A Novel Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
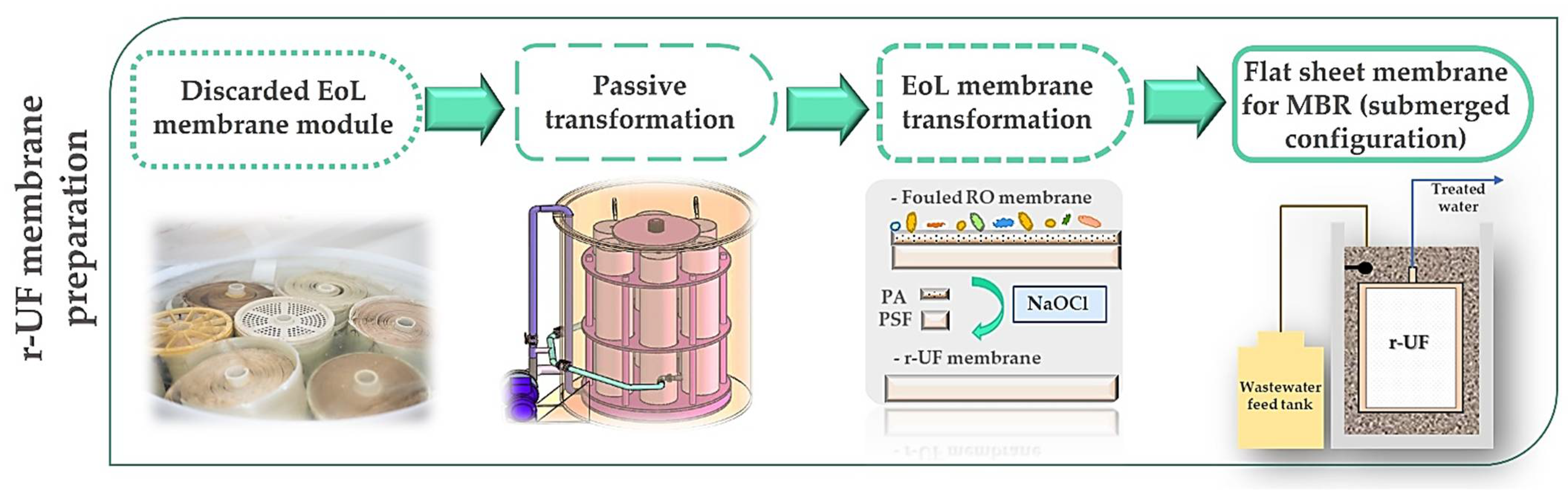
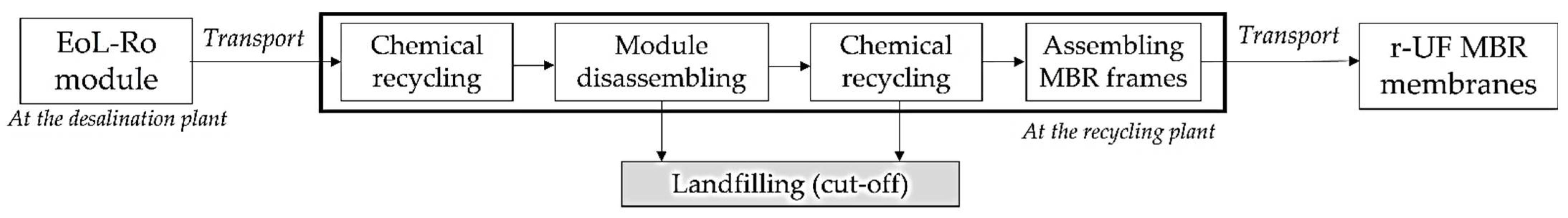
2.2. Membranes: Description and Characterization
2.3. Experimental Set-Up
2.4. Membrane Fouling Analysis
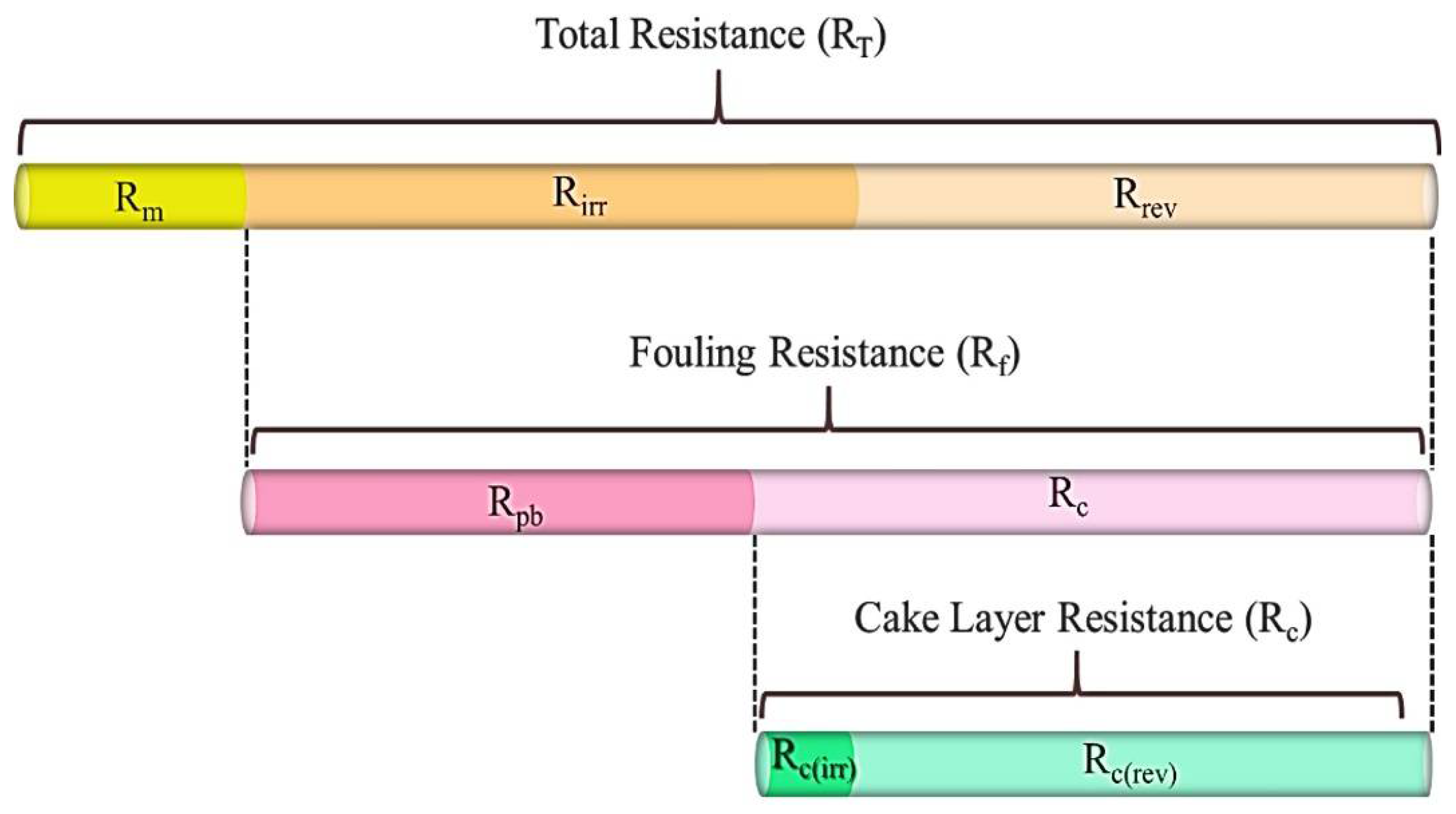
Membrane Resistance Analysis
2.5. Preliminary Economic Assessment
3. Results and Discussions
3.1. Characterization of the Studied Membranes: Permeability and Pore Size
3.2. Performance Efficiency of the Lab-Scale aMBR Unit
3.2.1. Permeate Quality
3.2.2. Membrane Permeability Stability and Preliminary Fouling Assessment
3.2.3. Evaluation of Fouling Mechanisms
3.3. Critical Factors Affecting Economic Sustainability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aMBR | Aerobic membrane bioreactor | MWCO | Molecular weight cut-off |
| BOD5 | Biological oxygen demand | NF | Nanofiltration |
| CAPEX | Capital expenditure | OPEX | Operational expenditure |
| CAS | Conventional activated sludge | PA | Polyamide |
| c−MF | Commercial microfiltration | PES | Polyethersulfone |
| COD | Chemical oxygen demand | PSF | Polysulfone |
| cSWW | Concentrated synthetic wastewater | RO | Reverse osmosis |
| DO | Dissolved oxygen | r-UF | Recycled ultrafiltration |
| EoL | End of life | SEC | Specific energy consumption |
| HRT | Hydraulic retention time | SRT | Solid retention time |
| MBR | Membrane bioreactor | TN | Total nitrogen |
| MF | Microfiltration | TOC | Total organic carbon |
