia, J.; Chung, T.S.; Paul, D.R. Physical aging and carbon dioxide plasticization of thin polyimide films in mixed gas permeation. J. Memb. Sci. 2014, 450, 457–468. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Nijmeijer, K.; Ribeiro, C.P.; Freeman, B.D.; Wessling, M. On the effects of plasticization in CO2/light gas separation using polymeric solubility selective membranes. J. Memb. Sci. 2011, 367, 33–44. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, K.; Liu, L.; Liu, M.; Qiu, W.; Webley, P.A. Enhancing plasticization-resistance of mixed-matrix membranes with exceptionally high CO2/CH4 selectivity through incorporating ZSM-25 zeolite. J. Memb. Sci. 2019, 583, 23–30. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.; Orsi, A.F.; Lozinska, M.M.; Johnson, T.; Jansen, K.M.B.; Wright, P.A.; Carta, M.; McKeown, N.B.; Kapteijn, F.; et al. Towards high performance metal–organic framework–microporous polymer mixed matrix membranes: Addressing compatibility and limiting aging by polymer doping. Chem. -A Eur. J. 2018, 24, 12796–12800. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, X.; Zhang, Y.; Tsuru, T. Enhanced permeation through CO2-stable dual-inorganic composite membranes with tunable nanoarchitectured channels. ACS Sustain. Chem. Eng. 2018, 6, 8515–8524. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hägg, M.B.; Gregersen, Ø.W. Cellulose nanocrystal/PVA nanocomposite membranes for CO2/CH4 separation at high pressure. J. Memb. Sci. 2018, 554, 275–281. [Google Scholar] [CrossRef]
- Venturi, D.; Grupkovic, D.; Sisti, L.; Baschetti, M.G. Effect of humidity and nanocellulose content on polyvinylamine-nanocellulose hybrid membranes for CO2 capture. J. Memb. Sci. 2018, 548, 263–274. [Google Scholar] [CrossRef]
- Khosravi, A.; Vatani, A.; Mohammadi, T. Application of polyhedral oligomeric silsesquioxane to the stabilization and performance enhancement of poly(4-methyl-2-pentyne) nanocomposite membranes for natural gas conditioning. J. Appl. Polym. Sci. 2017, 134, 19–23. [Google Scholar] [CrossRef]
- Molki, B.; Heidarian, P.; Mohammadi Aframehr, W.; Nasri-Nasrabadi, B.; Bahrami, B.; Ahmadi, M.; Komeily-Nia, Z.; Bagheri, R. Properties investigation of polyvinyl alcohol barrier films reinforced by calcium carbonate nanoparticles. Mater. Res. Express 2019, 6, 055311. [Google Scholar] [CrossRef]
- Molavi, H.; Shojaei, A.; Mousavi, S.A. Improving mixed-matrix membrane performance: Via PMMA grafting from functionalized NH2-UiO-66. J. Mater. Chem. A 2018, 6, 2775–2791. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Shen, Y.; Hou, J.; Zhang, Y.Y.; Fam, W.; Liu, J.; Bennett, T.D.; Chen, V. Ultraselective Pebax membranes enabled by templated microphase separation. ACS Appl. Mater. Interfaces 2018, 10, 20006–20013. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Ariyoshi, M.; Fujikawa, S. Thickness effect on CO2/N2 separation in double layer Pebax-1657®/PDMS membranes. Membranes 2018, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Ogieglo, W.; Puspasari, T.; Hota, M.K.; Wehbe, N.; Alshareef, H.N.; Pinnau, I. Nanohybrid thin-film composite carbon molecular sieve membranes. Mater. Today Nano 2020, 9, 100065. [Google Scholar] [CrossRef]
- Zhao, J.; He, G.; Liu, G.; Pan, F.; Wu, H.; Jin, W.; Jiang, Z. Manipulation of interactions at membrane interfaces for energy and environmental applications. Prog. Polym. Sci. 2018, 80, 125–152. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Gas permeation and selectivity characteristics of PSf based nanocomposite membranes. Polymer 2019, 180, 121692. [Google Scholar] [CrossRef]
- Borandeh, S.; Abdolmaleki, A.; Zamani Nekuabadi, S.; Sadeghi, M. Methoxy poly (ethylene glycol) methacrylate-TiO2/poly (methyl methacrylate) nanocomposite: An efficient membrane for gas separation. Polym. Technol. Mater. 2019, 58, 789–802. [Google Scholar] [CrossRef]
- Kausar, A. Design of poly(1-hexadecene-sulfone)/poly(1,4-phenylene sulfide) membrane containing nano-zeolite and carbon nanotube for gas separation. Int. J. Plast. Technol. 2017, 21, 96–107. [Google Scholar] [CrossRef]
- Wu, J.K.; Ye, C.C.; Liu, T.; An, Q.F.; Song, Y.H.; Lee, K.R.; Hung, W.S.; Gao, C.J. Synergistic effects of CNT and GO on enhancing mechanical properties and separation performance of polyelectrolyte complex membranes. Mater. Des. 2017, 119, 38–46. [Google Scholar] [CrossRef]
- Khorshidi, B.; Hosseini, S.A.; Ma, G.; McGregor, M.; Sadrzadeh, M. Novel nanocomposite polyethersulfone- antimony tin oxide membrane with enhanced thermal, electrical and antifouling properties. Polymer 2019, 163, 48–56. [Google Scholar] [CrossRef]
