Use of Fungal Mycelium as Biosupport in the Formation of Lichen-like Structure: Recovery of Algal Grown in Sugarcane Molasses for Lipid Accumulation and Balanced Fatty Acid Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Medium
2.2. Sugarcane Molasses
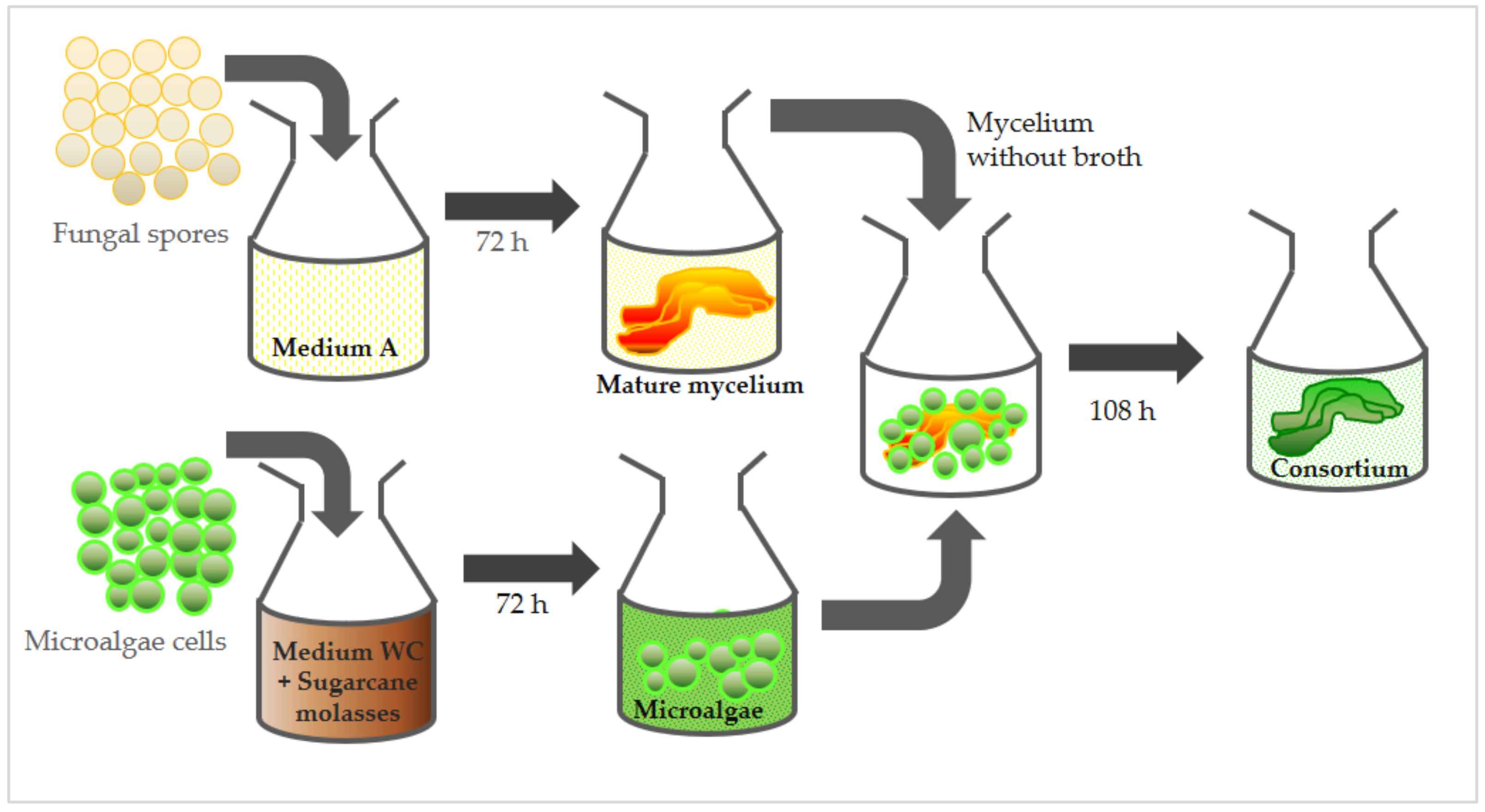
2.3. Lichenization Procedures—Microalgae and Fungus Consortium
2.4. Analytical Methods
2.5. Calculation of Biochemical Parameters Based on Cultivation Data
2.6. Recovery Efficiency of Microalgae Cells
3. Results and Discussion
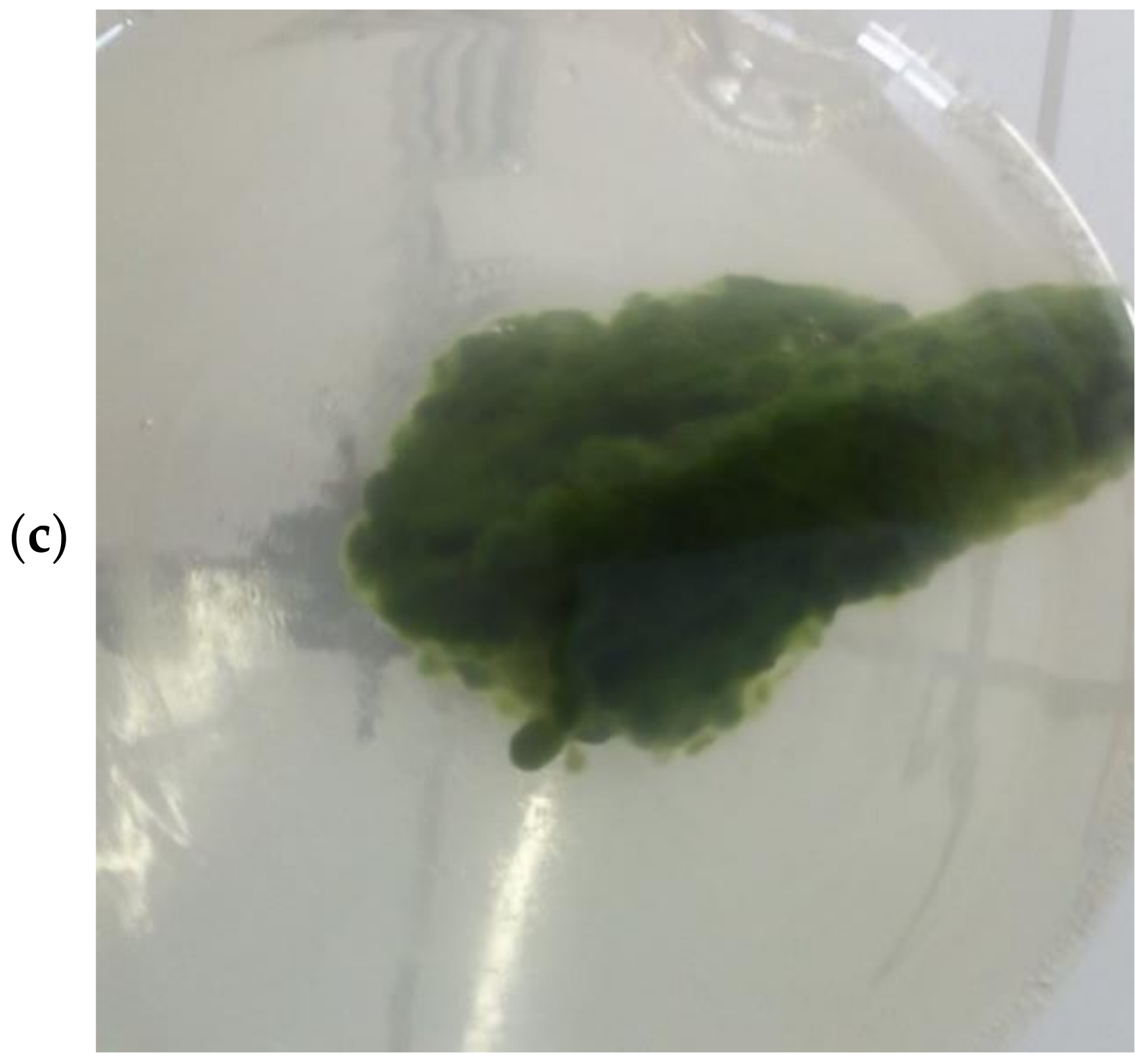
3.1. Growth Parameters of Microbial Species and Consortia, and Physical Characterization
3.2. Lipid Characteristics: Fatty Acid Profile
3.3. Evaluation of Harvesting Efficiency and Consortium Biomass Composition
3.4. Formation of the Lichen-like Structure at the Molecular Level
3.5. Potential Membrane-Related Applications and Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Pan, M.; Zhu, X.; Pan, G.; Angelidak, I. Integrated valorization system for simultaneous high strength organic wastewater treatment and astaxanthin production from Haematococcus pluvialis. Bioresour. Technol. 2021, 326, 124761. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mhatre, A.; Pandit, R.; Lali, A.M. Synergistic biorefinery of Scenedesmus obliquus and Ulva lactuca in poultry manure towards sustainable bioproduct generation. Bioresour. Technol. 2021, 297, 122462. [Google Scholar] [CrossRef]
- Zuccaro, G.; Del Mondo, A.; Pinto, G.; Pollio, A.; De Natale, A. Biorefinery-based approach to exploit mixed cultures of Lipomyces starkeyi and Chloroidium saccharophilum for single cell oil production. Energies 2021, 14, 1340. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Visigalli, S.; Barberis, M.G.; Turolla, A.; Canziani, R.; Zrimec, M.B.; Reinhardt, R.; Ficara, E. Electrocoagulation–flotation (ECF) for microalgae harvesting—A review. Sep. Purif. Technol. 2021, 271, 118684. [Google Scholar] [CrossRef]
- Das, P.K.; Rani, J.; Rawat, S.; Kumar, S. Microalgal co-cultivation for biofuel production and bioremediation: Current status and benefits. BioEnergy Res. 2021. [Google Scholar] [CrossRef]
