Recent Advancements in Polyphenylsulfone Membrane Modification Methods for Separation Applications
Abstract
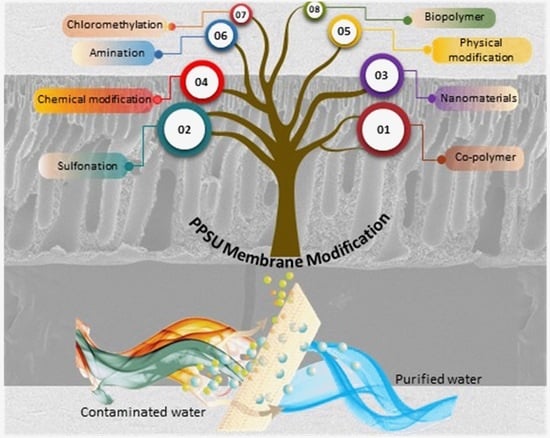
:1. Introduction
2. Polyphenylsulfone Characteristics
3. Bulk Modification
3.1. Polyphenylsulfone Sulfonation
3.2. Polyphenylsulfone Amination
3.3. Polyphenylsulfone Chloromethylation
4. Polymer Blending
4.1. Polyphenylsulfone Blended with the Polymer
4.2. Polyphenylsulfone Blended with the Nanomaterials
4.3. Polyphenylsulfone Blended with the Biopolymer
5. Polyphenylsulfone Surface Modification
5.1. Physical Modification
5.2. Chemical Modification
6. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decker, B.; Hartmann-Thompson, C.; Carver, P.I.; Keinath, S.E.; Santurri, P.R. Multilayer sulfonated polyhedral oligosilsesquioxane (S-POSS)-sulfonated polyphenylsulfone (S-PPSU) composite proton exchange membranes. Chem. Mater. 2010, 22, 942–948. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Sgreccia, E.; Licoccia, S.; Khadhraoui, M.; Denoyel, R.; Knauth, P. Composite proton-conducting hybrid polymers: Water sorption isotherms and mechanical properties of blends of sulfonated PEEK and substituted PPSU. Chem. Mater. 2008, 20, 4327–4334. [Google Scholar] [CrossRef]
- Park, A.M.; Turley, F.E.; Wycisk, R.J.; Pintauro, P.N. Electrospun and cross-linked nanofiber composite anion exchange membranes. Macromolecules 2014, 47, 227–235. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Zhang, J.; Tour, J.M.; Arnusch, C.J. Sulfur-Doped Laser-Induced Porous Graphene Derived from Polysulfone-Class Polymers and Membranes. ACS Nano 2018, 12, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Di Vona, M.L.; Sgreccia, E.; Tamilvanan, M.; Khadhraoui, M.; Chassigneux, C.; Knauth, P. High ionic exchange capacity polyphenylsulfone (SPPSU) and polyethersulfone (SPES) cross-linked by annealing treatment: Thermal stability, hydration level and mechanical properties. J. Memb. Sci. 2010, 354, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhou, Y.; Hao, J.; Bao, B.; Bian, X.; Jiang, X.; Pang, J.; Zhang, H.; Jiang, Z.; Jiang, L. A Charge-Density-Tunable Three/Two-Dimensional Polymer/Graphene Oxide Heterogeneous Nanoporous Membrane for Ion Transport. ACS Nano 2017, 11, 10816–10824. [Google Scholar] [CrossRef]
- Han, J.; Peng, Y.; Lin, B.; Zhu, Y.; Ren, Z.; Xiao, L.; Zhuang, L. Hydrophobic Side-Chain Attached Polyarylether-Based Anion Exchange Membranes with Enhanced Alkaline Stability. ACS Appl. Energy Mater. 2019, 2, 8052–8059. [Google Scholar] [CrossRef]
- Slonov, A.L.; Zhansitov, A.A.; Rzhevskaya, E.V.; Khakulova, D.M.; Sapaev, K.K.; Shetov, R.A.; Khashirova, S.Y. Influence of the Length and Concentration of Carbon and Glass Fibers on the Properties of Polyphenylene Sulfone. Fibre Chem. 2018, 50, 354–360. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, X.; Zhang, S.; Ren, J.; Yang, L.; Wang, Q.; Wang, G. Synthesis of sulfonated polyphenylsulfone as candidates for antifouling ultrafiltration membrane. Sep. Purif. Technol. 2012, 98, 298–307. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Saljoughi, E.; Shahtahmassebi, N. Preparation and characterization of modified polyphenylsulfone membranes with hydrophilic property for filtration of aqueous media. Polym. Adv. Technol. 2018, 29, 1632–1648. [Google Scholar] [CrossRef]
- Norrman, K.; Wang, Y.; Stamate, E.; Zhang, W. Controlling surface properties of electrospun polyphenylsulfone using plasma treatment and X-ray photoelectron spectroscopy. Heliyon 2019, 5, e01943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Liu, Q.; Li, X.; Pan, J.; Wei, L.; Wu, Y.; Peng, H.; Wang, Y.; Li, G.; Chen, C.; et al. An effective approach for alleviating cation-induced backbone degradation in aromatic ether-based alkaline polymer electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhang, Q.; Cui, Z.; Li, W.; Xing, W. Alkali resisting polyphenylsulfone ultrafiltration membrane with tailored microstructure. Polymer 2017, 124, 128–138. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Jansen, J.C.; Tasselli, F.; Tocci, E.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B. Novel polyphenylsulfone membrane for potential use in solvent nanofiltration. J. Memb. Sci. 2011, 379, 60–68. [Google Scholar] [CrossRef]
- Hong, S.H.; Cha, M.S.; Hong, S.-K.; Oh, S.-G.; Lee, J.Y. Structural Effect of the Hydrophobic Block on the Chemical Stability of Ion-Conducting Multiblock Copolymers for Flow Battery. ACS Sustain. Chem. Eng. 2019, 7, 17088–17099. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Zhang, L.; Chen, S.B.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Rheology and phase inversion behavior of polyphenylenesulfone (PPSU) and sulfonated PPSU for membrane formation. Polymer 2016, 99, 72–82. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Bhattacharjee, S. Rational design of phase inversion membranes by tailoring thermodynamics and kinetics of casting solution using polymer additives. J. Memb. Sci. 2013, 441, 31–44. [Google Scholar] [CrossRef]
- Nadour, M.; Boukraa, F.; Benaboura, A. Removal of Diclofenac, Paracetamol and Metronidazole using a carbon-polymeric membrane. J. Environ. Chem. Eng. 2019, 7, 103080. [Google Scholar] [CrossRef]
- Yang, Y.N.; Jun, W.; Zheng, Q.Z.; Cheng, X.S.; Zhang, H.X. The research of rheology and thermodynamics of organic-inorganic hybrid membrane during the membrane formation. J. Memb. Sci. 2008, 311, 200–207. [Google Scholar] [CrossRef]
- Hwang, L.L.; Tseng, H.H.; Chen, J.C. Fabrication of polyphenylsulfone/polyetherimide blend membranes for ultrafiltration applications: The effects of blending ratio on membrane properties and humic acid removal performance. J. Memb. Sci. 2011, 384, 72–81. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Chung, T.S.; Weber, M.; Widjojo, N.; Maletzko, C. Effects of polyethylene glycol on membrane formation and properties of hydrophilic sulfonated polyphenylenesulfone (sPPSU) membranes. J. Memb. Sci. 2017, 531, 27–35. [Google Scholar] [CrossRef]
- Ballengee, J.B.; Pintauro, P.N. Composite Fuel Cell Membranes from Dual-Nanofiber Electrospun Mats. Macromolecules 2011, 44, 7307–7314. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, J.D.; Miyatake, K. Effect of thermal crosslinking on the properties of sulfonated poly(phenylene sulfone)s as proton conductive membranes. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Q.; Lin, X.; Liu, J.Z.; Wang, H. Hydrophilic Nanowire Modified Polymer Ultrafiltration Membranes with High Water Flux. ACS Appl. Mater. Interfaces 2014, 6, 19161–19167. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Zhu, L.; Liu, C.; Cui, F.; Li, N.; Zhang, B. Graphene Oxide/Polyamide-Based Nanofiltration Membranes for Water Purification. ACS Appl. Nano Mater. 2020, 4, 673–682. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Huang, F.; Lin, S.; Zhang, H.; Cao, S.; Chen, L.; He, Z.; Lutes, R.; Yang, J.; et al. Preparation and Characterization of Cellulose-Based Nanofiltration Membranes by Interfacial Polymerization with Piperazine and Trimesoyl Chloride. ACS Sustain. Chem. Eng. 2018, 6, 13168–13176. [Google Scholar] [CrossRef]
- Liao, Z.; Fang, X.; Xie, J.; Li, Q.; Wang, D.; Sun, X.; Wang, L.; Li, J. Hydrophilic Hollow Nanocube-Functionalized Thin Film Nanocomposite Membrane with Enhanced Nanofiltration Performance. ACS Appl. Mater. Interfaces 2019, 11, 5344–5352. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, S.; Yan, L.; Liu, Y.; Tan, X. Preparation and properties of PPSU/GO mixed matrix membrane. Chin. J. Chem. Eng. 2017, 25, 408–414. [Google Scholar] [CrossRef]