- Atash Jameh, A.; Mohammadi, T.; Bakhtiari, O. Preparation of PEBAX-1074/modified ZIF-8 nanoparticles mixed matrix membranes for CO2 removal from natural gas. Sep. Purif. Technol. 2020, 231, 115900. [Google Scholar] [CrossRef]
- Fan, H.; Xia, H.; Kong, C.; Chen, L. Synthesis of thin amine-functionalized MIL-53 membrane with high hydrogen permeability. Int. J. Hydrog. Energy 2013, 38, 10795–10801. [Google Scholar] [CrossRef]
- Mohd Sidek, H.B.; Jo, Y.K.; Kim, I.Y.; Hwang, S.J. Stabilization of layered double oxide in hybrid matrix of graphene and layered metal oxide nanosheets: An effective way to explore efficient CO2 adsorbent. J. Phys. Chem. C 2016, 120, 23421–23429. [Google Scholar] [CrossRef]
- Hashemifard, S.A.; Ismail, A.F.; Matsuura, T. Mixed matrix membrane incorporated with large pore size halloysite nanotubes (HNTs) as filler for gas separation: Morphological diagram. Chem. Eng. J. 2011, 172, 581–590. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Nguyen, H.N.; Rodrigues, D.F.; Advincula, R.C. Electrospinning superhydrophobic and antibacterial PS/MWNT nanofibers onto multilayer gas barrier films. Macromol. Symp. 2017, 374, 1600138. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Wang, C.; Chang, H.-C.C.; Guo, R. Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J. Memb. Sci. 2020, 598, 117794. [Google Scholar] [CrossRef]
- Mozafari, M.; Rahimpour, A.; Abedini, R. Exploiting the effects of zirconium-based metal organic framework decorated carbon nanofibers to improve CO2/CH4 separation performance of thin film nanocomposite membranes. J. Ind. Eng. Chem. 2020, 85, 102–110. [Google Scholar] [CrossRef]
- You, J.; Won, S.; Jin, H.J.; Soo, Y.; Jae, J.; Yun, Y.S.; Wie, J.J. Nano-patching defects of reduced graphene oxide by cellulose nanocrystals in scalable polymer nanocomposites. Carbon 2020, 165, 18–25. [Google Scholar] [CrossRef]
- Araki, J. Electrostatic or steric?-preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter. 2013, 9, 4125–4141. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Hybridization of MWCNTs and reduced graphene oxide on random and electrically aligned nanocomposite membrane for selective separation of O2/N2 gas pair. J. Mater. Sci. 2018, 53, 15442–15464. [Google Scholar] [CrossRef]
- Dabbaghianamiri, M.; Beall, G.W. Self-assembling nanostructured intercalates: Via ion-dipole bonding. Dalt. Trans. 2018, 47, 3178–3184. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.C.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Thin film nanocomposite embedded with polymethyl methacrylate modified multi-walled carbon nanotubes for CO2 removal. RSC Adv. 2015, 5, 31683–31690. [Google Scholar] [CrossRef]
- Kim, E.S.; Deng, B. Fabrication of polyamide thin-film nano-composite (PA-TFN) membrane with hydrophilized ordered mesoporous carbon (H-OMC) for water purifications. J. Memb. Sci. 2011, 375, 46–54. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Chen, J.Y.; Twu, Y.K.; Chen, W.J. The effect of pH and ionic strength on the dispersion of carbon nanotubes in poly(acrylic acid) solutions. Polym. Int. 2012, 61, 554–559. [Google Scholar] [CrossRef]
- Khazaee, M.; Xia, W.; Lackner, G.; Mendes, R.G.; Rummeli, M.; Muhler, M.; Lupascu, D.C. Dispersibility of vapor phase oxygen and nitrogen functionalized multi-walled carbon nanotubes in various organic solvents. Sci. Rep. 2016, 6, 26208. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Sun, D.; Kang, S.; Liu, C.; Lu, Q.; Cui, L.; Hu, B. Effect of zeta potential and particle size on the stability of SiO2 nanospheres as carrier for ultrasound imaging contrast agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Yoo, M.J.; Kim, H.W.; Yoo, B.M.; Park, H.B. Highly soluble polyetheramine-functionalized graphene oxide and reduced graphene oxide both in aqueous and non-aqueous solvents. Carbon 2014, 75, 149–160. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhang, Z.; Yang, W.C. High-yield exfoliation of graphene using ternary-solvent strategy for detecting volatile organic compounds. Appl. Surf. Sci. 2016, 360, 323–328. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef]
- Asgari, M.; Sundararaj, U. Silane functionalization of sodium montmorillonite nanoclay: The effect of dispersing media on intercalation and chemical grafting. Appl. Clay Sci. 2018, 153, 228–238. [Google Scholar] [CrossRef]
- Gårdebjer, S.; Andersson, M.; Engström, J.; Restorp, P.; Persson, M.; Larsson, A. Using Hansen solubility parameters to predict the dispersion of nano-particles in polymeric films. Polym. Chem. 2016, 7, 1756–1764. [Google Scholar] [CrossRef]
- Chong, C.Y.; Lau, W.J.; Yusof, N.; Lai, G.S.; Ismail, A.F. Roles of nanomaterial structure and surface coating on thin film nanocomposite membranes for enhanced desalination. Compos. Part B Eng. 2019, 160, 471–479. [Google Scholar] [CrossRef]