| MLSS | Mixed liquor suspended solids | TP | Total phosphorous |
| L | Membrane permeability at (L·m− 2·h−1·bar−1) | Rm | Membrane resistance (m−1) |
| LS | Membrane permeability at a certain time (L·m−2·h−1·bar−1) | Rpb | Pore blocking resistance (m−1) |
| J | Flux (L·m−2·h−1) | Rq | Root mean square average of height deviation (nm) |
| R | Resistance (m−1) | Rrev | Reversible resistance (m−1) |
| Ra | Arithmetic average of absolute values of surface height deviations (nm) | RT | Total resistance (m−1) |
| Rc | Cake layer resistance (m−1) | t | Time (h) |
| Rc(ir) | Cake layer irreversible resistance (m−1) | T | Temperature (°C) |
| Rc(rev) | Cake layer reversible resistance (m−1) | TMP | Transmembrane pressure (Pa) |
| Rf | Fouling resistance (m−1) | µ | Dynamic viscosity (Pa·s) |
| Rir | Irreversible resistance (m−1) |
References
- MBR Global Capacity. Available online: https://www.thembrsite.com/membrane-bioreactor-global-capacity/ (accessed on 8 November 2021).
- Membrane Bioreactor Market by Membrane Type (Hollow Fiber, Flat Sheet, Multi-tubular), System Configuration (Submerged, External), Application (Municipal Wastewater Treatment and Industrial Wastewater Treatment), Region-Global Forecast to 2024; Markets and Markets Research Private Ltd.: Chicago, IL, USA, 2020; Available online: https://www.marketsandmarkets.com/Market-Reports/membrane-bioreactor-market-484.html?gclid=EAIaIQobChMI5eTZ4aD-9QIVFeJ3Ch33BwiJEAAYASAAEgIL1vD_BwE (accessed on 8 November 2021).