- Dorf, T.; Ferrer, I.; Ciurana, J. The effect of weld line on tensile strength of polyphenylsulfone (PPSU) in ultrasonic micro-moulding technology. Int. J. Adv. Manuf. Technol. 2019, 103, 2391–2400. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, Z.; Ma, R.; Zhang, W.; Li, J. Development of High-Antifouling PPSU Ultrafiltration Membrane by Using Compound Additives: Preparation, Morphologies, and Filtration Resistant Properties. Membranes 2016, 6, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saranya, R.; Arthanareeswaran, G.; Ismail, A.F.; Reddy, N.L.; Shankar, M.V.; Kweon, J. Efficient rejection of organic compounds using functionalized ZSM-5 incorporated PPSU mixed matrix membrane. RSC Adv. 2017, 7, 15536–15552. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, J.; Tang, Y.; Hou, J.; Yu, L.; Gao, C. A novel long-lasting antifouling membrane modified with bifunctional capsaicin-mimic moieties: Via in situ polymerization for efficient water purification. J. Mater. Chem. A 2016, 4, 10352–10362. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Cao, J.; Guan, J.; Xu, L.; Zhang, R.; He, M.; Zhang, Q.; Fan, L.; Jiang, Z. Synergy of the mechanical, antifouling and permeation properties of a carbon nanotube nanohybrid membrane for efficient oil/water separation. Nanoscale 2017, 9, 7508–7518. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, H.; Seyed Dorraji, M.S.; Rasoulifard, M.H.; Ahmadi, A.; Nooshiran-Zadeh, N. Tris(hydroxymethyl)aminomethane-grafted polyamine nanofiltration membrane: Enhanced antifouling and pH resistant properties. New J. Chem. 2020, 44, 6321–6330. [Google Scholar] [CrossRef]
- Ureña, N.; Pérez-Prior, M.T.; del Río, C.; Várez, A.; Sanchez, J.Y.; Iojoiu, C.; Levenfeld, B. Multiblock copolymers of sulfonated PSU/PPSU Poly(ether sulfone)s as solid electrolytes for proton exchange membrane fuel cells. Electrochim. Acta 2019, 302, 428–440. [Google Scholar] [CrossRef]
- Çalhan, A.; Deniz, S.; Kujawski, W.; Kujawa, J.; Knozowska, K.; Hasanoğlu, A. Silica Filled Polyphenylsulfone/Polydimethylsiloxane Composite Membranes for Pervaporation Separation of Biobutanol from ABE Mixtures. Chem. Eng. Process. Process Intensif. 2020, 156, 108099. [Google Scholar] [CrossRef]
- Medalsy, I.D.; Müller, D.J. Nanomechanical Properties of Proteins and Membranes Depend on Loading Rate and Electrostatic Interactions. ACS Nano 2013, 7, 2642–2650. [Google Scholar] [CrossRef]
- Rho, H.; Cho, J.; Westerhoff, P.; Chon, K. Intrinsic pKa of Nanofiltration Membrane Surfaces to Assess Fouling and Cleaning Behaviors Induced by Foulant–Membrane Electrostatic Interactions. Environ. Sci. Technol. 2020, 54, 7706–7714. [Google Scholar] [CrossRef]
- Kobayashi, D.; Nakahara, H.; Shibata, O.; Unoura, K.; Nabika, H. Interplay of Hydrophobic and Electrostatic Interactions between Polyoxometalates and Lipid Molecules. J. Phys. Chem. C 2017, 121, 12895–12902. [Google Scholar] [CrossRef]
- Rong, G.; Zhou, D.; Pang, J. Preparation of high-performance antifouling polyphenylsulfone ultrafiltration membrane by the addition of sulfonated polyaniline. J. Polym. Res. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Ali, F.A.A. Antimicrobial and antifouling properties of versatile PPSU/carboxylated GO nanocomposite membrane against Gram-positive and Gram-negative bacteria and protein. Environ. Sci. Pollut. Res. 2018, 25, 34103–34113. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.C.; Isloor, A.M.; Moslehyani, A.; Ismail, A.F. Preparation and characterization of PPSU membranes with BiOCl nanowafers loaded on activated charcoal for oil in water separation. J. Taiwan Inst. Chem. Eng. 2017, 77, 293–301. [Google Scholar] [CrossRef]
- Slonov, A.L.; Zhansitov, A.A.; Rzhevskaya, E.V.; Khakulova, D.M.; Sapaev, K.K.; Shetov, R.A.; Khashirova, S.Y. Effects of Length and Concentration of Carbon and Glass Fibers on Polyphenylene Sulfone Properties. Fibre Chem. 2019, 50, 402–407. [Google Scholar] [CrossRef]
- Zhong, P.; Fu, X.; Chung, T.-S.; Weber, M.; Maletzko, C. Development of Thin-Film Composite forward Osmosis Hollow Fiber Membranes Using Direct Sulfonated Polyphenylenesulfone (sPPSU) as Membrane Substrates. Environ. Sci. Technol. 2013, 47, 7430–7436. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Isloor, A.M.; Somasekhara Rao, T.; Ismail, A.F.; Farnood, R.; Nambissan, P.M.G. Removal of toxic arsenic from aqueous media using polyphenylsulfone/cellulose acetate hollow fiber membranes containing zirconium oxide. Chem. Eng. J. 2020, 393, 124367. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Tasselli, F.; Jansen, J.C.; Tocci, E.; Bazzarelli, F.; Bernardo, P.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B. Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes. J. Memb. Sci. 2011, 384, 89–96. [Google Scholar] [CrossRef]
- Jaafer, M.J.; Al-Najar, J.A.; Alsalhy, Q.F. Poly(phenyl sulfone) hollow fiber forward osmosis membrane for saline water desalination. Chem. Eng. Process. Process Intensif. 2020, 157, 108119. [Google Scholar] [CrossRef]
- Praneeth, K.; Suresh, K.B.; James, T.; Sridhar, S. Design of novel ultrafiltration systems based on robust polyphenylsulfone hollow fiber membranes for treatment of contaminated surface water. Chem. Eng. J. 2014, 248, 297–306. [Google Scholar]
- Arumugham, T.; Kaleekkal, N.J.; Doraiswamy, M. Development of new hybrid ultrafiltration membranes by entanglement of macromolecular PPSU-SO3H chains: Preparation, morphologies, mechanical strength, and fouling resistant properties. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Sgreccia, E.; Di Vona, M.L.; Knauth, P. Hybrid composite membranes based on SPEEK and functionalized PPSU for PEM fuel cells. Int. J. Hydrogen Energy 2011, 36, 8063–8069. [Google Scholar] [CrossRef]
- Dyck, A.; Fritsch, D.; Nunes, S.P. Proton-conductive membranes of sulfonated polyphenylsulfone. J. Appl. Polym. Sci. 2002, 86, 2820–2827. [Google Scholar] [CrossRef]
- Karlsson, L.E.; Jannasch, P. Polysulfone ionomers for proton-conducting fuel cell membranes: 2. Sulfophenylated polysulfones and polyphenylsulfones. Electrochim. Acta 2005, 50, 1939–1946. [Google Scholar] [CrossRef]
- Ramiro, J.; Egulazábal, J.I.; Nazábal, J. New miscible poly(ether imide)/poly(phenyl sulfone) blends. Macromol. Mater. Eng. 2006, 291, 707–713. [Google Scholar] [CrossRef]
- Di Vona, M.L.; D’Epifanio, A.; Marani, D.; Trombetta, M.; Traversa, E.; Licoccia, S. SPEEK/PPSU-based organic-inorganic membranes: Proton conducting electrolytes in anhydrous and wet environments. J. Memb. Sci. 2006, 279, 186–191. [Google Scholar] [CrossRef]
- Licoccia, S.; Di Vona, M.L.; D’Epifanio, A.; Ahmed, Z.; Bellitto, S.; Marani, D.; Mecheri, B.; de Bonis, C.; Trombetta, M.; Traversa, E. SPPSU-based hybrid proton conducting polymeric electrolytes for intermediate temperature PEMFCs. J. Power Sources 2007, 167, 79–83. [Google Scholar] [CrossRef]
- Weng, T.H.; Tseng, H.H.; Wey, M.Y. Preparation and characterization of PPSU/PBNPI blend membrane for hydrogen separation. Int. J. Hydrogen Energy 2008, 33, 4178–4182. [Google Scholar] [CrossRef]
- Weng, T.H.; Tseng, H.H.; Wey, M.Y. Effects of crosslinking modification on the O2/N2 separation characteristics of poly(phenyl sulfone)/poly(bisphenol A-co-4-nitrophthalic anhydride-co-1,3-phenylenediamine) blend membranes. J. Appl. Polym. Sci. 2010, 116, 1254–1263. [Google Scholar]
- Zhong, P.S.; Widjojo, N.; Chung, T.S.; Weber, M.; Maletzko, C. Positively charged nanofiltration (NF) membranes via UV grafting on sulfonated polyphenylenesulfone (sPPSU) for effective removal of textile dyes from wastewater. J. Memb. Sci. 2012, 417–418, 52–60. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhou, Z.; Ren, J.; Geng, Z.; Luan, J.; Wang, G. Novel sulfonated thin-film composite nanofiltration membranes with improved water flux for treatment of dye solutions. J. Memb. Sci. 2012, 394–395, 218–229. [Google Scholar] [CrossRef]