- Ng, Z.C.; Chong, C.Y.; Lau, W.J.; Karaman, M.; Ismail, A.F. Boron removal and antifouling properties of thin-film nanocomposite membrane incorporating PECVD-modified titanate nanotubes. J. Chem. Technol. Biotechnol. 2019, 94, 2772–2782. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Gas separation performance of thin film nanocomposite membranes incorporated with polymethyl methacrylate grafted multi-walled carbon nanotubes. Int. Biodeterior. Biodegrad. 2015, 102, 339–345. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Zhou, T.; Chen, C.; Zhong, F.; Xia, Y.; Xie, P.; Zhang, C. Synergistic functionalization of h-BN by mechanical exfoliation and PEI chemical modification for enhancing the corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2020, 142, 105541. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Zhao, H.; Jiang, Y.; Liu, G.; Gao, J. Enhanced dispersibility of metal-organic frameworks (mofs) in the organic phase: Via surface modification for tfn nanofiltration membrane preparation. RSC Adv. 2020, 10, 4045–4057. [Google Scholar] [CrossRef] [Green Version]
- Benko, A.; Duch, J.; Gajewska, M.; Marzec, M.; Bernasik, A.; Nocuń, M.; Piskorz, W.; Kotarba, A. Covalently bonded surface functional groups on carbon nanotubes: From molecular modeling to practical applications. Nanoscale 2021, 13, 10152–10166. [Google Scholar] [CrossRef]
- Katayama, Y.; Bentz, K.C.; Cohen, S.M. Defect-free MOF-based mixed-matrix membranes obtained by corona cross-linking. ACS Appl. Mater. Interfaces 2019, 11, 13029–13037. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Xu, J.Z.; Liu, X.Y.; Zhong, G.J.; Li, Z.M. Role of surface chemical groups on carbon nanotubes in nucleation for polymer crystallization: Interfacial interaction and steric effect. Polymer 2013, 54, 6479–6488. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Yang, W.; Xiao, C.; Wei, M. Effects of different amine-functionalized graphene on the mechanical, thermal, and tribological properties of polyimide nanocomposites synthesized by in situ polymerization. Polymer 2018, 140, 56–72. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Chen, C.; Zhong, F.; Li, H.; Chen, J.; Zhou, T. Non-covalently functionalized boron nitride by graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124337. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, X.; Shen, Y. xiao Cation-dependent structural instability of graphene oxide membranes and its effect on membrane separation performance. Desalination 2016, 399, 40–46. [Google Scholar] [CrossRef]
- Alpatova, A.L.; Shan, W.; Babica, P.; Upham, B.L.; Rogensues, A.R.; Masten, S.J.; Drown, E.; Mohanty, A.K.; Alocilja, E.C.; Tarabara, V.V. Single-walled carbon nanotubes dispersed in aqueous media via non-covalent functionalization: Effect of dispersant on the stability, cytotoxicity, and epigenetic toxicity of nanotube suspensions. Water Res. 2010, 44, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Hussein, O.A.; Habib, K.; Saidur, R.; Muhsan, A.S.; Shahabuddin, S.; Alawi, O.A. The influence of covalent and non-covalent functionalization of GNP based nanofluids on its thermophysical, rheological and suspension stability properties. RSC Adv. 2019, 9, 38576–38589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Quan, L.; Xu, L. Effects of amino-functionalized carbon nanotubes on the crystal structure and thermal properties of polyacrylonitrile homopolymer microspheres. Polymers 2017, 9, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Dong, S.; Wang, Z.; Xu, J.; Xu, R.; Wang, J. Preparation of mixed matrix composite membrane for hydrogen purification by incorporating ZIF-8 nanoparticles modified with tannic acid. Int. J. Hydrog. Energy 2020, 45, 7444–7454. [Google Scholar] [CrossRef]
- Butler, E.L.; Petit, C.; Livingston, A.G. Poly(piperazine trimesamide) thin film nanocomposite membrane formation based on MIL-101: Filler aggregation and interfacial polymerization dynamics. J. Memb. Sci. 2020, 596, 117482. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Investigation on nanocomposite membrane of multiwalled carbon nanotube reinforced polycarbonate blend for gas separation. J. Nanomater. 2016, 2016, 7089530. [Google Scholar] [CrossRef]
- Swapna, V.P.; Nambissan, P.M.G.; Thomas, S.P.; Vayyaprontavida Kaliyathan, A.; Jose, T.; George, S.C.; Thomas, S.; Stephen, R. Free volume defects and transport properties of mechanically stable polyhedral oligomeric silsesquioxane embedded poly(vinyl alcohol)-poly(ethylene oxide) blend membranes. Polym. Int. 2019, 68, 1280–1291. [Google Scholar] [CrossRef]