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Memb. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, R.; Simón, P.; Moragas, L.; Arce, A.; Rodriguez-Roda, I. Cost comparison of full-scale water reclamation technologies with an emphasis on membrane bioreactors. Water Sci. Technol. 2017, 75, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, Z.; Zinatizadeh, A.A.; Zinadini, S. Milk processing wastewater treatment in an MBR: A comparative study on the use of two synthetic anti-fouling PES-UF membranes. J. Environ. Chem. Eng. 2019, 7, 103369. [Google Scholar] [CrossRef]
- Maneewan, P.; Sajomsang, W.; Singto, S.; Lohwacharin, J.; Suwannasilp, B.B. Fouling mitigation in an anaerobic membrane bioreactor via membrane surface modification with tannic acid and copper. Environ. Pollut. 2021, 291, 118205. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; Blanco, A.; García-Pacheco, R.; Landaburu-Aguirre, J.; García-Calvo, E. Prospective Life Cycle Assessment and economic analysis of direct recycling of end-of-life reverse osmosis membranes based on Geographic Information Systems. J. Clean. Prod. 2020, 282, 124400. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Lawler, W.; Landaburu-Aguirre, J.; García-Calvo, E.; Le-clech, P. End-of-Life membranes: Challenges and opportunities. In Comprehensive Membrane Science and Engineering, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- European Comission First Circular Economy Action Plan. Available online: https://ec.europa.eu/environment/circular-economy/ (accessed on 7 June 2021).