- Kim, J.D.; Donnadio, A.; Jun, M.S.; Di Vona, M.L. Crosslinked SPES-SPPSU membranes for high temperature PEMFCs. Int. J. Hydrogen Energy 2013, 38, 1517–1523. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.S.; Weber, M.; Maletzko, C.; Warzelhan, V. A sulfonated polyphenylenesulfone (sPPSU) as the supporting substrate in thin film composite (TFC) membranes with enhanced performance for forward osmosis (FO). Chem. Eng. J. 2013, 220, 15–23. [Google Scholar] [CrossRef]
- Jansen, J.C.; Darvishmanesh, S.; Tasselli, F.; Bazzarelli, F.; Bernardo, P.; Tocci, E.; Friess, K.; Randova, A.; Drioli, E.; Van der Bruggen, B. Influence of the blend composition on the properties and separation performance of novel solvent resistant polyphenylsulfone/polyimide nanofiltration membranes. J. Memb. Sci. 2013, 447, 107–118. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Effect of TiO2 nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J. Mater. Chem. B 2014, 2, 7502–7514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, E.; Wang, G.; Yu, P.; Zhao, Q.; Yao, F. Poly(phenyl sulfone) anion exchange membranes with pyridinium groups for vanadium redox flow battery applications. J. Power Sources 2015, 282, 328–334. [Google Scholar] [CrossRef]
- Sani, N.A.A.; Lau, W.J.; Ismail, A.F. Polyphenylsulfone-based solvent resistant nanofiltration (SRNF) membrane incorporated with copper-1,3,5-benzenetricarboxylate (Cu-BTC) nanoparticles for methanol separation. RSC Adv. 2015, 5, 13000–13010. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Shahtahmassebi, N.; Saljoughi, E. Hydrophilicity improvement in polyphenylsulfone nanofibrous filtration membranes through addition of polyethylene glycol. Appl. Surf. Sci. 2015, 359, 252–258. [Google Scholar] [CrossRef]
- Yong, W.F.; Lee, Z.K.; Chung, T.S. Blends of a polymer of intrinsic microporosity and partially sulfonated polyphenylenesulfone for Gas separation. ChemSusChem 2016, 9, 1953–1962. [Google Scholar] [CrossRef]
- Saranya, R.; Kumar, M.; Tamilarasan, R.; Ismail, A.F.; Arthanareeswaran, G. Functionalised activated carbon modified polyphenylsulfone composite membranes for adsorption enhanced phenol filtration. J. Chem. Technol. Biotechnol. 2016, 91, 748–761. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Rana, D.; Doraiswamy, M. Separation of oil/water emulsions using nano MgO anchored hybrid ultrafiltration membranes for environmental abatement. J. Appl. Polym. Sci. 2016, 133, 1–12. [Google Scholar] [CrossRef]
- Sani, N.A.A.; Lau, W.J.; Nordin, N.A.H.M.; Ismail, A.F. Influence of organic solvents and operating conditions on the performance of polyphenylsulfone (PPSU)/copper-1,3,5-benzenetricarboxylate (Cu-BTC) solvent resistant nanofiltration (SRNF) membranes. Chem. Eng. Res. Des. 2016, 115, 66–76. [Google Scholar] [CrossRef]
- Kim, J.D.; Ghil, L.J. Annealing effect of highly sulfonated polyphenylsulfone polymer. Int. J. Hydrogen Energy 2016, 41, 11794–11800. [Google Scholar] [CrossRef]
- Yam-Cervantes, M.A.; Santiago-García, J.L.; Loría-Bastarrachea, M.I.; Duarte-Aranda, S.; Alberto Ruiz-Treviño, F.; Aguilar-Vega, M. Sulfonated polyphenylsulfone asymmetric membranes: Effect of coagulation bath (acetic acid-NaHCO3/isopropanol) on morphology and antifouling properties. J. Appl. Polym. Sci. 2017, 134, 44502. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, T.; Liu, Z.; Su, Y.; Yu, H.; Ma, J.; Yang, Y.; Jiang, Z. Synthesis and properties of sulfonated biphenyl poly(ether sulfone) and its mixed-matrix membranes containing carbon nanotubes for gas separation. J. Appl. Polym. Sci. 2017, 134, 44995. [Google Scholar] [CrossRef]
- Alam, J.; Alhoshan, M.; Shukla, A.K.; Aldalbahi, A.; Ali, F.A.A.; Dass, L.A.; Muthumareeswaran, M.R. κ-Carrageenan as a promising pore-former for the preparation of a highly porous polyphenylsulfone membrane. Mater. Lett. 2017, 204, 108–111. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Balkanloo, P.G. Clay-hyperbranched epoxy/polyphenylsulfone nanocomposite membranes. Iran. Polym. J. 2017, 26, 711–720. [Google Scholar] [CrossRef]
- Golpour, M.; Pakizeh, M. Development of a new nanofiltration membrane for removal of kinetic hydrate inhibitor from water. Sep. Purif. Technol. 2017, 183, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Lawrence Arockiasamy, D.; Alhoshan, M.; Alam, J.; Muthumareeswaran, M.R.; Figoli, A.; Arun Kumar, S. Separation of proteins and antifouling properties of polyphenylsulfone based mixed matrix hollow fiber membranes. Sep. Purif. Technol. 2017, 174, 529–543. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of high flux ultrafiltration polyphenylsulfone membranes applying the systems with upper and lower critical solution temperatures: Effect of polyethylene glycol molecular weight and coagulation bath temperature. J. Memb. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Asadi Tashvigh, A.; Luo, L.; Chung, T.S.; Weber, M.; Maletzko, C. A novel ionically cross-linked sulfonated polyphenylsulfone (sPPSU) membrane for organic solvent nanofiltration (OSN). J. Memb. Sci. 2018, 545, 221–228. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Isaichykova, Y.A.; Ovcharova, A.A. Preparation of High-Flux Ultrafiltration Polyphenylsulfone Membranes. Pet. Chem. 2018, 58, 747–759. [Google Scholar] [CrossRef]
- Golpour, M.; Pakizeh, M. Preparation and characterization of new PA-MOF/PPSU-GO membrane for the separation of KHI from water. Chem. Eng. J. 2018, 345, 221–232. [Google Scholar] [CrossRef]
- Asadi Tashvigh, A.; Luo, L.; Chung, T.S.; Weber, M.; Maletzko, C. Performance enhancement in organic solvent nanofiltration by double crosslinking technique using sulfonated polyphenylsulfone (sPPSU) and polybenzimidazole (PBI). J. Memb. Sci. 2018, 551, 204–213. [Google Scholar] [CrossRef]
- Naderi, A.; Asadi Tashvigh, A.; Chung, T.S.; Weber, M.; Maletzko, C. Molecular design of double crosslinked sulfonated polyphenylsulfone /polybenzimidazole blend membranes for an efficient hydrogen purification. J. Memb. Sci. 2018, 563, 726–733. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Rana, D. Fabrication of novel aromatic amine functionalized nanofiltration (NF) membranes and testing its dye removal and desalting ability. Polym. Test. 2018, 72, 1–10. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Ali, F.A.A.; Muthumareeswaran, M.R.; Mishra, U.; Ansari, M.A. Removal of heavy metal ions using a carboxylated graphene oxide-incorporated polyphenylsulfone nanofiltration membrane. Environ. Sci. Water Res. Technol. 2018, 4, 438–448. [Google Scholar] [CrossRef]
- Moideen, I.K.; Isloor, A.M.; Qaiser, A.A.; Ismail, A.F.; Abdullah, M.S. Separation of heavy metal and protein from wastewater by sulfonated polyphenylsulfone ultrafiltration membrane process prepared by glycine betaine enriched coagulation bath. Korean J. Chem. Eng. 2018, 35, 1281–1289. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Shi, M.; Liu, L.; Tong, Y.; Huang, L.; Li, W.; Xing, W. Advanced porous polyphenylsulfone membrane with ultrahigh chemical stability and selectivity for vanadium flow batteries. J. Appl. Polym. Sci. 2019, 136, 47752. [Google Scholar] [CrossRef]
- Nayak, M.C.; Isloor, A.M.; Prabhu, B.; Norafiqah, N.I.; Asiri, A.M. Novel polyphenylsulfone (PPSU)/nano tin oxide (SnO2) mixed matrix ultrafiltration hollow fiber membranes: Fabrication, characterization and toxic dyes removal from aqueous solutions. React. Funct. Polym. 2019, 139, 170–180. [Google Scholar]