- Pragya, N.; Pandey, K.K.; Sahoo, P.K. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Gultom, S.; Hu, B. Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies 2013, 6, 5921–5939. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.E.R.; Bento, H.B.S.; Carvalho, A.K.F.; Rajendran, A.; Hu, B.; De Castro, H.F. Critical applications of Mucor circinelloides within a biorefinery context. Crit. Rev. Biotechnol. 2019, 39, 555–570. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour. Technol. 2012, 114, 529–535. [Google Scholar] [CrossRef]
- Li, T.; Li, C.-T.; Butler, K.; Hays, S.G.; Guarnieri, M.T.; Oyler, G.A.; Betenbaugh, M.J. Mimicking lichens: Incorporation of yeast strains together with sucrose-secreting cyanobacteria improves survival, growth, ROS removal, and lipid production in a stable mutualistic co-culture production platform. Biotechnol. Biofuels 2017, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Olson, D.G.; Mc Bride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef]
- Loures, C.; Amaral, M.S.; Da Rós, P.C.M.; Zorn, S.M.F.E.; De Castro, H.F.; Silva, M.B. Simultaneous esterification and transesterification of microbial oil from Chlorella minutissima by acid catalysis route: A comparison between homogeneous and heterogeneous catalysts. Fuel 2018, 211, 261–268. [Google Scholar] [CrossRef]
- Zorn, S.M.F.E.; Reis, C.E.R.; Bento, H.S.B.; Carvalho, A.K.F.; Silva, M.B.; De Castro, H.F. In situ transesterification of marine microalgae biomass via heterogeneous acid catalysis. BioEnergy Res. 2020, 13, 1260–1268. [Google Scholar] [CrossRef]
- Jiru, T.M.; Steyn, L.; Pohl, C.; Abate, D. Production of single cell oil from cane molasses by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 as a biodiesel feedstock. Chem. Cent. J. 2018, 12, 91. [Google Scholar] [CrossRef]
- Bento, H.B.S.; Carvalho, A.K.F.; Reis, C.E.R.; De Castro, H.F. Single cell oil production and modification for fuel and food applications: Assessing the potential of sugarcane molasses as culture medium for filamentous fungus. Ind. Crop. Prod. 2020, 145, 112141. [Google Scholar] [CrossRef]
- Tinôco, D.; Castro, A.M.; Seldin, L.; Freire, D.M.G. Production of (2R,3R)-butanediol by Paenibacillus polymyxa PM 3605 from crude glycerol supplemented with sugarcane molasses. Process. Biochem. 2021, 106, 88–95. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Valle, G.F.; Bento, H.B.S.; Carvalho, A.K.F.; Alves, T.A.; De Castro, H.F. Sugarcane by-products within the biodiesel production chain: Vinasse and molasses as feedstock for oleaginous fungi and conversion to ethyl esters. Fuel 2020, 277, 118064. [Google Scholar] [CrossRef]
