Isoporous Polyvinylidene Fluoride Membranes with Selective Skin Layers via a Thermal-Vapor Assisted Phase Separation Method for Industrial Purification Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Preparation
2.2. Characterization
2.3. Separation Performance Evaluation
3. Results and Discussion
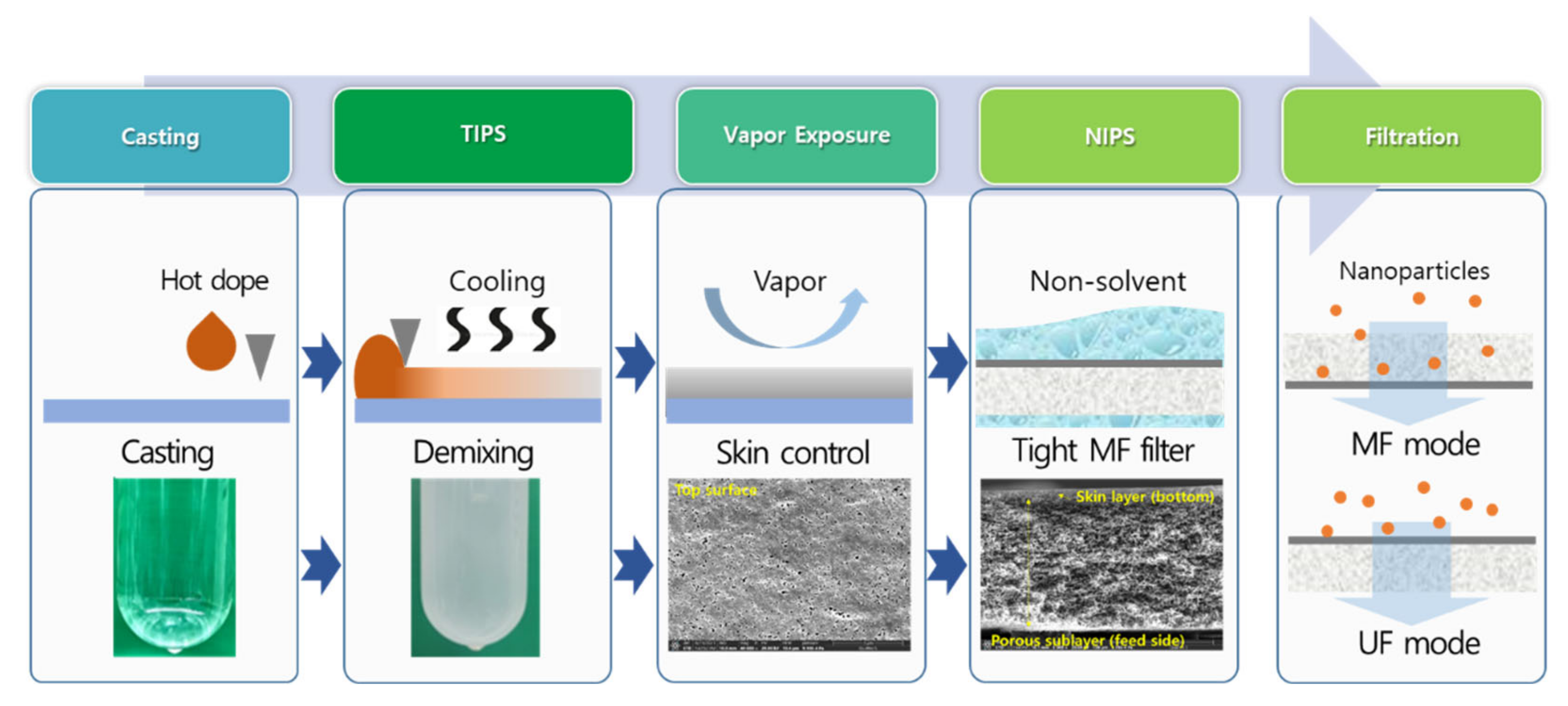
3.1. Membrane Fabrication
3.2. Effects of Additives on the Membrane Properties
3.3. Effects of Water Vapor Exposure on the Membrane Properties
3.4. Filtration Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Mulder, M.; Mulder, J. Basic Principles of Membrane Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Eyal, A.M.; Bressler, E. Industrial separation of carboxylic and amino acids by liquid membranes: Applicability, process considerations, and potential advantage. Biotechnol. Bioeng. 1993, 41, 287–295. [Google Scholar] [CrossRef]
- Cuperus, F.P.; Nijhuis, H.H. Applications of membrane technology to food processing. Trends Food Sci. Technol. 1993, 4, 277–282. [Google Scholar] [CrossRef]
- Turan, M.; Ates, A.; Inanc, B. Fouling of reverse osmosis and nanofiltration membranes by diary industry effluents. Water Sci. Technol. 2002, 45, 355–360. [Google Scholar] [CrossRef]
- Pabby, A.K.; Rizvi, S.S.; Requena, A.M.S. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Singh, N.; Cheryan, M. Membrane Technology in Corn Refining and Bioproduct-Processing. Starch-Stärke 1998, 50, 16–23. [Google Scholar] [CrossRef]
- Leos, J.Z.; Zydney, A.L. Microfiltration and Ultrafiltration: Principles and Applications; Routledge: London, UK, 2017. [Google Scholar]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration membrane processes: A review of research trends over the past decade. J. Water Process Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 42042. [Google Scholar] [CrossRef]