- Arumugham, T.; Amimodu, R.G.; Kaleekkal, N.J.; Rana, D. Nano CuO/g-C3N4 sheets-based ultrafiltration membrane with enhanced interfacial affinity, antifouling and protein separation performances for water treatment application. J. Environ. Sci. 2019, 82, 57–69. [Google Scholar] [CrossRef]
- Wang, Y.; Górecki, R.P.; Stamate, E.; Norrman, K.; Aili, D.; Zuo, M.; Guo, W.; Hélix-Nielsen, C.; Zhang, W. Preparation of super-hydrophilic polyphenylsulfone nanofiber membranes for water treatment. RSC Adv. 2019, 9, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Rao, T.S.; Isloor, A.M.; Ibrahim, G.P.S.; Ismail, N.; Ismail, A.F.; Asiri, A.M. Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. Int. J. Biol. Macromol. 2019, 129, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Arthanareeswaran, G. Silver nano-particle coated hydroxyapatite nano-composite membrane for the treatment of palm oil mill effluent. J. Water Process Eng. 2019, 31, 100844. [Google Scholar] [CrossRef]
- Chandrashekhar Nayak, M.; Isloor, A.M.; Lakshmi, B.; Marwani, H.M.; Khan, I. Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: Fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arab. J. Chem. 2019, 13, 4661–4672. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ali, F.A.A.; Alhoshan, M. Efficient soluble anionic dye removal and antimicrobial properties of ZnO embedded-polyphenylsulfone membrane. Water Environ. J. 2021, 35, 670–684. [Google Scholar] [CrossRef]
- Gronwald, O.; Frost, I.; Ulbricht, M.; Kouchaki Shalmani, A.; Panglisch, S.; Grünig, L.; Handge, U.A.; Abetz, V.; Heijnen, M.; Weber, M. Hydrophilic poly(phenylene sulfone) membranes for ultrafiltration. Sep. Purif. Technol. 2020, 250, 117107. [Google Scholar] [CrossRef]
- Dehban, A.; Kargari, A.; Ashtiani, F.Z. Preparation and optimization of antifouling PPSU/PES/SiO2 nanocomposite ultrafiltration membranes by VIPS-NIPS technique. J. Ind. Eng. Chem. 2020, 88, 292–311. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Arathi Krishnan, P.V.; Isloor, A.M.; Shyma, P.C. Fabrication of PPSU/PANI hollow fiber membranes for humic acid removal. Mater. Today Proc. 2020, 41, 541–548. [Google Scholar] [CrossRef]
- Mohamad Nor, N.A.; Nakao, H.; Jaafar, J.; Kim, J.D. Crosslinked carbon nanodots with highly sulfonated polyphenylsulfone as proton exchange membrane for fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 9979–9988. [Google Scholar] [CrossRef]
- Xiao, S.; Huo, X.; Fan, S.; Zhao, K.; Yu, S.; Tan, X. Design and synthesis of Al-MOF/PPSU mixed matrix membrane with pollution resistance. Chin. J. Chem. Eng. 2020, 29, 110–120. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Ibrahim, G.P.S.; Ismail, A.F.; Asiri, A.M. Improved separation of dyes and proteins using membranes made of polyphenylsulfone/cellulose acetate or acetate phthalate. Environ. Chem. Lett. 2020, 18, 881–887. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ali, F.A.A.; Alhoshan, M. A highly permeable zinc-based MOF/polyphenylsulfone composite membrane with elevated antifouling properties. Chem. Commun. 2020, 56, 5231–5234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Nagaraja, H.S.; Ismail, A.F.; Susanti, R. Effect of binary zinc-magnesium oxides on polyphenylsulfone/cellulose acetate derivatives hollow fiber membranes for the decontamination of arsenic from drinking water. Chem. Eng. J. 2021, 405, 126809. [Google Scholar] [CrossRef]
- Alam, J.; Shukla, A.K.; Ansari, M.A.; Abdulraqeb, F.; Ali, A.; Alhoshan, M. Dye Separation and Antibacterial Activities of Polyaniline Thin Film-Coated Poly (phenyl sulfone) Membranes. Membranes 2021, 11, 25–40. [Google Scholar] [CrossRef]
- Jutemar, E.P.; Takamuku, S.; Jannasch, P. Sulfonated poly(arylene ether sulfone) ionomers containing di- and tetrasulfonated arylene sulfone segments. Polym. Chem. 2011, 2, 181–191. [Google Scholar] [CrossRef]
- Chen, R.; Li, G. New sulfonated poly(arylene ether sulfone) copolymers containing phenyl side chains as proton exchange membranes. New J. Chem. 2016, 40, 3755–3762. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, C.; Ye, Y.S.; Xue, Z.; Zhou, X.; Xie, X. The enhanced actuation response of an ionic polymer-metal composite actuator based on sulfonated polyphenylsulfone. Polym. Chem. 2014, 5, 6097–6107. [Google Scholar] [CrossRef]
- Nor, N.A.M.; Jaafar, J.; Kim, J.D. Improved properties of sulfonated octaphenyl polyhedral silsequioxane cross-link with highly sulfonated polyphenylsulfone as proton exchange membrane. J. Solid State Electrochem. 2020, 24, 1185–1195. [Google Scholar] [CrossRef]
- Yoshida-Hirahara, M.; Takahashi, S.; Yoshizawa-Fujita, M.; Takeoka, Y.; Rikukawa, M. Synthesis and investigation of sulfonated poly(p-phenylene)-based ionomers with precisely controlled ion exchange capacity for use as polymer electrolyte membranes. RSC Adv. 2020, 10, 12810–12822. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Han, G.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Oil/water separation via ultrafiltration by novel triangle-shape tri-bore hollow fiber membranes from sulfonated polyphenylenesulfone. J. Memb. Sci. 2015, 476, 162–170. [Google Scholar] [CrossRef]
- Tang, Y.; Widjojo, N.; Shi, G.M.; Chung, T.S.; Weber, M.; Maletzko, C. Development of flat-sheet membranes for C1–C4 alcohols dehydration via pervaporation from sulfonated polyphenylsulfone (sPPSU). J. Memb. Sci. 2012, 415–416, 686–695. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Merrington, A.; Carver, P.I.; Keeley, D.L.; Rousseau, J.L.; Hucul, D.; Bruza, K.J.; Thomas, L.S.; Keinath, S.E.; Nowak, R.M.; et al. Proton-conducting polyhedral oligosilsesquioxane nanoadditives for sulfonated polyphenylsulfone hydrogen fuel cell proton exchange membranes. J. Appl. Polym. Sci. 2008, 110, 958–974. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Luchetti, L.; Spera, G.P.; Sgreccia, E.; Knauth, P. Synthetic strategies for the preparation of proton-conducting hybrid polymers based on PEEK and PPSU for PEM fuel cells. Comptes Rendus Chim. 2008, 11, 1074–1081. [Google Scholar] [CrossRef]
- Jo, Y.J.; Choi, E.Y.; Kim, S.W.; Kim, C.K. Fabrication and characterization of a novel polyethersulfone/aminated polyethersulfone ultrafiltration membrane assembled with zinc oxide nanoparticles. Polymer 2016, 87, 290–299. [Google Scholar] [CrossRef]
- Jyothi, M.S.; Nayak, V.; Padaki, M.; Geetha Balakrishna, R.; Soontarapa, K. Aminated polysulfone/TiO2 composite membranes for an effective removal of Cr(VI). Chem. Eng. J. 2016, 283, 1494–1505. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Dizman, C.; Altinkok, C.; Tasdelen, M.A. Synthesis of self-curable polysulfone containing pendant benzoxazine units via CuAAC click chemistry. Des. Monomers Polym. 2017, 20, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Di Vona, M.L.; Narducci, R.; Pasquini, L.; Pelzer, K.; Knauth, P. Anion-conducting ionomers: Study of type of functionalizing amine and macromolecular cross-linking. Int. J. Hydrogen Energy 2014, 39, 14039–14049. [Google Scholar] [CrossRef]
- Sarath, C.C.; Shanks, R.A.; Thomas, S. Chapter 1—Polymer Blends. In Nanostructured Polymer Blends; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Oxford, UK, 2014; pp. 1–14. ISBN 978-1-4557-3159-6. [Google Scholar]
- Sabzi, F. Chapter 24—Gas Transport Through Polymer Blends. In Transport Properties of Polymeric Membranes; Thomas, S., Wilson, R., Kumar, S.A., George, S.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 517–532. ISBN 978-0-12-809884-4. [Google Scholar]
- Utracki, L.A. Polymer blends: Fundamentals. In Polypropylene; Karger-Kocsis, J., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 601–605. ISBN 978-94-011-4421-6. [Google Scholar]