- Carvalho, A.K.F.; Bento, H.B.S.; Reis, C.E.R.; De Castro, H.F. Sustainable enzymatic approaches in a fungal lipid biorefinery based in sugarcane bagasse hydrolysate as carbon source. Bioresour. Technol. 2019, 276, 269–275. [Google Scholar] [CrossRef]
- Darki, B.Z.; Seyfabadi, J.; Fayazi, S. Effect of nutrients on total lipid content and fatty acids profile of Scenedesmus obliquus. Braz. Arch. Biol. Technol. 2017, 60, e160304. [Google Scholar] [CrossRef] [Green Version]
- Cheung, S.L.; Allen, D.G.; Short, S.M. Specific quantification of Scenedesmus obliquus and Chlorella vulgaris in mixed-species algal biofilms. Bioresour. Technol. 2020, 295, 122251. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, B.G.; Orsat, V.; Lefsrud, M.; Wu, B.S. A comprehensive study on the effect of light quality imparted by light-emitting diodes (LEDs) on the physiological and biochemical properties of the microalgal consortia of Chlorella variabilis and Scenedesmus obliquus cultivated in dairy wastewater. Bioprocess. Biosyst. Eng. 2020, 43, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.K.F.; Rivaldi, J.D.; Barbosa, J.C.; De Castro, H.F. Biosynthesis, characterization and enzymatic transesterification of single cell oil of Mucor circinelloides—A sustainable pathway for biofuel production. Bioresour. Technol. 2015, 181, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Hu, B. Mycoalgae biofilm: Development of a novel platform technology using algae and fungal cultures. Biotechnol. Biofuels 2016, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillard, R.R.; Lorenzen, C.J. Yellow-green algae with chlorophyllide C1,2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, A.K.; Hamilton, K.J.; Rebers, A.P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, J.; Zhang, W.; Hu, B. A new cultivation method for microbial oil production: Cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnol. Biofuels 2011, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.T.; Yeh, K.L.; Chen, C.L.; Chang, J.S. Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Talebi, A.F.; Motashami, S.K.; Tabatabaei, M.; Tohidfar, M.; Bagheri, A.; Zeinalabedini, M.; Mirzaei, H.H.; Mirzajanzadeh, M.; Shafaroudi, S.M.; Bakhtiari, S. Fatty acids profiling: A selective criterion for screening microalgae strains for biodiesel production. Algal Res. 2013, 2, 258–267. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Chawpraknoi, A.; Chantarit, S.; Cheirsilp, B.; Prasertsan, P. A rapid method for harvesting and immobilization of oleaginous microalgae using pellet-forming filamentous fungi and the application in phytoremediation of secondary effluent. Int. J. Phytoremediation 2018, 20, 1017–1024. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Cheirsilp, B.; Prasertsan, P.; Kato, Y.; Asano, Y. Photoautotrophic cultivation of oleaginous microalgae and co-pelletization with filamentous fungi for cost-effective harvesting process and improved lipid yield. Aquac. Int. 2018, 26, 1493–1509. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [Green Version]
- Pei, X.-Y.; Ren, H.-Y.; Liu, B.-F. Flocculation performance and mechanism of fungal pellets on harvesting of microalgal biomass. Bioresour. Technol. 2021, 321, 124463. [Google Scholar]
- Arora, N.; Patel, A.; Mehtani, J.; Pruthi, A.P.; Pruthi, V.; Poluri, M.K. Co-culturing of oleaginous microalgae and yeast: Paradigm shift towards enhanced lipid productivity. Environ. Sci. Pollut. Res. 2019, 26, 16952–16973. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Gong, G.; Zhang, X.; Tan, T. Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour. Technol. 2014, 164, 93–99. [Google Scholar] [CrossRef]
- Xue, F.; Miao, J.; Zhang, X.; Tan, T. A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl. Biochem. Biotechnol. 2010, 160, 498–503. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Rajendran, A.; Silva, M.B.; Hu, B.; De Castro, H.F. The application of microbial consortia in a biorefinery context: Understanding the importance of artificial lichens. In Sustainable Biotechnology—Enzymatic Resources of Renewable Energy, 1st ed.; Singh, O., Chandel, A., Eds.; Springer International Publishing: New York, NY, USA, 2018; Volume 1, pp. 423–437. [Google Scholar]