- García-Pacheco, R.; Gabarro, J.; Suquet, J.; Galizia, A.; Godo-Pla, L.; Molina, F.; Campos, E.; Landaburu, J.; Monclús, H.; Comas, J. Landfill leachate treatment using second-hand membranes. In 7th MSA ECR Membrane Symposium; University of Technology Sydney: Sydney, Australia, 2020; pp. 1–4. [Google Scholar]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Molina, S.; Rodríguez-Sáez, L.; Teli, S.B.; García-Calvo, E. Transformation of end-of-life RO membranes into NF and UF membranes: Evaluation of membrane performance. J. Memb. Sci. 2015, 495, 305–315. [Google Scholar] [CrossRef]
- Morón-López, J.; Nieto-Reyes, L.; Senán-Salinas, J.; Molina, S.; El-Shehawy, R. Recycled desalination membranes as a support material for biofilm development: A new approach for microcystin removal during water treatment. Sci. Total Environ. 2019, 647, 785–793. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular economy in membrane technology: Using end-of-life reverse osmosis modules for preparation of recycled anion exchange membranes and validation in electrodialysis. J. Memb. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Terrero-Rodríguez, P.; Campos, E.; Molina-Serrano, F.; Rabadán, J.; Zarzo, D.; García-Calvo, E. Validation of recycled membranes for treating brackish water at pilot scale. Desalination 2018, 433, 199–208. [Google Scholar] [CrossRef]
- Molina, S.; García Pacheco, R.; Rodríguez Sáez, L.; García-Calvo, E.; Campos Pozuelo, E.; Zarco Martínez, D.; González de la Campa, J.; De Abajo González, J. Transformation of end-of-life RO membrane into recycled NF and UF membranes, surface characterization. In Proceedings of the International Desalination Association World Congress on Desalination and Water Reuse, San Diego, CA, USA, 30 August–4 September 2015. [Google Scholar]
- Da Costa, P.R.; Alkmin, A.R.; Amaral, M.C.S.; de França Neta, L.S.; Cerqueira, A.C.; Santiago, V.M.J. Ageing effect on chlorinated polyethylene membrane of an MBR caused by chemical cleaning procedures. Desalin. Water Treat. 2015, 53, 1460–1470. [Google Scholar] [CrossRef]
- Rodríguez-Sáez, L.; Landaburu-Aguirre, J.; Molina, S.; García-Payo, M.C.; García-Calvo, E. Study of surface modification of recycled ultrafiltration membranes using statistical design of experiments. Surf. Interfaces 2021, 23, 100978. [Google Scholar] [CrossRef]
- Patsios, S.I.; Karabelas, A.J. An investigation of the long-term filtration performance of a membrane bioreactor (MBR): The role of specific organic fractions. J. Memb. Sci. 2011, 372, 102–115. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public, Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Memb. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Yin, X.; Tian, L. Membrane fouling in a submerged membrane bioreactor (MBR) under sub-critical flux operation: Membrane foulant and gel layer characterization. J. Memb. Sci. 2008, 325, 238–244. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: www.R-project.org/ (accessed on 8 November 2021).
- Di Bella, G.; Di Trapani, D.; Judd, S. Fouling mechanism elucidation in membrane bioreactors by bespoke physical cleaning. Sep. Purif. Technol. 2018, 199, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, G.; Durante, F.; Torregrossa, M.; Viviani, G.; Mercurio, P.; Cicala, A. The role of fouling mechanisms in a membrane bioreactor. Water Sci. Technol. 2007, 55, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Lawler, W.; Bradford-Hartke, Z.; Cran, M.J.; Duke, M.; Leslie, G.; Ladewig, B.P.; Le-Clech, P. Towards new opportunities for reuse, recycling and disposal of used reverse osmosis membranes. Desalination 2012, 299, 103–112. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780080465104. [Google Scholar]
- BOE MARM BOE. Spanish Water Reuse Royal Decree 1620/2007; Ministry of the Presidence: Madrid, Spain, 2007; pp. 50639–50661.