- Ryan, A.J. Designer polymer blends. Nat. Mater. 2002, 1, 8–10. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Feng, S.; Kondo, S.; Kaseyama, T.; Nakazawa, T.; Kikuchi, T.; Selyanchyn, R.; Fujikawa, S.; Christiani, L.; Sasaki, K.; Nishihara, M. Development of polymer-polymer type charge-transfer blend membranes for fuel cell application. J. Memb. Sci. 2018, 548, 223–231. [Google Scholar] [CrossRef]
- Niaounakis, M. Chapter 3—Blending. In Biopolymers: Processing and Products; Niaounakis, M., Ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 117–185. ISBN 978-0-323-26698-7. [Google Scholar]
- Rajeswari, A.; Stobel Christy, E.J.; Pius, A. Chapter 5—Biopolymer blends and composites: Processing technologies and their properties for industrial applications. In Biopolymers and Their Industrial Applications; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 105–147. ISBN 978-0-12-819240-5. [Google Scholar]
- Heeres, H.J.; van Maastrigt, F.; Picchioni, F. Polymeric Blends with Biopolymers. In Handbook of Biopolymer-Based Materials: From Blends and Composites to Gels and Complex Networks; Wiley-VCH Verlag GmbH & Co. KGaA: New York, NY, USA, 2013; pp. 143–171. ISBN 9783527328840. [Google Scholar]
- Robeson, L.M. Polymer Blends in Membrane Transport Processes. Ind. Eng. Chem. Res. 2010, 49, 11859–11865. [Google Scholar] [CrossRef]
- Islam, M.S.; Islam, M.M. 2-Physical and chemical properties of sustainable polymers and their blends. In Advances in Sustainable Polymer Composites; Rahman, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 37–57. ISBN 978-0-12-820338-5. [Google Scholar]
- García, M.G.; Marchese, J.; Ochoa, N.A. Aliphatic-aromatic polyimide blends for H2 separation. Int. J. Hydrogen Energy 2010, 35, 8983–8992. [Google Scholar] [CrossRef]
- Jones, P.J.; Paslay, L.C.; Morgan, S.E. Effects of chain conformation on miscibility, morphology, and mechanical properties of solution blended substituted polyphenylene and polyphenylsulfone. Polymer 2010, 51, 738–747. [Google Scholar] [CrossRef]
- White, J.L.; Wachowicz, M. Chapter 7 Polymer Blend Miscibility. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2008; Volume 64, pp. 189–209. ISBN 0066-4103. [Google Scholar]
- Goh, S.H. Miscible Polymer Blends. In Polymer Blends Handbook; Utracki, L.A., Wilkie, C.A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1915–2151. ISBN 978-94-007-6064-6. [Google Scholar]
- Rana, D.; Bag, K.; Bhattacharyya, S.N.; Mandal, B.M. Miscibility of poly(styrene-co-butyl acrylate) with poly(ethyl methacrylate): Existence of both UCST and LCST. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 369–375. [Google Scholar] [CrossRef]
- Olabisi, O. Interpretations of polymer-polymer miscibility. J. Chem. Educ. 1981, 58, 944–950. [Google Scholar] [CrossRef] [Green Version]
- Arrighi, V.; Cowie, J.M.G.; Fuhrmann, S.; Youssef, A. Miscibility Criterion in Polymer Blends and its Determination. Encycl. Polym. Blends 2010, 1, 153–198. [Google Scholar] [CrossRef]
- Higgins, J.S.; Lipson, J.E.G.; White, R.P. A simple approach to polymer mixture miscibility. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1009–1025. [Google Scholar] [CrossRef] [Green Version]
- Hopfenberg, H.B.; Paul, D.R. Chapter 10—Transport Phenomena in Polymer Blends. In Polymer Blends; Paul, D.R., Newman, S., Eds.; Academic Press: Cambridge, MA, USA, 1978; pp. 445–489. ISBN 978-0-12-546801-5. [Google Scholar]
- Fu, Z.; Wang, H.; Zhao, X.; Horiuchi, S.; Li, Y. Immiscible polymer blends compatibilized with reactive hybrid nanoparticles: Morphologies and properties. Polymer 2017, 132, 353–361. [Google Scholar] [CrossRef]
- Abtal, E.; Prud’Homme, R.E. Orientation of miscible and immiscible polymer blends. Polym. Eng. Sci. 1992, 32, 1857–1862. [Google Scholar] [CrossRef]
- Alkhodairi, H.; Russell, S.T.; Pribyl, J.; Benicewicz, B.C.; Kumar, S.K. Compatibilizing Immiscible Polymer Blends with Sparsely Grafted Nanoparticles. Macromolecules 2020, 53, 10330–10338. [Google Scholar] [CrossRef]
- Marischal, L.; Cayla, A.; Lemort, G.; Campagne, C.; Éric, D. Selection of immiscible polymer blends filled with carbon nanotubes for heating applications. Polymers 2019, 11, 1827. [Google Scholar] [CrossRef] [Green Version]
- Aseeri, J.; Alandis, N.M.; Mekhamer, W.; Alam, M. Miscibility studies of polystyrene/polyvinyl chloride blend in presence of organoclay. Open Chem. 2019, 17, 927–935. [Google Scholar] [CrossRef]
- Ajitha, A.R.; Thomas, S. Chapter 1—Introduction: Polymer blends, thermodynamics, miscibility, phase separation, and compatibilization. In Compatibilization of Polymer Blends; Ajitha, A.R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–29. ISBN 978-0-12-816006-0. [Google Scholar]
- Kordjazi, Z.; Ajji, A. Partially miscible polymer blends of ethyl cellulose and hydroxyl terminated polybutadiene. Polymer 2020, 211, 123067. [Google Scholar] [CrossRef]
- Pan, P.; Bao, J.; Han, L.; Xie, Q.; Shan, G.; Bao, Y. Stereocomplexation of high-molecular-weight enantiomeric poly(lactic acid)s enhanced by miscible polymer blending with hydrogen bond interactions. Polymer 2016, 98, 80–87. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Saljoughi, E.; Shahtahmassebi, N. Preparation of amorphous polyphenylsulfone nanofiltration membrane via thermally-induced lamination. J. Non. Cryst. Solids 2021, 551, 120416. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Saljoughi, E.; Shahtahmassebi, N. Novel high flux nanofibrous composite membrane based on polyphenylsulfone thin barrier layer on nanofibrous support. Fibers Polym. 2017, 18, 1531–1544. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Shahtahmassebi, N.; Saljoughi, E. Preparation and characterization of polyphenylsulfone nanofibrous membranes for the potential use in liquid filtration. Desalin. Water Treat. 2016, 57, 16250–16259. [Google Scholar] [CrossRef]
- Jones, P.J.; Cook, R.D.; McWright, C.N.; Nalty, R.J.; Choudhary, V.; Morgan, S.E. Polyhedral oligomeric silsesquioxane-polyphenylsulfone nanocomposites: Investigation of the melt-flow enhancement, thermal behavior, and mechanical properties. J. Appl. Polym. Sci. 2011, 121, 2945–2956. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Rahaman, M.; Alrehaili, A.; Alhoshan, M.; Aldalbahi, A. A Facile Approach for Elimination of Electroneutral/Anionic Organic Dyes from Water Using a Developed Carbon-Based Polymer Nanocomposite Membrane. Water. Air. Soil Pollut. 2020, 231, 104–119. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Muthumareeswaran, M.R. Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci. Rep. 2017, 7, 41976–41987. [Google Scholar] [CrossRef] [Green Version]
- Sani, N.A.A.; Lau, W.J.; Ismail, A.F. Morphologies and separation characteristics of polyphenylsulfone-based solvent resistant nanofiltration membranes: Effect of polymer concentration in casting solution and membrane pretreatment condition. Korean J. Chem. Eng. 2015, 32, 743–752. [Google Scholar] [CrossRef]
- Ali, Q.; Taweepreda, W.; Techato, K. Preparation and characterization of polymer electrolyte membrane from chloroacetate chitosan/chitosan blended with epoxidized natural rubber. Polym. Test. 2020, 82, 106294. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Memb. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef]
- Sun, F.Y.; Wang, X.M.; Li, X.Y. Effect of biopolymer clusters on the fouling property of sludge from a membrane bioreactor (MBR) and its control by ozonation. Process Biochem. 2011, 46, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Yang, H.; Xu, Z.; Li, F. Surface Modification of Polyacrylonitrile Membrane by Chemical Reaction and Physical Coating: Comparison between Static and Pore-Flowing Procedures. ACS Omega 2018, 3, 4231–4241. [Google Scholar] [CrossRef]