- Quan, S.; Li, S.W.; Xiao, Y.C.; Shao, L. CO2-selective mixed matrix membranes (MMMs) containing graphene oxide (GO) for enhancing sustainable CO2 capture. Int. J. Greenh. Gas Control 2017, 56, 22–29. [Google Scholar] [CrossRef]
- Habib, N.; Shamair, Z.; Tara, N.; Nizami, A.-S.; Akhtar, F.H.; Ahmad, N.M.; Gilani, M.A.; Bilad, M.R.; Khan, A.L. Development of highly permeable and selective mixed matrix membranes based on Pebax® 1657 and NOTT-300 for CO2 capture. Sep. Purif. Technol. 2020, 234, 116101. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Uddin, F. Modified Bruggeman models for prediction of CO2 permeance in polycarbonate/silica nanocomposite membranes. Can. J. Chem. Eng. 2017, 95, 2398–2409. [Google Scholar] [CrossRef]
- Rigotti, D.; Checchetto, R.; Tarter, S.; Caretti, D.; Rizzuto, M.; Fambri, L.; Pegoretti, A. Polylactic acid-lauryl functionalized nanocellulose nanocomposites: Microstructural, thermo-mechanical and gas transport properties. Express Polym. Lett. 2019, 13, 858–876. [Google Scholar] [CrossRef]
- Silva-Leyton, R.; Quijada, R.; Bastías, R.; Zamora, N.; Olate-Moya, F.; Palza, H. Polyethylene/graphene oxide composites toward multifunctional active packaging films. Compos. Sci. Technol. 2019, 184, 107888. [Google Scholar] [CrossRef]
- Ren, X.; Kanezashi, M.; Nagasawa, H.; Xu, R.; Zhong, J.; Tsuru, T. Ceramic-supported polyhedral oligomeric silsesquioxane-organosilica nanocomposite membrane for efficient gas separation. Ind. Eng. Chem. Res. 2019, 58, 21708–21716. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quemener, D. MOF-mixed matrix membranes: Precise dispersion of MOF particles with better compatibility via a particle fusion approach for enhanced gas separation properties. J. Memb. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Zhang, X.F.; Feng, Y.; Wang, Z.; Jia, M.; Yao, J. Fabrication of cellulose nanofibrils/UiO-66-NH2 composite membrane for CO2/N2 separation. J. Memb. Sci. 2018, 568, 10–16. [Google Scholar] [CrossRef]
- Casanova, S.; Liu, T.Y.; Chew, Y.M.J.; Livingston, A.; Mattia, D. High flux thin-film nanocomposites with embedded boron nitride nanotubes for nanofiltration. J. Memb. Sci. 2020, 597, 117749. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Sanaeepur, H.; Omidkhah, M.; Kargari, A. “Ship-in-a-bottle”, a new synthesis strategy for preparing novel hybrid host-guest nanocomposites for highly selective membrane gas separation. J. Mater. Chem. A 2018, 6, 1751–1771. [Google Scholar] [CrossRef]
- Ahmadizadegan, H.; Esmaielzadeh, S. Preparation and application of novel bionanocomposite green membranes for gas separation. Polym. Bull. 2019, 76, 4903–4927. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Highly permeable and selective graphene oxide-enabled thin film nanocomposite for carbon dioxide separation. Int. J. Greenh. Gas Control 2017, 64, 257–266. [Google Scholar] [CrossRef]
- Ahmadizadegan, H.; Esmaielzadeh, S. Novel polyester/SiO2 nanocomposite membranes: Synthesis, properties and morphological studies. Solid State Sci. 2018, 80, 81–91. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Gurr, P.A.; Tan, S.S.L.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrathin metal–organic framework nanosheets as a gutter layer for flexible composite gas separation membranes. ACS Nano 2018, 12, 11591–11599. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, P.; Duan, S.; She, H.; Huang, J.; Wang, Q. In-situ incorporation of copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci. Bull. 2019, 64, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Dalod, A.R.M.; Grendal, O.G.; Skjærvø, S.L.; Inzani, K.; Selbach, S.M.; Henriksen, L.; van Beek, W.; Grande, T.; Einarsrud, M.-A. Controlling oriented attachment and in situ functionalization of TiO2 nanoparticles during hydrothermal synthesis with APTES. J. Phys. Chem. C 2017, 121, 11897–11906. [Google Scholar] [CrossRef]
- Drohmann, C.; Beckman, E.J. Phase behavior of polymers containing ether groups in carbon dioxide. J. Supercrit. Fluids 2002, 22, 103–110. [Google Scholar] [CrossRef]
- Lee, B.-S. Effect of specific interaction of CO2 with poly(ethylene glycol) on phase behavior. J. CO2 Util. 2018, 28, 228–234. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Memb. Sci. 2019, 570–571, 343–354. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, J.; Liu, C.; Gao, Y.; Li, N.; Xie, Z. Enhancing the CO2 separation performance of SPEEK membranes by incorporation of polyaniline-decorated halloysite nanotubes. J. Memb. Sci. 2019, 573, 602–611. [Google Scholar] [CrossRef]
- Prasad, B.; Mandal, B. Graphene-incorporated biopolymeric mixed-matrix membrane for enhanced CO2 separation by regulating the support pore filling. ACS Appl. Mater. Interfaces 2018, 10, 27810–27820. [Google Scholar] [CrossRef]