- Zamalloa, C.; Gultom, S.O.; Rajendran, A.; Hu, B. Ionic effects on microalgae harvest via microalgae fungi co-pelletization. Biocatal. Agric. Biotechnol. 2017, 9, 145–155. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; García-Depraect, O. Membrane-based harvesting processes for microalgae and their valuable-related molecules: A review. Membranes 2021, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, S.; Ma, H.; Ma, B.; Guo, Z.; You, H.; Mei, J.; Hou, X.; Liang, Z.; Li, Z. Effect of different influent conditions on biomass production and nutrient removal by aeration microalgae membrane bioreactor (ICFB-MMBR) system for mariculture wastewater treatment. Membranes 2021, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, P.; Kim, H.G.; Kim, J. Membrane fouling mechanisms in combined microfiltration-coagulation of algal rich water applying ceramic membranes. Membranes 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Haupt, A.; Lerch, A. Forward osmosis application in manufacturing industries: A short review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Drexler, I.L.; Yeh, D.H. Membrane applications for microalgae cultivation and harvesting: A review. Rev. Environ. Sci. Bio/Technol. 2014, 13, 487–504. [Google Scholar] [CrossRef]



| Parameters | Cultivation without SM | Cultivation with SM | Filamentous Fungus | ||
|---|---|---|---|---|---|
| Consortium | Microalgae | Consortium | Microalgae | ||
| X (mg·L−1) | 1000 ± 0.7 | 524 ± 0.7 | 1270 ± 0.7 | 1042 ± 0.7 | 900 ± 0.5 |
| P (mg·L−1) | 230.0 ± 0.3 | 149.2 ± 0.3 | 482.6 ± 0.3 | 322.0 ± 0.3 | 64.8 ± 0.3 |
| P (%) | 23.0 ± 0.4 | 28.5 ± 0.3 | 38.2 ± 0.5 | 30.9 ± 0.4 | 7.2 ± 0.3 |
| QX (mg·L−1·day−1) | 133.3 ± 0.4 | 69.8 ± 0.4 | 169.3 ± 0.4 | 138.9 ± 0.4 | 120 ± 0.4 |
| QP (mg·L−1·day−1) | 30.7 ± 0.4 | 19.9 ± 0.4 | 64.4 ± 0.5 | 42.9 ± 0.4 | 8.6 ± 0.4 |
| Fatty Acid Profile | Cultivation without SM | Cultivation with SM | Filamentous Fungus | |||
|---|---|---|---|---|---|---|
| Consortium | Microalgae | Consortium | Microalgae | |||
| C8:0 | Caprylic acid | 3.7 ± 0.17 | 10.7 ± 0.18 | 0.4 ± 0.25 | 0.12 ± 0.25 | 18.1 ± 0.23 |
| C16:0 | Palmitic acid | 30.7 ± 0.15 | 31.7 ± 0.30 | 18.9 ± 0.18 | 33.1 ± 0.18 | 24.0 ± 0.29 |
| C18:0 | Stearic acid | 0 | 0 | 1.6 ± 0.18 | 1.49 ± 0.18 | 4.2 ± 0.17 |
| SFA | Saturated Fatty Acids | 34.4 | 42.4 | 20.9 | 34.7 | 46.3 |
| C16:1 | Palmitoleic acid | 0 | 0 | 9.7 ± 0.18 | 1.23 ± 0.25 | 0 |
| C18:1 | Oleic acid | 15.3 ± 0.22 | 13.5 ± 0.31 | 22.1 ± 0.31 | 30.56 ± 0.30 | 0 |