- Lau, W.-J.; Ong, C.-S.; Nordin, N.A.H.M.; Sani, N.A.A.; Mokhtar, N.M.; Jamshidi Gohari, R.; Emadzadeh, D.; Fauzi Ismail, A. Surface Modification of Polymeric Membranes for Various Separation Processes. Surf. Treat. Biol. Chem. Phys. Appl. 2017, 34, 115–180. [Google Scholar]
- Jullok, N.; Darvishmanesh, S.; Luis, P.; Van der Bruggen, B. The potential of pervaporation for separation of acetic acid and water mixtures using polyphenylsulfone membranes. Chem. Eng. J. 2011, 175, 306–315. [Google Scholar] [CrossRef]
- Mys, N.; Geiregat, M.; Deruyck, F.; Verberckmoes, A.; Cardon, L. Physicochemical processing of polyphenylsulfone: Optimization of processing parameters for microsphere production via spray drying using a response surface model. Adv. Polym. Technol. 2018, 37, 3747–3758. [Google Scholar] [CrossRef]
- Lu, Z.; Dunn, M.L. Van der Waals adhesion of graphene membranes. J. Appl. Phys. 2010, 107, 44301. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Chapter 13—Van der Waals Forces between Particles and Surfaces. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 253–289. ISBN 978-0-12-375182-9. [Google Scholar]
- He, M.; Fan, X.; Yang, Z.; Zhang, R.; Liu, Y.; Fan, L.; Zhang, Q.; Su, Y.; Jiang, Z. Antifouling high-flux membranes via surface segregation and phase separation controlled by the synergy of hydrophobic and hydrogen bond interactions. J. Memb. Sci. 2016, 520, 814–822. [Google Scholar] [CrossRef]
- Alayemieka, E.; Lee, S.; Lee, S.; Kim, D. A power-law model of electrostatic interactions of colloidal particles in cake layer on membrane surface. Desalination 2010, 250, 793–797. [Google Scholar] [CrossRef]
- Li, Q.; Shi, W.; Yang, Q. Polarization Induced Covalent Bonding: A New Force of Heavy Metal Adsorption on Charged Particle Surface. J. Hazard. Mater. 2021, 412, 125168. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cui, Z.; Dong, N.; Li, B.; Zhang, Y.; Wang, J.; Jiang, Z. Enhanced optical limiting properties of composite films consisting of hyperbranched phthalocyanine and polyphenylsulfone with high linear transmittance. Synth. Met. 2020, 265, 116405. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, H.; Li, B.; Jiang, Z.; Zhang, Y.; Li, Z. New Type of Eco-Friendly Polymeric Dye by Covalently Bonding Anthraquinone into Polyphenylsulfone. Macromol. Mater. Eng. 2019, 304, 1–9. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Moeini, N.; Yadav, M.; Ahmad, S. Chapter 12—Antimicrobial polymer nanocomposite films and coatings. In Handbook of Polymer Nanocomposites for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 379–397. ISBN 978-0-12-821497-8. [Google Scholar]
- Reis, R.; Dumée, L.F.; Tardy, B.L.; Dagastine, R.; Orbell, J.D.; Schutz, J.A.; Duke, M.C. Towards Enhanced Performance Thin-film Composite Membranes via Surface Plasma Modification. Sci. Rep. 2016, 6, 29206. [Google Scholar] [CrossRef]
- Puppolo, M.M.; Hughey, J.R.; Weber, B.; Dillon, T.; Storey, D.; Cerkez, E.; Jansen-Varnum, S. Plasma modification of microporous polymer membranes for application in biomimetic dissolution studies. AAPS Open 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Membrane Surface Modification by Plasma-Induced Polymerization of Acrylamide for Improved Surface Properties and Reduced Protein Fouling. Langmuir 2003, 19, 79–85. [Google Scholar] [CrossRef]
- Kormunda, M.; Ryšánek, P.; Hájková, P.; Štěpanovská, E.; Čapková, P.; Pavlík, J. Effect of low energy plasma treatment on surface chemistry and phase composition of electrospun polyvinylidene fluoride membrane. Surf. Interfaces 2021, 22, 100900. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Grant, D.; Kumar, A.; Bazaka, K.; Jacob, M.V. Chapter 8—Plasma Treatment of Polymeric Membranes. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., Praveen, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240. ISBN 978-0-12-813152-7. [Google Scholar]
- Fatyeyeva, K.; Dahi, A.; Chappey, C.; Langevin, D.; Valleton, J.M.; Poncin-Epaillard, F.; Marais, S. Effect of cold plasma treatment on surface properties and gas permeability of polyimide films. RSC Adv. 2014, 4, 31036–31046. [Google Scholar] [CrossRef]
- Poncin-Epaillard, F.; Brosse, J.C.; Falher, T. Cold Plasma Treatment: Surface or Bulk Modification of Polymer Films? Macromolecules 1997, 30, 4415–4420. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, M.-X.; Dai, Z.-W.; Tian, J.; Xu, Z.-K. Fabrication of Glycosylated Surface on Polymer Membrane by UV-Induced Graft Polymerization for Lectin Recognition. Langmuir 2006, 22, 9345–9349. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, S.; Liu, J.; He, J.; Li, J.; Wang, L.; Lei, J. Fabrication and characterization of a defect-free mixed matrix membrane by facile mixing PPSU with ZIF-8 core–shell microspheres for solvent-resistant nanofiltration. J. Memb. Sci. 2019, 589, 117261. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide TFC membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.S.; Ali, F.A.A.; Mishra, U.; Hamid, A.A. Thin-Film Nanocomposite Membrane Incorporated with Porous Zn-Based Metal-Organic Frameworks: Toward Enhancement of Desalination Performance and Chlorine Resistance. ACS Appl. Mater. Interfaces 2021, 13, 28818–28831. [Google Scholar] [CrossRef]














| Description | Methods of Modification | Modifier Agents | Process of Membrane | Application | Performance | Ref. |
|---|---|---|---|---|---|---|
| Proton-conductive sPPSU membranes | Sulfonation | SO3 and (CH3)3 SiSO3Cl | Solvent evaporation | Electrochemical | (CH3)3SiClSO3 gave a homogeneous sPPSU with better control of the DS values as high as 1.0; asymmetric structure; high mechanical stability; proton conductivity about 55 mS/cm at 80 °C | [51] |
| Proton-conducting fuel cell sphPPSU membranes | Sulfophenylation | BuLi (metalating agent) and 2-sulfobenzoic acid cyclic anhydride | Vacuum dry | Fuel cells | sphPPSU showed DS values as 0.9; membranes have high thermal stability (300 and 350 °C); the proton conductivity about 60 mS/cm at 70 °C | [52] |
| PEI/PPSU sheet | Blending | PEI | Direct injection molding | Plasticization | PEI/PPSU blends are miscible; elasticity and yield stress changed linearly with PEI-rich blends composition | [53] |
| Proton exchange SPEEK/SiSPPSU membranes | Silylation and sulfonation; and blending | PhSiCl3 and H2SO4; SPEEK | Solvent evaporation | Fuel cells | SiSPPSU showed DS values as 2.0; exhibited high and stable conductivity values at 120 °C when dry (6.1 × 10−3 S/cm) and wet conditions (6.4 × 10−2 S/cm) | [54] |
| sPPSU-proton conducting membrane | Sulfonation | H2SO4 and ClSO3Si (CH3)3 | Sol-gel processes | Fuel cells | sPPSU reached the conductivity values as high as 1.1 × 10−2 S cm−1 at 130 °C | [55] |
| PPSU/PBNPI membrane | Blending | PBNPI | Solvent evaporation | Hydrogen separation | The gases H2, CO2 and CH4 permeability increased up to 50% | [56] |
| PPSU/PBNPI membrane | Blending; immersion method | PBNPI; p-xylylenediamine (crosslinking reagent) | Solvent evaporation | Gas permeation | O2 and N2 permeation rates of 23.2 and 22.42 | [57] |
| sPOSS/sPPSU composite proton exchange membranes | Blending | sPOSS | Dry | Fuel cells | sPOSS/sPPSU composites multilayered structure and reduce brittleness; conductivity 1 × 10−2 S cm−1 at 90 °C | [1] |
| Ionic exchange sPPSU/sPES membrane | Sulfonation; Blending | H2SO4; sPES | Solvent evaporation and dry | Fuel cells | The membrane surfaces show the smoother about 2 nm; stress–strain values 80 MPa and 7% | [5] |
| SPEEK/SiPPSU composite membranes | Silylation; Blending | SPEEK | Dry | Fuel cells | The presence of silicon enhances the temperature of loss of sulfonic acid groups; composites show superior behavior in terms of mechanical properties (higher elastic modulus and tensile strength) | [50] |