- Qazvini, O.T.; Babarao, R.; Telfer, S.G. Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework. Nat. Commun. 2021, 12, 197. [Google Scholar] [CrossRef]
- Ansaloni, L.; Zhao, Y.; Jung, B.T.; Ramasubramanian, K.; Baschetti, M.G.; Ho, W.S.W. Facilitated transport membranes containing amino-functionalized multi-walled carbon nanotubes for high-pressure CO2 separations. J. Memb. Sci. 2015, 490, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Isfahani, A.P.; Muchtar, A.; Sakurai, K.; Shrestha, B.B.; Qin, D.; Yamaguchi, D.; Sivaniah, E.; Ghalei, B. Pebax/ionic liquid modified graphene oxide mixed matrix membranes for enhanced CO2 capture. J. Memb. Sci. 2018, 565, 370–379. [Google Scholar] [CrossRef]
- Nematollahi, M.H.; Babaei, S.; Abedini, R. CO2 separation over light gases for nano-composite membrane comprising modified polyurethane with SiO2 nanoparticles. Korean J. Chem. Eng. 2019, 36, 763–779. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.-V. CO2-selective PEO–PBT (PolyActiveTM)/graphene oxide composite membranes. Chem. Commun. 2015, 51, 14187–14190. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Stevens, K.A.; Park, J.S.; Moon, J.D.; Liu, Q.; Freeman, B.D.; Guo, R. Highly CO2-selective gas separation membranes based on segmented copolymers of poly(ethylene oxide) reinforced with pentiptycene-containing polyimide hard segments. ACS Appl. Mater. Interfaces 2016, 8, 2306–2317. [Google Scholar] [CrossRef]
- Kargari, A.; Rezaeinia, S. State-of-the-art modification of polymeric membranes by PEO and PEG for carbon dioxide separation: A review of the current status and future perspectives. J. Ind. Eng. Chem. 2020, 84, 1–22. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 capture by functionalized graphene oxide nanosheets as fillers to fabricate multi-permselective mixed matrix membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Jie, X.; Liu, D.; Cao, Y. Improved interfacial affinity and CO2 separation performance of asymmetric mixed matrix membranes by incorporating postmodified MIL-53(Al). ACS Appl. Mater. Interfaces 2016, 8, 22696–22704. [Google Scholar] [CrossRef]
- Zheng, B.; Bai, J.; Duan, J.; Wojtas, L.; Zaworotko, M.J. Enhanced CO2 binding affinity of a high-uptake rht-type metal−organic framework decorated with acylamide groups. J. Am. Chem. Soc. 2011, 133, 748–751. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Effect of the ZIF-8 distribution in mixed-matrix membranes based on Matrimid® 5218-PEG on CO2 separation. Chem. Eng. Technol. 2019, 42, 744–752. [Google Scholar] [CrossRef]
- Iskra, A.; Gentleman, A.S.; Cunningham, E.M.; Mackenzie, S.R. Carbon dioxide binding to metal oxides: Infrared spectroscopy of NbO2+(CO2)n and TaO2+(CO2)n complexes. Int. J. Mass Spectrom. 2019, 435, 93–100. [Google Scholar] [CrossRef]
- Sheng, D.; Zhang, Y.; Han, Y.; Xu, G.; Song, Q.; Hu, Y.; Liu, X.; Shan, D.; Cheng, A. A zinc(ii) metal-organic framework with high affinity for CO2 based on triazole and tetrazolyl benzene carboxylic acid. CrystEngComm 2019, 21, 3679–3685. [Google Scholar] [CrossRef]
- Zhang, N.; Peng, D.; Wu, H.; Ren, Y.; Yang, L.; Wu, X.; Wu, Y.; Qu, Z.; Jiang, Z.; Cao, X. Significantly enhanced CO2 capture properties by synergy of zinc ion and sulfonate in Pebax-pitch hybrid membranes. J. Memb. Sci. 2018, 549, 670–679. [Google Scholar] [CrossRef]
- Saeedi Dehaghani, A.H.; Pirouzfar, V.; Alihosseini, A. Novel nanocomposite membranes-derived poly(4-methyl-1-pentene)/functionalized titanium dioxide to improve the gases transport properties and separation performance. Polym. Bull. 2020, 77, 6467–6489. [Google Scholar] [CrossRef]
- Li, S.; Zeng, X.; Chen, H.; Fang, W.; He, X.; Li, W.; Huang, Z.H.; Zhao, L. Porous hexagonal boron nitride nanosheets from g-C3N4 templates with a high specific surface area for CO2 adsorption. Ceram. Int. 2020, 46, 27627–27633. [Google Scholar] [CrossRef]
- Peng, D.; Wang, S.; Tian, Z.; Wu, X.; Wu, Y.; Wu, H.; Xin, Q.; Chen, J.; Cao, X.; Jiang, Z. Facilitated transport membranes by incorporating graphene nanosheets with high zinc ion loading for enhanced CO2 separation. J. Memb. Sci. 2017, 522, 351–362. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158–159, 20–29. [Google Scholar] [CrossRef]
- Torralba-Calleja, E.; Skinner, J.; Gutiérrez-Tauste, D. CO2 capture in ionic liquids: A review of solubilities and experimental methods. J. Chem. 2013, 2013, 473584. [Google Scholar] [CrossRef] [Green Version]
- Taheri, M.; Zhu, R.; Yu, G.; Lei, Z. Ionic liquid screening for CO2 capture and H2S removal from gases: The syngas purification case. Chem. Eng. Sci. 2021, 230, 116199. [Google Scholar] [CrossRef]