- Van der Bruggen, B. Microfiltration, ultrafiltration, nanofiltration, reverse osmosis, and forward osmosis. In Fundamental Modeling of Membrane Systems: Membrane and Process Performance; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25–70. ISBN 9780128134849. [Google Scholar]
- Molina, S.; García-Pacheco, R.; Rodríguez-Sáez, L.; García-Calvo, E.; Campos-Pozuelo, E.; Zarzo-Martínez, D.; de la Campa, J.G.; de Abajo, J. Transformation of end-of-life RO membranes into recycled NF and UF. In Proceedings of the IDA World Congress, San Diego, CA, USA, 4 September 2015. [Google Scholar]
- Adham, S.; DeCarolis, J.F. Optimization of Various MBR Systems for Water Reclamation: Phase III; U.S. Department of the Interior Bureau of Reclamation Technical Service Center: Denver, CO, USA, 2004. [Google Scholar]
- Li, S.; Chen, H.; Zhao, X.; Lucia, L.A.; Liang, C.; Liu, Y. Impact factors for flux decline in ultrafiltration of lignocellulosic hydrolysis liquor. Sep. Purif. Technol. 2020, 240, 116597. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Zulhairun, A.K.; Ismail, A.F. Antifouling property of oppositely charged titania nanosheet assembled on thin film composite reverse osmosis membrane for highly concentrated oily saline water treatment. Membranes 2020, 10, 237. [Google Scholar] [CrossRef]
- Molina, S.; Landaburu-Aguirre, J.; Rodríguez-Sáez, L.; García-Pacheco, R.; de la Campa, J.G.; García-Calvo, E. Effect of sodium hypochlorite exposure on polysulfone recycled UF membranes and their surface characterization. Polym. Degrad. Stab. 2018, 150, 46–56. [Google Scholar] [CrossRef]
- Woo, S.H.; Min, B.R.; Lee, J.S. Change of surface morphology, permeate flux, surface roughness and water contact angle for membranes with similar physicochemical characteristics (except surface roughness) during microfiltration. Sep. Purif. Technol. 2017, 187, 274–284. [Google Scholar] [CrossRef]
- Rai, P.; Rai, C.; Majumdar, G.C.; DasGupta, S.; De, S. Resistance in series model for ultrafiltration of mosambi (Citrus sinensis (L.) Osbeck) juice in a stirred continuous mode. J. Memb. Sci. 2006, 283, 116–122. [Google Scholar] [CrossRef]
- Bugge, T.V.; Jørgensen, M.K.; Christensen, M.L.; Keiding, K. Modeling cake buildup under TMP-step filtration in a membrane bioreactor: Cake compressibility is significant. Water Res. 2012, 46, 4330–4338. [Google Scholar] [CrossRef]
- Jørgensen, M.K.; Bugge, T.V.; Christensen, M.L.; Keiding, K. Modeling approach to determine cake buildup and compression in a high-shear membrane bioreactor. J. Memb. Sci. 2012, 409, 335–345. [Google Scholar] [CrossRef]
- Le-clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Cucurachi, S.; Van Der Giesen, C.; Guinée, J. Ex-ante LCA of Emerging Technologies. Procedia CIRP 2018, 69, 463–468. [Google Scholar] [CrossRef]
- Lo, C.H.; McAdam, E.; Judd, S. The cost of a small membrane bioreactor. Water Sci. Technol. 2015, 72, 1739–1746. [Google Scholar] [CrossRef] [Green Version]