| PPSU/PEI membranes | Blending | PEI; PEG 200 | Wet phase inversion | Ultrafiltration | Asymmetric and spongelike structure; water contact angle decreases significantly upto 64° and EWC 59.37%; IEP shifted pH 8 and shown positive charge; flux 545.54 kg m−2 h−1; rejection 56% | [20] |
| sPPSU positively charged membrane | UV grafting | [2-(methacryloyloxy)ethyl]trimethyl ammonium chloride; diallyldimethylammonium chloride | Nanofiltration; textile dyes | Spongelike morphology; MWCO 1627–1674 Da; PWP of 9–14 LMH bar−1; rejection of MgCl2 (95%) and Safranin O dye (99.9%) | [58] | |
| PPSU thin-film composite membrane | Oxygen plasma (pretreatment); surface modification | 2,5-bis(4-amino- 2-trifluoromethyl-phenoxy)benzenesulfonic acid; 4,4-bis(4-amino-2-trifluoromethyl-phenoxy)biphenyl-4,4-disulfonic acid | interfacial polymerization | Nanofiltration; dye removal | Water flux 63.9 and 71.3 L/m2 h; dye rejection 48–80% | [59] |
| sPPSU/sPES membranes | Sulfonation; Blending | H2SO4; sPES | Crosslinking; heat and dry | Fuel cells | Maximum conductivity of 0.12 S/cm | [60] |
| sPPSU TFC membranes | Surface modification | MPD;TMC | Interfacial polymerization | Forward osmosis | Water flux up to 54 LMH with 8.8 gMH salt reverse flux under PRO mode | [61] |
| PPSU/PI solvent resistant membrane | Blending | PI | Phase inversion; solvent evaporation | Nanofiltration | Asymmetric structure with a dense skin layer; highest flux for alcohol and alkanes was achieved for a 50/50 wt.% blend; | [62] |
| PPSU/TiO2 nanocomposites membrane | Blending | TiO2 | Solvent evaporation | Biomedical | Nanocomposites shown active inhibition against E. coli and S. aureus bacteria with and without UV irradiation; the stiffness, strength, toughness, hardness and heat distortion temperature increases | [63] |
| Anion exchange PyPPSU membrane | Blending | 1-methyl-2-pyrrolidone | Solvent evaporation | Vanadium redox flow battery | Vanadium ions permeability (0.07 × 10−7–0.15 × 10−7 cm2 min−1); coulombic efficiency of 97.8% and energy efficiency of 80.2% | [64] |
| PPSU solvent resistant membrane | Blending | Cu-BTC | Phase inversion | Nanofiltration; methanol–dye separation | Improve tensile strength 29%; methanol flux 135 L m−2 h−1 | [65] |
| PPSU nanofibrous membrane | Blending | PEG 400 | Electrospinning | Wastewater treatments | Water contact angle 8.9°; porosity 72.4%; water flux 7920 L/m2h | [66] |
| PPSU membranes | Blending | sPPSU | Phase inversion | Ultrafiltration | Porosity 48%; MWCO 70 kDa; pure water flux 218 L m−2 h−1; FRR 79%; BSA rejection 85% | [49] |
| sPPSU/PIM-1 membrane | Blending | sDCDPS; PIM-1 | Slower solvent evaporation | Gas Separation | The tensile strength up to 72 MPa and extension at break 3.5%; the gas separation performance above the Robeson upper bounds for O2/N2, CO2/N2, CO2/CH4 | [67] |
| PPSU/FAC composite membrane | Blending | FAC | Phase inversion | Phenol filtration | Fragmented surface and spongy porous linkages; contact angle 43.8°; porosity 30%; pure water flux 26 Lm−2 h−1, phenol rejection 96.4% | [68] |
| MgO/sPPSU/PPSU membranes | Blending | MgO; sPPSU | Phase inversion | Ultrafiltration; Oil separation | Porosity 65% and MWCO 70 kDa; contact angle 48°; FRR 85% and HA rejection 63% and castor oil rejection 99% | [69] |
| PPSU/Cu-BTC solvent resistant nanofiltration | Blending | Cu-BTC | Phase inversion | Nanofiltration; dye and methanol separation | Contact angle 61°, and porosity 62%; Flux 19 L/m2 h and rejection of methanol 93% | [70] |
| sPPSU proton exchange membrane | Sulfonation; Blending | H2SO4 | Solvent evaporation | Fuel cells | Conductivity of 0.1 S/cm and power density of 471 mW/cm2 at 80 °C | [71] |
| PPSU membrane | Blending | PVP; PEG; Tween 80 | Phase inversion | Ultrafiltration | Water flux 148 L/m2 h; BSA rejection increased from 53.2% to 81.5% | [30] |
| sPPSU asymmetric membranes | Sulfonation; Blending | TMSClS | Phase inversion | Ultrafiltration | Decomposition temperature at 510 °C; contact angle 33°, and porosity 51%; FRR 70% | [72] |
| sPPSU/f-SWCNTs mixed-matrix membranes | Sulfonation; Blending | 3,3′-disulfonated 4,4′-dichlorodiphenyl sulfone; f-SWCNTs | Phase inversion | Gas separation | Enhanced the permeability for N2, O2, He, and CO2 and the selectivity for O2/N2 and O2/CO2 | [73] |
| Porous PPSU membrane | Blending | Carrageenan | Phase inversion | Ultrafiltration | Contact angle 43° and porosity 78%; zeta potential −24 mV at pH 7; permeability increased up to 29 Lm−2 h−1 bar−1 | [74] |
| PPSU/GO mixed matrix membrane | Blending | GO; PEG1000 | Phase inversion | Ultrafiltration | Hydrophilicity and the thermal stability improved; pure water flux 132 L·m−2·h−1 and the rejection 96.8% | [28] |
| PPSU/Zeolite mixed matrix membrane | Blending | Fe-ZSM-5; Cu-ZSM-5 | Phase inversion | Organic compounds removal | Surface roughness increased (Ra- 18.52 nm); zeta potential about −57.2 mV at pH 7; water flux of 62 L·m−2·h−1, lignin rejection up to 88.5% | [31] |
| PPSU/BiOCl-AC membrane | Blending | BiOCl-AC; PVP | Phase inversion | Ultrafiltration; oil separation | Asymmetric structures with thick top layer; contact angle 67°; pure water flux 465 L·m−2·h−1; rejection diesel fuel 80% and 90% of crude oil | [42] |
| Alkali resisting PPSU membrane | Blending | PVP- 10, 55, 360, and 1300 kDa | Phase inversion | Ultrafiltration | Asymmetric and fingerlike structure; Tensile strength upto 2.53 MPa for 10 kDa; MWCO ranged from 2 kDa to 175 kDa; pure water flux 69 L·m−2·h−1; better anti-alkali property in NaOH solution (pH = 13) | [13] |
| HBE–MMT/PPSU nanocomposite membrane | Blending | Functionalized montmorillonite | Phase inversion | Water treatment | Contact angle 53.6°; pure water flux about 380 L·m−2·h−1 at 5 bar; rejection of salt 40–50% | [75] |
| Polyamide TFN PPSU membrane | Blending; Surface modification | GO (support layer); PIP and TMC | Interfacial polymerization | Nanofiltration; l kinetic hydrate inhibitor (KHI) removal | KHI rejection of 99% and permeation flux of 32.7 L/m2 h (at 9 bar and feed concentration of 0.5 wt.% KHI) | [76] |
| sPPSU/TiO2 mixed matrix hollow fiber membranes | Blending | TiO2 | Phase inversion | Ultrafiltration | Pure water flux 60 L·m−2·h−1; contact angle 67°; rejection of BSA 91% | [77] |
| PPSU membrane | Blending | PEG 400; PEG 20000 | Phase inversion | Filtration of aqueous media | Porosity 72%; tensile Strength at Break 7.75 MPa and elongation at Break 50.14%; Pure water flux 19 L·m−2·h−1 (PEG400) and 183 L·m−2·h−1 (PEG20000); 100% turbidity rejection | [10] |
| PPSU membrane | Blending | PEG 400; PEG 2000; PEG 6000; PEG 20000; PEG 35000; PEG 40000 | Phase inversion | Ultrafiltration | Contact angle 50° to 90°; pure water flux of 486 Lm−2 h−1; human serum albumin rejection 90% | [78] |
| Ionic crosslinked sPPSU membrane | Surface modification | HPEI | Coating | Nanofiltration; organic solvent filtration | Ethanol permeability 1.47 L m−2 h−1 bar−1; rejection of 99.9% to Rose Bengal dye | [79] |
| High-Flux PPSU membranes | Blending | PEG 6000–40000 | Phase inversion | Ultrafiltration | Pure water flux 500–1000 L m–2 h–1 at 0.1 MPa; 90% rejection of human serum albumin (PEG20000) | [80] |
| PA-MOF/PPSU-GO TFN membrane | Blending; Surface modification | GO (support layer); MOF; PIP and TMC | Interfacial polymerization | Nanofiltration | Permeate flux 59.9 L/m2·h; KHI rejection 96%; FRR 97.8% and an excellent long-term stability | [81] |