- Seon Bang, H.; Jang, S.; Soo Kang, Y.; Won, J. Dual facilitated transport of CO2 using electrospun composite membranes containing Ionic liquid. J. Memb. Sci. 2015, 479, 77–84. [Google Scholar] [CrossRef]
- Sang, Y.; Huang, J. Benzimidazole-based hyper-cross-linked poly(ionic liquid)s for efficient CO2 capture and conversion. Chem. Eng. J. 2020, 385, 123973. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Pyrrolidinium-based polymeric ionic liquid materials: New perspectives for CO2 separation membranes. J. Memb. Sci. 2013, 428, 260–266. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Zeng, S.; Bai, L.; Gao, H.; Deng, J.; Yang, Q.; Zhang, S. Pebax-based composite membranes with high gas transport properties enhanced by ionic liquids for CO2 separation. RSC Adv. 2017, 7, 6422–6431. [Google Scholar] [CrossRef] [Green Version]
- Carlisle, T.K.; Bara, J.E.; Lafrate, A.L.; Gin, D.L.; Noble, R.D. Main-chain imidazolium polymer membranes for CO2 separations: An initial study of a new ionic liquid-inspired platform. J. Memb. Sci. 2010, 359, 37–43. [Google Scholar] [CrossRef]
- Chae, I.S.; Hong, G.H.; Song, D.; Kang, Y.S.; Kang, S.W. Enhanced olefin and CO2 permeance through mesopore-confined ionic liquid membrane. Macromol. Res. 2019, 27, 250–254. [Google Scholar] [CrossRef]
- De Clippel, F.; Khan, A.L.; Cano-Odena, A.; Dusselier, M.; Vanherck, K.; Peng, L.; Oswald, S.; Giebeler, L.; Corthals, S.; Kenens, B.; et al. CO2 reverse selective mixed matrix membranes for H2 purification by incorporation of carbon-silica fillers. J. Mater. Chem. A. 2013, 1, 945–953. [Google Scholar] [CrossRef]
- Abedini, R.; Omidkhah, M.; Dorosti, F. Hydrogen separation and purification with poly (4-methyl-1-pentyne)/MIL 53 mixed matrix membrane based on reverse selectivity. Int. J. Hydrog. Energy 2014, 39, 7897–7909. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Enhancing hydrogen gas separation performance of thin film composite membrane through facilely blended polyvinyl alcohol and PEBAX. Int. J. Hydrog. Energy 2021, 46, 19737–19748. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, X.; Wei, Z.; Xie, Q.; Zhang, Y.; Tan, X.; Hongyong, Z.; Xiaoli, D.; Zhengang, W.E.I.; Qian, X.I.E. H2/CO2 gas transport performance in poly (ethylene oxide) reverse-selective membrane with star-like structures. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 195–200. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S. Polycarbonate/silica nanocomposite membranes: Fabrication, characterization, and performance evaluation. J. Appl. Polym. Sci. 2017, 134, 45310. [Google Scholar] [CrossRef]
- Khalilinejad, I.; Kargari, A.; Sanaeepur, H. Preparation and characterization of (Pebax 1657 + silica nanoparticle)/PVC mixed matrix composite membrane for CO2/N2 separation. Chem. Pap. 2017, 71, 803–818. [Google Scholar] [CrossRef]
- Borandeh, S.; Abdolmaleki, A.; Zamani nekuabadi, S.; Sadeghi, M. Poly(vinyl alcohol)/methoxy poly(ethylene glycol) methacrylate-TiO2 nanocomposite as a novel polymeric membrane for enhanced gas separation. J. Iran. Chem. Soc. 2019, 16, 523–533. [Google Scholar] [CrossRef]
- Salahshoori, I.; Nasirian, D.; Rashidi, N.; Hossain, M.K.; Hatami, A.; Hassanzadeganroudsari, M. The effect of silica nanoparticles on polysulfone–polyethylene glycol (PSF/PEG) composite membrane on gas separation and rheological properties of nanocomposites. Polym. Bull. 2021, 78, 3227–3258. [Google Scholar] [CrossRef]
- Azizi, N.; Arzani, M.; Mahdavi, H.R.; Mohammadi, T. Synthesis and characterization of poly(ether-block-amide) copolymers/multi-walled carbon nanotube nanocomposite membranes for CO2/CH4 separation. Korean J. Chem. Eng. 2017, 34, 2459–2470. [Google Scholar] [CrossRef]
- Park, H.J.; Bhatti, U.H.; Nam, S.C.; Park, S.Y.; Lee, K.B.; Baek, I.H. Nafion/TiO2 nanoparticle decorated thin film composite hollow fiber membrane for efficient removal of SO2 gas. Sep. Purif. Technol. 2019, 211, 377–390. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, S.; Weidman, J.R.; Guo, R. Preparation and gas separation performance of mixed-matrix membranes based on triptycene-containing polyimide and zeolite imidazole framework (ZIF-90). Polymer 2017, 131, 209–216. [Google Scholar] [CrossRef]
- Zhou, B.; Li, C.Y.; Qi, N.; Jiang, M.; Wang, B.; Chen, Z.Q. Pore structure of mesoporous silica (KIT-6) synthesized at different temperatures using positron as a nondestructive probe. Appl. Surf. Sci. 2018, 450, 31–37. [Google Scholar] [CrossRef]