- Huehmer, R.P. MF/UF pretreatment in seawater desalination: Applications and trends. In Proceedings of the World Congress in Desalination and Reuse, International Desalination Association, IDAWC/DB09-253, Dubai, United Arab Emirates, 7–12 November 2009. [Google Scholar]
- Tang, Y.; Lin, Y.; Ma, W.; Wang, X. A review on microporous polyvinylidene fluoride membranes fabricated via thermally induced phase separation for MF/UF application. J. Membr. Sci. 2021, 639, 119759. [Google Scholar] [CrossRef]
- Al-Maas, M.; Hussain, A.; Matar, J.M.; Ponnamma, D.; Hassan, M.K.; Al-Maadeed, M.A.A.; Alamgir, K.; Adham, S. Validation and application of a membrane filtration evaluation protocol for oil-water separation. J. Water Process Eng. 2021, 43, 102185. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Duek, A.; Gitis, V. Maximal pore size in UF membranes. J. Membr. Sci. 2012, 394, 89–97. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Firoozpour, L.; Vanneste, J.; Luis, P.; Degrève, J.; Van der Bruggen, B. Performance of solvent resistant nanofiltration membranes for purification of residual solvent in the pharmaceutical industry: Experiments and simulation. Green Chem. 2011, 13, 3476–3483. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Eisnor, J.D.; O’Leary, K.C.; Gagnon, G.A. Evaluation of particle removal at water treatment plants in Nova Scotia. Water Qual. Res. J. 2001, 36, 105–119. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chang, J.-S.; Vigneswaran, S.; Kandasamy, J. Pharmaceutical wastewater treatment by membrane bioreactor process–a case study in southern Taiwan. Desalination 2008, 234, 393–401. [Google Scholar] [CrossRef]
- XpressKleen™ G2 Filters & XpressKleen™ G2 KC Assemblies Product Guideline. Available online: https://shop.pall.com/us/en/microelectronics/semiconductor/wet-etch-cleans-3/zidhhtg9041 (accessed on 10 January 2022).