- Hashisho, J.; El-Fadel, M.; Al-Hindi, M.; Salam, D.; Alameddine, I. Hollow fiber vs. flat sheet MBR for the treatment of high strength stabilized landfill leachate. Waste Manag. 2016, 55, 249–256. [Google Scholar] [CrossRef]
- Fudala-Ksiazek, S.; Pierpaoli, M.; Luczkiewicz, A. Efficiency of landfill leachate treatment in a MBR/UF system combined with NF, with a special focus on phthalates and bisphenol A removal. Waste Manag. 2018, 78, 94–103. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; Landaburu-Aguirre, J.; Contreras-Martinez, J.; García-Calvo, E. Life Cycle Assessment application for emerging membrane recycling technologies: From reverse osmosis into forward osmosis. Resour. Conserv. Recycl. 2021, 179, 106075. [Google Scholar] [CrossRef]
- Lawler, W.; Antony, A.; Cran, M.; Duke, M.; Leslie, G.; Le-Clech, P. Production and characterisation of UF membranes by chemical conversion of used RO membranes. J. Memb. Sci. 2013, 447, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Skouteris, G.; Arnot, T.C.; Jraou, M.; Feki, F.; Sayadi, S. Modeling Energy Consumption in Membrane Bioreactors for Wastewater Treatment in North Africa. Water Environ. Res. 2014, 86, 232–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, J.A.; Túa, L.; Rueda, A.; Montaño, B.; Rodríguez, M.; Prats, D. Monitoring and analysis of the energy cost of an MBR. Desalination 2010, 250, 997–1001. [Google Scholar] [CrossRef]
- Fenu, A.; Roels, J.; Wambecq, T.; De Gussem, K.; Thoeye, C.; De Gueldre, G.; Van De Steene, B. Energy audit of a full scale MBR system. Desalination 2010, 262, 121–128. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Ul Haq, I. A review on the mechanism, impacts and control methods of membrane fouling in MBR system. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]




| Membrane Material | Nominal Permeability (20 °C) | Nominal Pore Size | Effective Membrane Area | Ra | Rq | Contact Angle | |
|---|---|---|---|---|---|---|---|
| c-MF | Chlorinated polyethylene | 1300 L·m−2·h−1·bar−1 | 0.4 µm | 0.11 m2 | 184 ± 21 nm | 234 ± 26 nm | 104° [16] |
| r-UF | PES | 255 L·m−2·h−1·bar−1 | 12 nm | 0.11 m2 | 4.7 ± 0.6 nm [17] | 6.3 ± 1.2 nm [17] | 68° [15] |
| Permeate Quality | Removal (%) | p-Value | |||
| (Ia) c-MF | (II a) r-UF | (Ia) c-MF | (II a) r-UF | r-UF-c-MF | |
| Turbidity (NTU) | 0.14 ± 0.01 | 0.04 ± 0.02 | - | - | 0.000246 * |
| TOC (mg/L) | 3.00 ± 0.26 | 1.82 ± 0.12 | 98.2 ± 0.2 | 98.9 ± 0.1 | 0.0006 * |
| Total N (mg/L) | 26.01 ± 1.79 | 24.23 ± 2.59 | 17.2 ± 5.7 | 22.85 ± 9.5 | 0.40 |
| Total P (mg/L) | 3.68 ± 0.29 | 3.26 ± 0.65 | 29.9 ± 5.4 | 37.9 ± 14.2 | 0.50 |
| COD (mg/L) | 5.05 ± 0.64 | 5.93 ± 0.88 | 99.1 ± 0.2 | 98.8 ± 0.3 | 0.216 |
| BOD5 (mg/L) | 1.25 ± 0.35 | <1 | 99.5 ± 0.1 | 99.7 ± 0.1 | 0.293 |
| Permeate Quality | Removal (%) | p-Value | |||
| (Ib) c-MF | (II b) r-UF | (Ib) c-MF | (II b) r-UF | r-UF-c-MF | |
| Turbidity (NTU) | 0.29 ± 0.32 | 0.01 ± 0.05 | - | - | 0.0919 |
| TOC (mg/L) | 2.28 ± 0.38 | 1.57 ± 0.20 | 98.6 ± 0.2 | 99.0 ± 0.1 | 0.01 * |
| Total N (mg/L) | 22.21 ± 3.56 | 17.40 ± 6.34 | 29.3 ± 11.3 | 51.2 ± 14.3 | 0.02 * |
| Total P (mg/L) | 3.58 ± 0.75 | 3.43 ± 0.31 | 31.8 ± 14.3 | 38.6 ± 6.6 | 0.39 |