- Abate, S.; Genovese, C.; Perathoner, S.; Centi, G. Pd-Ag thin film membrane for H2 separation. Part 2. Carbon and oxygen diffusion in the presence of CO/CO2 in the feed and effect on the H2 permeability. Int. J. Hydrog. Energy 2010, 35, 5400–5409. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Speight, J.D.; Book, D. Hydrogen separation from blended natural gas and hydrogen by Pd-based membranes. Int. J. Hydrog. Energy 2019, 44, 29092–29099. [Google Scholar] [CrossRef]
- Patel, A.K.; Acharya, N.K. Metal coated and nanofiller doped polycarbonate membrane for hydrogen transport. Int. J. Hydrog. Energy 2018, 43, 21675–21682. [Google Scholar] [CrossRef]
- Czyperek, M.; Zapp, P.; Bouwmeester, H.J.M.; Modigell, M.; Ebert, K.; Voigt, I.; Meulenberg, W.A.; Singheiser, L.; Stöver, D. Gas separation membranes for zero-emission fossil power plants: MEM-BRAIN. J. Memb. Sci. 2010, 359, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Sadykov, V.A.; Krasnov, A.V.; Fedorova, Y.E.; Lukashevich, A.I.; Bespalko, Y.N.; Eremeev, N.F.; Skriabin, P.I.; Valeev, K.R.; Smorygo, O.L. Novel nanocomposite materials for oxygen and hydrogen separation membranes. Int. J. Hydrog. Energy 2020, 45, 13575–13585. [Google Scholar] [CrossRef]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Synergistic effect of incorporating ZIF-302 and graphene oxide to polysulfone to develop highly selective mixed-matrix membranes for carbon dioxide separation from wet post-combustion flue gases. J. Ind. Eng. Chem. 2016, 36, 154–162. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, C.; Zhao, L.; Li, Y.; Xiang, D. Synergistic effect of α-ZrP and graphene oxide nanofillers on the gas barrier properties of PVA films. J. Appl. Polym. Sci. 2018, 135, 46455. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, L.; Wang, X.; Liu, T. Graphene oxide-assisted dispersion of pristine multiwalled carbon nanotubes in aqueous media. J. Phys. Chem. C 2010, 114, 11435–11440. [Google Scholar] [CrossRef]
- Huang, D.; Xin, Q.; Ni, Y.; Shuai, Y.; Wang, S.; Li, Y.; Ye, H.; Lin, L.; Ding, X.; Zhang, Y. Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation. RSC Adv. 2018, 8, 6099–6109. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, P.J.; Álvarez, V.H.; Marrucho, I.M.; Aznar, M.; Coutinho, J.A.P. High carbon dioxide solubilities in trihexyltetradecylphosphonium-based ionic liquids. J. Supercrit. Fluids 2010, 52, 258–265. [Google Scholar] [CrossRef]
- Voskian, S.; Brown, P.; Halliday, C.; Rajczykowski, K.; Hatton, T.A. Amine-based ionic liquid for CO2 capture and electrochemical or thermal regeneration. ACS Sustain. Chem. Eng. 2020, 8, 8356–8361. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Zhang, Y.; Chen, W.; Wang, Q.; Li, S.; Zhang, S.; Li, W. Sustainable ionic liquid organic solution with efficient recyclability and low regeneration energy consumption for CO2 capture. Sep. Purif. Technol. 2021, 275, 119123. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Sun, C.; Chen, L.; Wang, Q.; Li, S.; Li, W. Novel amino-functionalized ionic liquid/organic solvent with low viscosity for CO2 capture. Environ. Sci. Technol. 2020, 54, 3520–3529. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Song, Z.; Zhou, T. Data-driven ionic liquid design for CO2 capture: Molecular structure optimization and DFT verification. Ind. Eng. Chem. Res. 2021, 60, 9992–10000. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, J.; Yoo, H.; Lee, Y. Machine learning-based approach for tailor-made design of ionic liquids: Application to CO2 capture. Sep. Purif. Technol. 2021, 275, 119117. [Google Scholar] [CrossRef]
- Wang, J.; Song, Z.; Cheng, H.; Chen, L.; Deng, L.; Qi, Z. Multilevel screening of ionic liquid absorbents for simultaneous removal of CO2 and H2S from natural gas. Sep. Purif. Technol. 2020, 248, 117053. [Google Scholar] [CrossRef]
- Jimenez-Solomon, M.F.; Song, Q.; Jelfs, K.E.; Munoz-Ibanez, M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–767. [Google Scholar] [CrossRef] [Green Version]
- Amini, Z.; Asghari, M. Preparation and characterization of ultra-thin poly ether block amide/nanoclay nanocomposite membrane for gas separation. Appl. Clay Sci. 2018, 166, 230–241. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Agrawal, K.V.; Coronas, J. Ultrathin permselective membranes: The latent way for efficient gas separation. RSC Adv. 2020, 10, 12653–12670. [Google Scholar] [CrossRef]
- Wang, M.; Guo, W.; Jiang, Z.; Pan, F. Reducing active layer thickness of polyamide composite membranes using a covalent organic framework interlayer in interfacial polymerization. Chin. J. Chem. Eng. 2020, 28, 1039–1045. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhao, S.; Wang, J.; Wang, S.; Wang, Z.; Guiver, M.D. A highly permeable aligned montmorillonite mixed-matrix membrane for CO2 separation. Angew. Chem. -Int. Ed. 2016, 55, 9321–9325. [Google Scholar] [CrossRef]