- Kuriyel, R.; Zydney, A.L. Sterile filtration and virus filtration. In Downstream Processing of Proteins; Springer: Berlin/Heidelberg, Germany, 2000; pp. 185–194. [Google Scholar]
- Kim, I.-S.; Choi, Y.-W.; Kang, Y.; Sung, H.-M.; Sohn, K.-W.; Kim, Y.-S. Improvement of virus safety of an antihemophilc factor IX by virus filtration process. J. Microbiol. Biotechnol. 2008, 18, 1317–1325. [Google Scholar] [PubMed]
- Bakhshayeshirad, M. Performance Characteristics of Virus Filtration Membranes: Protein Fouling and Virus Retention. Ph.D. Thesis, Penn State University, State College, PA, USA, 2011. [Google Scholar]
- Wickramasinghe, S.R.; Stump, E.D.; Grzenia, D.L.; Husson, S.M.; Pellegrino, J. Understanding virus filtration membrane performance. J. Membr. Sci. 2010, 365, 160–169. [Google Scholar] [CrossRef]
- DiLeo, A.J.; Allegrezza, A.E.; Builder, S.E. High resolution removal of virus from protein solutions using a membrane of unique structure. Bio/Technology 1992, 10, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kosiol, P.; Hansmann, B.; Ulbricht, M.; Thom, V. Determination of pore size distributions of virus filtration membranes using gold nanoparticles and their correlation with virus retention. J. Membr. Sci. 2017, 533, 289–301. [Google Scholar] [CrossRef]
- Bolton, G.; Cabatingan, M.; Rubino, M.; Lute, S.; Brorson, K.; Bailey, M. Normal-flow virus filtration: Detection and assessment of the endpoint in bioprocessing. Biotechnol. Appl. Biochem. 2005, 42, 133–142. [Google Scholar]
- Fallahianbijan, F.; Giglia, S.; Carbrello, C.; Bell, D.; Zydney, A.L. Impact of protein fouling on nanoparticle capture within the Viresolve® Pro and Viresolve® NFP virus removal membranes. Biotechnol. Bioeng. 2019, 116, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Tam, S.-Y.; Lu, Y.; Brinker, C.J. Dual-layer asymmetric microporous silica membranes. J. Membr. Sci. 2000, 169, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Bonyadi, S.; Chung, T.S. Flux enhancement in membrane distillation by fabrication of dual layer hydrophilic–hydrophobic hollow fiber membranes. J. Membr. Sci. 2007, 306, 134–146. [Google Scholar] [CrossRef]
- Sui, Y.; Wang, Z.; Gao, X.; Gao, C. Antifouling PVDF ultrafiltration membranes incorporating PVDF-g-PHEMA additive via atom transfer radical graft polymerizations. J. Membr. Sci. 2012, 413, 38–47. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.; Zereshki, S.; Mansourpanah, Y. Preparation and characterization of modified nano-porous PVDF membrane with high antifouling property using UV photo-grafting. Appl. Surf. Sci. 2009, 255, 7455–7461. [Google Scholar] [CrossRef]
- Qin, H.; Sun, C.; He, C.; Wang, D.; Cheng, C.; Nie, S.; Sun, S.; Zhao, C. High efficient protocol for the modification of polyethersulfone membranes with anticoagulant and antifouling properties via in situ cross-linked copolymerization. J. Membr. Sci. 2014, 468, 172–183. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Mansourpanah, Y. Nano-porous polyethersulfone (PES) membranes modified by acrylic acid (AA) and 2-hydroxyethylmethacrylate (HEMA) as additives in the gelation media. J. Membr. Sci. 2010, 364, 380–388. [Google Scholar] [CrossRef]
- Menut, P.; Su, Y.; Chinpa, W.; Pochat-Bohatier, C.; Deratani, A.; Wang, D.; Huguet, P.; Kuo, C.; Lai, J.; Dupuy, C. A top surface liquid layer during membrane formation using vapor-induced phase separation (VIPS)—Evidence and mechanism of formation. J. Membr. Sci. 2008, 310, 278–288. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.-P.; Bouyer, D.; Drioli, E.; Kiadeh, N.T.H. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2020, 597, 117601. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Lu, X.; Xiao, C. Morphology changes of polyvinylidene fluoride membrane under different phase separation mechanisms. J. Membr. Sci. 2008, 320, 477–482. [Google Scholar] [CrossRef]
- Abdulla AlMarzooqi, F.; Roil Bilad, M.; Ali Arafat, H. Improving liquid entry pressure of polyvinylidene fluoride (PVDF) membranes by exploiting the role of fabrication parameters in vapor-induced phase separation VIPS and non-solvent-induced phase separation (NIPS) processes. Appl. Sci. 2017, 7, 181. [Google Scholar] [CrossRef]