| MUFA | Monounsaturated Fatty Acids | 15.3 | 13.5 | 31.8 | 31.7 | 0 |
| C18:2 | Linoleic acid | 31.1 ± 0.20 | 36.9 ± 0.18 | 16.1 ± 0.25 | 12.5 ± 0.30 | 22.9 ± 0.16 |
| C18:3 | Linolenic acid | 19.2 ± 0.20 | 7.2 ± 0.18 | 31.3 ± 0.25 | 21.0 ± 0.30 | 30.8 ± 0.27 |
| PUFA | Polyunsaturated Fatty Acids | 50.3 | 44.1 | 47.4 | 33.5 | 53.7 |
| Microalgae Species | Fungal Species | Harvesting Efficiency | Reference |
|---|---|---|---|
| Scenedesmus sp. | Trichoderma reesei QM 9414 | >94% | [34] |
| S. obliquus SIT06 | Cunninghamella echinulata TPU 4652 | 92.7% | [35] |
| S. quadricauda | Aspergillus fumigatus | >90% | [36] |
| Scenedesmussp. | Aspergillus niger | 99.4% | [37] |
| S. obliquus CCMA-UFSCar 604 | M. circinelloides URM 4182 | 99.7% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorn, S.; Carvalho, A.; Bento, H.; Gambarato, B.; Pedro, G.; da Silva, A.; Gonçalves, R.; Da Rós, P.; Silva, M. Use of Fungal Mycelium as Biosupport in the Formation of Lichen-like Structure: Recovery of Algal Grown in Sugarcane Molasses for Lipid Accumulation and Balanced Fatty Acid Profile. Membranes 2022, 12, 258. https://doi.org/10.3390/membranes12030258
Zorn S, Carvalho A, Bento H, Gambarato B, Pedro G, da Silva A, Gonçalves R, Da Rós P, Silva M. Use of Fungal Mycelium as Biosupport in the Formation of Lichen-like Structure: Recovery of Algal Grown in Sugarcane Molasses for Lipid Accumulation and Balanced Fatty Acid Profile. Membranes. 2022; 12(3):258. https://doi.org/10.3390/membranes12030258
Chicago/Turabian StyleZorn, Savienne, Ana Carvalho, Heitor Bento, Bruno Gambarato, Guilherme Pedro, Ana da Silva, Rhyan Gonçalves, Patrícia Da Rós, and Messias Silva. 2022. "Use of Fungal Mycelium as Biosupport in the Formation of Lichen-like Structure: Recovery of Algal Grown in Sugarcane Molasses for Lipid Accumulation and Balanced Fatty Acid Profile" Membranes 12, no. 3: 258. https://doi.org/10.3390/membranes12030258
APA StyleZorn, S., Carvalho, A., Bento, H., Gambarato, B., Pedro, G., da Silva, A., Gonçalves, R., Da Rós, P., & Silva, M. (2022). Use of Fungal Mycelium as Biosupport in the Formation of Lichen-like Structure: Recovery of Algal Grown in Sugarcane Molasses for Lipid Accumulation and Balanced Fatty Acid Profile. Membranes, 12(3), 258. https://doi.org/10.3390/membranes12030258