| sPPSU/PBI membrane | Blending; crosslinking | PBI; DBX (crosslinker) | Heat and solvent evaporation | Nanofiltration; organic solvent removal | Permeability 11.8 Lm−2 h−1 bar−1; rejection of tetracycline 97%. | [82] |
| Double crosslinked sPPSU/PBI membrane | Blending; crosslinking | PBI; DBX (crosslinker) | Heat and solvent evaporation | Nanofiltration; hydrogen purification | H2 permeability of 46.2 Barrer and a high H2/CO2 selectivity of 9.9 at 150 °C | [83] |
| Amine functionalized PPSU membrane | Amination; Blending | SnCl2; HNO3 | Phase inversion | Nanofiltration; dye removal | Pore size of 0.72 nm; positively charged active layers; contact angles 31°; pure water flux ∼54 Lm−2 h−1; CaCl2 and AlCl3 multivalent salts rejection 89% and 93.5%; crystal violet dye rejection > 99% | [84] |
| High-performance PPSU/sPANI membrane | Blending | sPANI | Nonsolvent induced phase separation | Ultrafiltration | Contact angle was 57°; porosity 81%; BSA adsorption value of 3.6 μg/cm2; water flux of 260 L/m2 h; BSA rejection 95% | [40] |
| PPSU/carboxylated GO nanocomposite membrane | Blending | Carboxylated GO | Phase inversion | Nanofiltration; heavy metal removal | Surface charge of −70 mV; flux of 27 L m−2 h−1; rejection of As(V) 96%, Cr(VI) 93%, Zn2+(81%), Cd2+ (74%), Pb2+ (73%) | [85] |
| sPPSU membrane | Sulfonation | H2SO4 | Phase inversion | Ultrafiltration; heavy metal and protein separation | Water flux of 190.33 Lm−2 h−1 and FRR of 86.56%; protein rejection of 66.3%, 74.0% and 91.2% for trypsin, pepsin, and BSA; Cd2+ and Pb2+ ions rejection of 75.2% and 87.6%; | [86] |
| PPSU/carboxylated GO nanocomposite membrane | Blending | Carboxylated GO | Phase inversion | Ultrafiltration; Antimicrobial and antifouling | Bacteriostasis rates of 74.2%,81.1% and 41.9% against E. coli, P. aeruginosa and S. aureus; FRR 95.3% | [87] |
| Porous PPSU/sPEEK membrane | Blending | sPEEK | Solvent evaporation | Vanadium flow batteries | Contact angle 47°; tensile strength 2.78 MPa; proton conductivity of 14.3 mS cm−1 at 15 °C | [88] |
| PPSU/SnO2 mixed matrix hollow fiber membrane | Blending | SnO2 | Vacuum evaporation | Ultrafiltration; dyes removal | Contact angle 63°; porosity 84%; pure water flux 362.9 L/m2 h; dyes rejection about >94% for RB-5, and >73% for RO-16 | [89] |
| PPSU/CuO/g-C3N4 membrane | Blending | CuO/g-C3N4 | Nonsolvent induced phase inversion | Ultrafiltration; antifouling and protein separation | Smooth surfaces Ra-9.8 nm; increase pores on the top layer as well as in the sublayer; contact angle 48°; water flux 202 L/m2h; BSA protein rejection 96%; FRR 79% | [90] |
| Super-hydrophilic PPSU TFC membrane | Surface modification | MPD and TMC | Electrospun; plasma treatments; interfacial polymerization | Forward osmosis | Contact angle 0°; Osmotic water flux 14 L/m2h | [91] |
| PPSU hollow fiber membranes | Blending | CA; CAP | Dry-wet spinning | Ultrafiltration; arsenic removal | Contact angle 60° and 43°; arsenic removal 34% and 41%; pure water permeability 61.47 L/m2h bar and 69.60 L/m2 h bar; FRR 88.67% | [92] |
| PPSU/silver-hydroxyapatite nanocomposite membrane | Blending | silver-hydroxyapatite | Phase inversion | Ultrafiltration; organic matter removal | Porous and honeycomblike structure; contact angle 60°; rejection 89% | [93] |
| Proton exchange sulfonated PPSU/PSU membrane | Sulfonation | Trimethylsilyl chlorosulfonate; | Vacuum dry | Fuel cells | Proton conductivity 34.1 mS cm−1 at 70 °C; power density of 400 mW cm−2; current density of 1100 mA cm−2 | [35] |
| PPSU/Ag-MWCNTs nanocomposite membrane | Blending | Ag-MWCNTs | Phase inversion | Nanofiltration; ion removal and antibacterial activity | Zeta potential −78 mV; contact angle 49°; porosity 73%; rejection of Na2HAsO4 99.5% and Na2Cr2O7 100% | [87] |
| PPSU/MWCNTs membrane | Blending | MWCNTs | Phase inversion | Ultrafiltration; heavy metals removal | Dense skin layer on top and a porous supportive sub-layer; surface roughness Ra 21 nm; contact angle 61°; porosity 50%; flux 186 L/m2 h rejection of Pb2+ (>98%), Hg2+ (>76%) and Cd2+ (>72%) | [94] |
| PPSU/ZnO nanocomposite membrane | Blending | ZnO | Phase inversion | Nanostructured- hybrid membranes; anionic dye; antimicrobial; wastewater treatment | Pore size 0.75 nm; zeta potential –65.7 mV at pH 7; methyl orange dye rejection 98% with a water flux 19 L/m2h; antibacterial activity of E. coli (6.2) and S. aureus (6.8) | [95] |
| Hydrophilic PPSU membranes | Blending | 1,2-propandiol; PVP | Nonsolvent induced phase separation | Ultrafiltration | Contact angles of 46.4°;Water flux 674 kg m−2 bar−1h−1 | [96] |
| PPSU/PES/SiO2 nanocomposite membrane | Blending | PES; SiO2 | Vapor induced phase separation; nonsolvent induced phase separation | Ultrafiltration | Water flux 76.65 L/m2·h; BSA retention of 82.01%; | [97] |
| Silica filled PPSU/PDMS Composite Membranes | Surface modification | PDMS; Silica | Coating | Biobutanol Separation | Weight loss starts from 400 °C; contact angle ∼130°; flux 536 g. m−2 h−1; butanol separation factor 30.6 | [36] |
| PPSU/PANI hollow fiber membrane | Blending | PANI | Dry-jet wet spinning | Humic acid removal | Zeta potential −16 mV at pH 9; Water flux 127 L/m2h; Humic acid rejection 98%; | [98] |
| Proton exchange sPPSU membrane | Sulfonation | H2SO4; CNDs (crosslinker) | Vacuum dry | Fuel cells | Proton conductivity 10−2 S/cm at 120 °C. | [99] |
| PPSU/Al-MOF mixed matrix membrane | Blending | Al-MOF | Phase inversion | Ultrafiltration,; dye separation; antifouling | Contact angle 63°; surface roughness Ra 21.9 nm; pure water flux 47 L·m−2·h−1; FRR 93%; rejection of organic dye methyl violet 93.8% | [100] |
| PPSU/CA/ZrO2 hollow fiber membranes | Blending | CA; ZrO2 | Dry-wet spinning | Arsenic Removal | Surface roughness Ra 43 nm; contact angle 48°; permeability of 89.94 L/m2h bar; removal of arsenic 87% | [45] |
| PPSU/CA hollow fiber membrane | Blending | CA | Dry–wet spinning | Removal of dyes | Permeability 64.47 L/m2 h bar; removal of Reactive black 5 dye 95% | [101] |
| PPSU/Zn-MOF composite membrane | Blending | Zn-MOF | Phase inversion | Ultrafiltration; antifouling | Asymmetric structure and dense microporous active skin layer; surface roughness Ra 13.88 nm; porosity 72%; tensile strength 7.9 MPa; permeability 33 L m−2 h−1 bar−1; FRR 98% | [102] |
| PPSU/CA/ZnO-MgO hollow fiber membrane | Blending | CA; ZnO-MgO | Dry–wet phase inversion | Arsenic removal | contact angle 60°; permeability 69.58 L/m2h bar; arsenic rejection 81.31%; FRR 91% | [103] |
| PANI coated PPSU Membranes | Surface modification | PANI | Coating | Dye separation; antibacterial activities | Surface roughness Ra-3.15 nm; contact angle 55°; zeta potential −1.7 mV at pH 6; permeability 53 L·m−2·h−1·bar−1; rejection of methylene blue dye 96%; bacteriostasis of E. coli 95% and S. aureus 88% | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, A.K.; Alam, J.; Alhoshan, M. Recent Advancements in Polyphenylsulfone Membrane Modification Methods for Separation Applications. Membranes 2022, 12, 247. https://doi.org/10.3390/membranes12020247
Shukla AK, Alam J, Alhoshan M. Recent Advancements in Polyphenylsulfone Membrane Modification Methods for Separation Applications. Membranes. 2022; 12(2):247. https://doi.org/10.3390/membranes12020247
Chicago/Turabian StyleShukla, Arun Kumar, Javed Alam, and Mansour Alhoshan. 2022. "Recent Advancements in Polyphenylsulfone Membrane Modification Methods for Separation Applications" Membranes 12, no. 2: 247. https://doi.org/10.3390/membranes12020247
APA StyleShukla, A. K., Alam, J., & Alhoshan, M. (2022). Recent Advancements in Polyphenylsulfone Membrane Modification Methods for Separation Applications. Membranes, 12(2), 247. https://doi.org/10.3390/membranes12020247