| COD (mg/L) | 7.97 ± 1.73 | 4.52 ± 1.34 | 98.3 ± 0.4 | 98.9 ± 0.3 | 0.05 |
| BOD5 (mg/L) | <1 | <1 | 99.7 ± 0.1 | 99.6 ± 0.0 | 1 |
| Membrane | Flux (L·m−2·h−1) | Data Series | Permeability Decline Rate (L·m−2·h−1·bar−1·d−1) | R2 | p-Value |
|---|---|---|---|---|---|
| c-MF | 12 | Days 1–7 | 43.9 ± 7.9 | 0.864 | 0.0025 |
| c-MF | 14 | Days 8–22 | 51.6 ± 4.3 | 0.941 | 7.70 × 10−7 |
| r-UF | 12 | Days 1–10 | 5.3 ± 0.7 | 0.835 | 0.00022 |
| r-UF | 14 | Days 11–19 | 15.8 ± 1.9 | 0.911 | 6.35 × 10−5 |
| Membrane Type | Membrane Fouling Mechanisms | |||
|---|---|---|---|---|
| Cake Layer | Pore Blocking | Reversible | Irreversible | |
| c-MF | 58.6 ± 16.6% | 41.4 ± 6.1% | 8.5 ± 8.5% | 91.5 ± 14.1% |
| r-UF | 66.4 ± 4.4% | 33.6 ± 1.9% | 0.0 ± 2.5% | 100.0 ± 3.8% |
| Commercial Membrane Modules/Frames | Sheet Dimensions (mm) | Number of Sheets Cut | Area Recovered (m2) | Area Recovered per Module (%) | Cost (EUR·m−2) |
|---|---|---|---|---|---|
| Recycled Toray TM 720 | 960 × 845 | 1 | 37 | - | - |
| Kubota-510 SINAP-80 | 490 × 1000 | 1 | 22.2 | 60 | 6.89 |
| Kubota-203 | 226 × 316 | 8 | 25.9 | 70 | 5.91 |
| SINAP-25 | 340 × 470 | 2 | 14.4 | 39 | 10.56 |
| SINAP-10 | 220 × 320 | 8 | 25.5 | 69 | 5.99 |
| Cost Type | Process | Cost per Module (EUR·Module−1) | Cost Contribution | Source |
|---|---|---|---|---|
| CAPEX + OPEX | Module transformation to the r-UF membrane, characterization, and logistics | 80 | 51.96% | [7] |
| OPEX-Labor | Disassembling and sheet cutting | 51.17 | 33.24% | Own data |
| OPEX-Labor | Re-assembling in new frames | 11.37 | 7.39% | Own data |
| OPEX-Energy | Electricity use during the processing | 0.03 | 0.02% | [44] |
| Total cost | Recycling of one module | 153.95 | 100% |
| Parameter | Effect (Δ%) | Ratio (Δ% Effect/Δ% Parameter) |
|---|---|---|
| Reduce 25% of area recovered | 33 | 1.32 |
| Change 25% of transformation cost | 13 | 0.52 |
| Change 25% of labor cost | 12 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sáez, L.; Patsios, S.I.; Senán-Salinas, J.; Landaburu-Aguirre, J.; Molina, S.; García-Calvo, E. A Novel Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Proof-of-Concept Study. Membranes 2022, 12, 218. https://doi.org/10.3390/membranes12020218
Rodríguez-Sáez L, Patsios SI, Senán-Salinas J, Landaburu-Aguirre J, Molina S, García-Calvo E. A Novel Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Proof-of-Concept Study. Membranes. 2022; 12(2):218. https://doi.org/10.3390/membranes12020218
Chicago/Turabian StyleRodríguez-Sáez, Laura, Sotiris I. Patsios, Jorge Senán-Salinas, Junkal Landaburu-Aguirre, Serena Molina, and Eloy García-Calvo. 2022. "A Novel Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Proof-of-Concept Study" Membranes 12, no. 2: 218. https://doi.org/10.3390/membranes12020218
APA StyleRodríguez-Sáez, L., Patsios, S. I., Senán-Salinas, J., Landaburu-Aguirre, J., Molina, S., & García-Calvo, E. (2022). A Novel Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Proof-of-Concept Study. Membranes, 12(2), 218. https://doi.org/10.3390/membranes12020218