- Zhao, J.; Chong, J.Y.; Shi, L.; Wang, R. Explorations of combined nonsolvent and thermally induced phase separation (N-TIPS) method for fabricating novel PVDF hollow fiber membranes using mixed diluents. J. Membr. Sci. 2019, 572, 210–222. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; Di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, S.D.; Kim, J.F.; Choi, H.; Kim, Y.; Nam, S.-E.; Park, Y.-I.; Park, H. Tailoring the porous structure of hollow fiber membranes for osmotic power generation applications via thermally assisted nonsolvent induced phase separation. J. Membr. Sci. 2019, 579, 329–341. [Google Scholar] [CrossRef]
- Ma, W.; Cao, Y.; Gong, F.; Liu, C.; Tao, G.; Wang, X. Poly (vinylidene fluoride) membranes prepared via nonsolvent induced phase separation combined with the gelation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 479, 25–34. [Google Scholar] [CrossRef]
- Hong, P.-D.; Chou, C.-M. Phase separation and gelation behaviors in poly (vinylidene fluoride)/tetra (ethylene glycol) dimethyl ether solutions. Polymer 2000, 41, 8311–8320. [Google Scholar] [CrossRef]
- Chen, J.-S.; Tu, S.-L.; Tsay, R.-Y. A morphological study of porous polylactide scaffolds prepared by thermally induced phase separation. J. Taiwan Inst. Chem. Eng. 2010, 41, 229–238. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Wan, L.-S.; Xu, Z.-K. Structure and performance of polyacrylonitrile membranes prepared via thermally induced phase separation. J. Membr. Sci. 2012, 409–410, 355–364. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous membrane formation via thermally-induced phase separation. II. Liquid—liquid phase separation. J. Membr. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Khuman, P.; Singh, W.B.K.; Devi, S.D.; Naorem, H. Viscosity-Temperature Behavior of Hydroxypropyl Cellulose Solution in Presence of an Electrolyte or a Surfactant: A Convenient Method to Determine the Cloud Point of Polymer Solutions. J. Macromol. Sci. Part A 2014, 51, 924–930. [Google Scholar] [CrossRef]
- Nazem-Bokaee, H.; Fallahianbijan, F.; Chen, D.; O’Donnell, S.M.; Carbrello, C.; Giglia, S.; Bell, D.; Zydney, A.L. Probing pore structure of virus filters using scanning electron microscopy with gold nanoparticles. J. Membr. Sci. 2018, 552, 144–152. [Google Scholar] [CrossRef]








| Dope | PVDF Concentration (wt%) | EGBE Concentration (wt%) | PEG200 Concentration (wt%) | Solvent |
|---|---|---|---|---|
| PNE | 20 | 10 | 0 | NMP |
| PDE | 20 | 10 | 0 | DMAc |
| PTE-0 | 20 | 0 | 0 | TEP |
| PTE-1 | 20 | 10 | 0 | TEP |
| PTE-1A | 15 | 10 | 0 | TEP |
| PTE-1B | 18 | 10 | 0 | TEP |
| PTE-1C | 20 | 5 | 0 | TEP |
| PTE-1D | 20 | 15 | 0 | TEP |
| PTE-2 | 20 | 8 | 2 | TEP |
| PTE-3 | 20 | 5 | 5 | TEP |
| PTE-4 | 20 | 0 | 10 | TEP |
| Position | Au Concentration (wt%) | |
|---|---|---|
| MF Mode (Skin = Bottom) | UF Mode (Skin = Top) | |
| Top surface | 30.5 | 93.1 |
| Middle part | 7.53 | 0.38 |
| Bottom surface | 0.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.H.; Kwon, S.; Yoo, Y.; Kim, I.-C.; Park, H.; Park, Y.-I.; Yang, S.Y.; Nam, S.-E.; Cho, Y.H. Isoporous Polyvinylidene Fluoride Membranes with Selective Skin Layers via a Thermal-Vapor Assisted Phase Separation Method for Industrial Purification Applications. Membranes 2022, 12, 250. https://doi.org/10.3390/membranes12030250
Choi DH, Kwon S, Yoo Y, Kim I-C, Park H, Park Y-I, Yang SY, Nam S-E, Cho YH. Isoporous Polyvinylidene Fluoride Membranes with Selective Skin Layers via a Thermal-Vapor Assisted Phase Separation Method for Industrial Purification Applications. Membranes. 2022; 12(3):250. https://doi.org/10.3390/membranes12030250
Chicago/Turabian StyleChoi, Da Han, Sei Kwon, Youngmin Yoo, In-Chul Kim, Hosik Park, You-In Park, Sung Yun Yang, Seung-Eun Nam, and Young Hoon Cho. 2022. "Isoporous Polyvinylidene Fluoride Membranes with Selective Skin Layers via a Thermal-Vapor Assisted Phase Separation Method for Industrial Purification Applications" Membranes 12, no. 3: 250. https://doi.org/10.3390/membranes12030250