- Gao, H.; Bai, L.; Han, J.; Yang, B.; Zhang, S.; Zhang, X. Functionalized ionic liquid membranes for CO2 separation. Chem. Commun. 2018, 54, 12671–12685. [Google Scholar] [CrossRef]


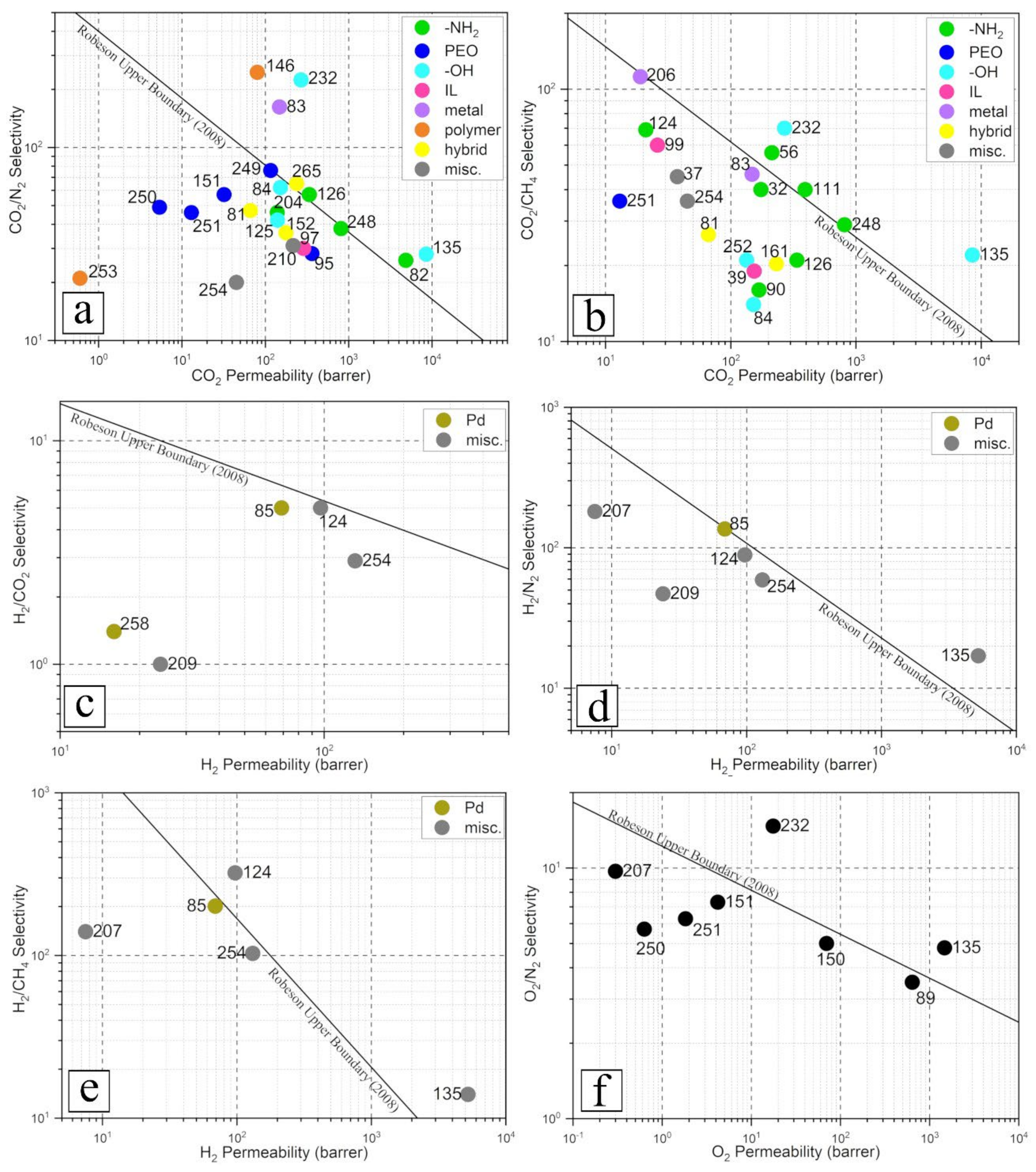
| Base Polymer | Filler (Loading) | Modification | Test Conditions | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | Pt-Pd (surface coating) | metal dope | 2 bar, 35 °C pure gas | 16 | 21 * | 1.4 | - | - | −8 * | - | - | [258] |
| Matrimid | Pd@ZIF-8 (20 wt.%) | encapsulation of Pd in ZIF-8 cage | 5 bar, 25 °C pure gas | 69 | 140 * 54 ϯ | 5 | 136 | 201 | 73 * 52 ϯ | 47 * 56 ϯ | 62 * 59 ϯ | [85] |
| 6FDA-TP | ZIF-90 (40 wt.%) | condensation polymerization of 6FDA with TP | 9.8 bar, 35 °C pure gas | 131 | 122 * | 2.9 | 59 | 103 | −3.3 * | −1.7 * | −8.8 * | [254] |
| PE | GOTMS-SiO2 (10 wt.%) | GOTMS as coupling agent | 4 bar, 25 °C pure gas | 24 | 23 * | 1 | 47 | - | −5 * | −11 * | - | [209] |
| XTR-PI | Am-BN (1 wt.%) | ball-mill with urea | -bar, 25 °C pure gas | 97 | −56 * | 5 | 89 | 322 | 364 * | 365 * | 1210 * | [124] |
| PI | MPS-TiO2 (20 wt.%) | grafting of TiO2 | 3.5 bar, 35 °C pure gas | 7.5 | 102 * | - | 181 | 140 | - | 3 * | −42 * | [207] |
| PIM | OH-pDCX (5 wt.%) | hydroxylation via Friedel-Crafts reaction | 2 bar, 25 °C pure gas | 5230 | 16 * | - | 17 | 14 | - | 25 * | 27 * | [135] |
| Base Polymer | Filler (Loading) | Modification | Test Conditions | Ref | ||||
|---|---|---|---|---|---|---|---|---|
| PDMS | PDMS-SiO2 (10 wt.%) | priming SiO2 with host polymer | 2 bar, r.t. pure gas | 640 | 8 * | 3.5 | 42 * | [89] |
| PIM | OH-pDCX (5 wt.%) | hydroxylation via Friedel-Crafts reaction | 2 bar, 25 °C pure gas | 1470 | 13 * | 4.8 | 20 * | [135] |
| PSF | GOTMS-SiO2 (20 wt.%) | adsorption | 10 bar, 30 °C pure gas | 1.8 | 43 * | 6.3 | 17 * | [251] |
| PVA | MPEG-TiO2 (3 wt.%) | grafting of MPEG via radical polymerization | 10 bar, 35 °C pure gas | 0.63 | 2000 * | 5.7 | 72 * | [250] |
| PMMA | MPEG-TiO2 (5 wt.%) | grafting of MPEG via radical polymerization | 10 bar, 35 °C pure gas | 4.2 | 1508 * | 7.3 | 98 * | [151] |
| PMP | hydrolyzed TNT (2 wt.%) | treatment of TNT with strong base | 2 bar, 25 °C pure gas | 17.6 | 418 * | 14.7 | 143 * | [232] |
| PI | MPS-TiO2 (20 wt.%) | grafting of TiO2 | 3.5 bar, 35 °C pure gas | 0.3 | 81 * | 9.7 | −7.6 * | [207] |
| Base Polymer (Filler Loading) | Base Filler | Secondary Filler (Method) | Test Conditions | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PHS/PPS (10 wt.%) | zeolite | CNT (mixing under reflux) | 0.68 bar, 27 °C pure gas | 177 | 10 ϯ | 36.1 | - | 10 ϯ | - | [152] |
| Matrimid-5218 (20 wt.%) | ZIF-8 | GO (in situ ZIF-8 growth with GO) | 1 bar, 30 °C pure gas | 238 | 358 * 34 ϯ | 65 | - | 80 * 55 ϯ | [265] | |
| PMP/PEBAX-1657 (3 wt.%) | carboxylated CNF | UiO-66-NH2 (in situ growth) | 6 bar, 25 °C CO2:CH4 = 50:50 vol. ratio | 232 | 31 ϯ | - | 20 | - | 93 ϯ | [161] |
| PA (0.25 mg/mL) | ACNT | GO (mixing) | 6 bar, 30 °C pure gas | 66.3 | 23 * 20 ϯ | 47.1 | 26.5 | 39 * 23 ϯ | 35 * 19 ϯ | [81] |
| Base Polymer (filler loading) | Base Filler | Secondary Filler (method) | Test Conditions | Ref | ||||||
| PSF (0.1 wt.%) | GO-NH4+ | mSiO2 | 1 bar, 5–50 °C pure gas | 70 | 1406 * 801 ϯ | 5 | 115 * 33 ϯ | [150] | ||
| PSF | MWCNT | rGO | 1 bar, 15–45 °C pure gas | 722 | 45 ϯ | 2.4 | 34 ϯ | [164] | ||
| Base Polymer (filler loading) | Base Filler | Secondary Filler (method) | Test Conditions | Ref | ||||||
| PVDF (40 wt.%) | zeolite 4A | Cu nanosheet (growth of Cu shell on zeolite core) | 1 bar, 25 °C feed gas: SO2 liquid sorbent: NaOH (30 L/h) | 9 × 10−4 | 107 * | 74 | 124 * | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.C.; Goh, P.S.; Ismail, A.F.; Kang, H.S.; Guo, Q.; Jiang, X.; Ma, J. The State-of-the-Art Functionalized Nanomaterials for Carbon Dioxide Separation Membrane. Membranes 2022, 12, 186. https://doi.org/10.3390/membranes12020186
Wong KC, Goh PS, Ismail AF, Kang HS, Guo Q, Jiang X, Ma J. The State-of-the-Art Functionalized Nanomaterials for Carbon Dioxide Separation Membrane. Membranes. 2022; 12(2):186. https://doi.org/10.3390/membranes12020186
Chicago/Turabian StyleWong, Kar Chun, Pei Sean Goh, Ahmad Fauzi Ismail, Hooi Siang Kang, Qingjie Guo, Xiaoxia Jiang, and Jingjing Ma. 2022. "The State-of-the-Art Functionalized Nanomaterials for Carbon Dioxide Separation Membrane" Membranes 12, no. 2: 186. https://doi.org/10.3390/membranes12020186
APA StyleWong, K. C., Goh, P. S., Ismail, A. F., Kang, H. S., Guo, Q., Jiang, X., & Ma, J. (2022). The State-of-the-Art Functionalized Nanomaterials for Carbon Dioxide Separation Membrane. Membranes, 12(2), 186. https://doi.org/10.3390/membranes12020186









