Effect of Protocatechuic Acid Ethyl Ester on Biomembrane Models: Multilamellar Vesicles and Monolayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Multilamellar Vesicles Preparation
2.3. Encapsulation Efficiency
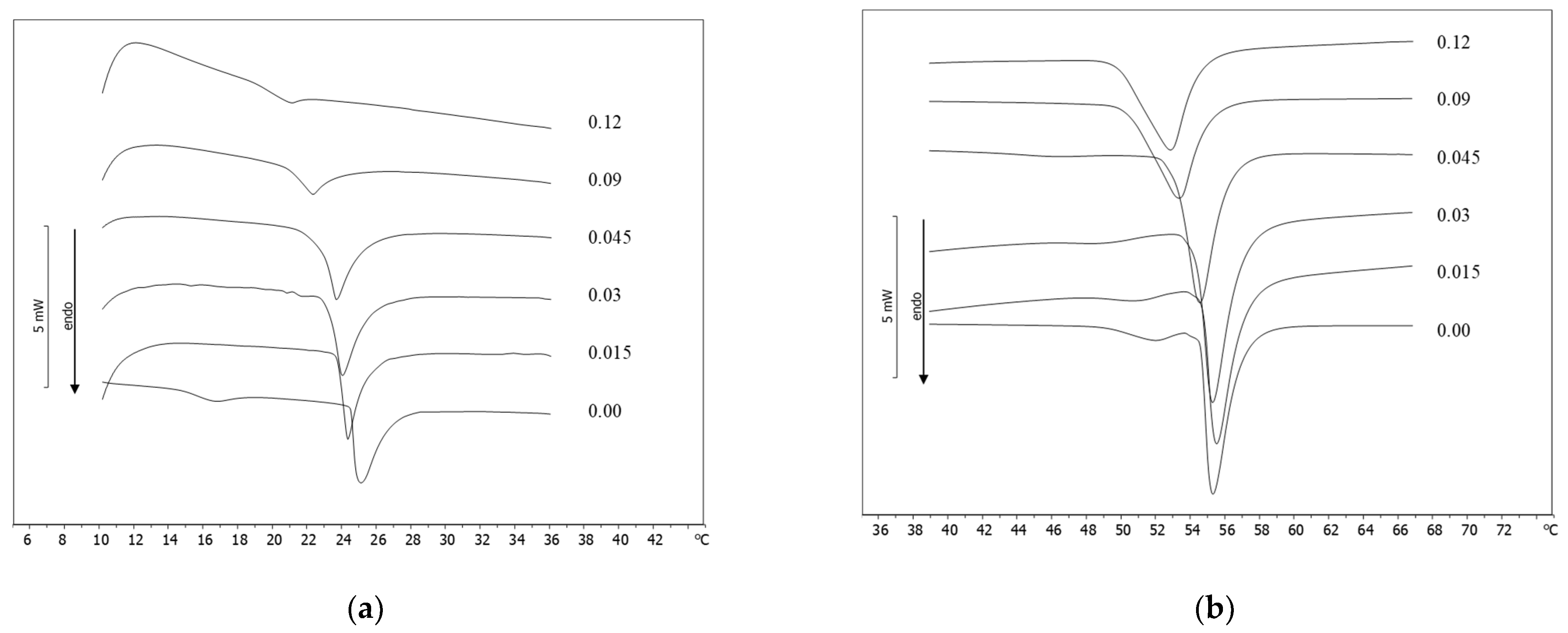
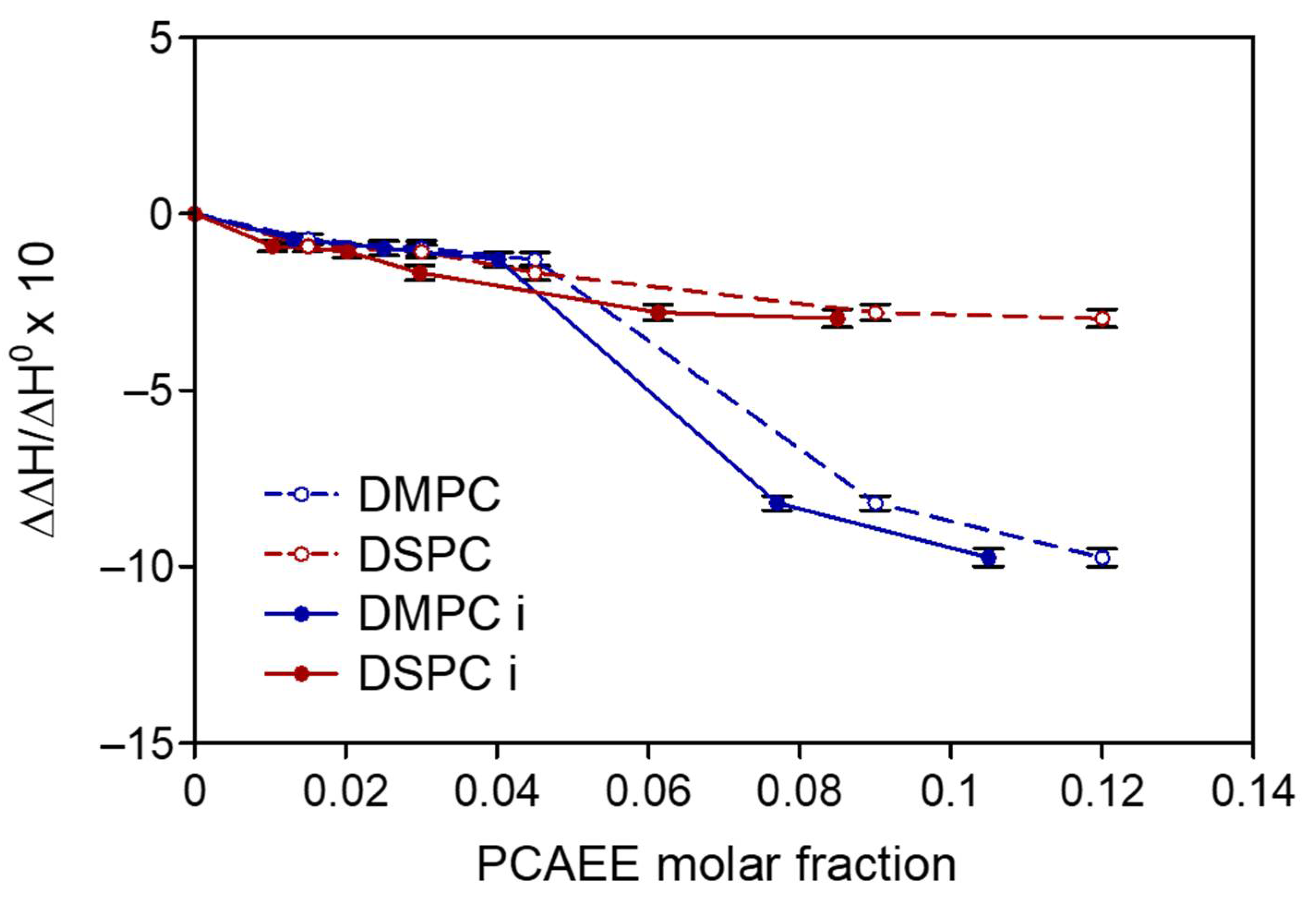
2.4. DSC Analysis
2.5. Interaction between MLV and Protocatechuic Acid Ethyl Ester
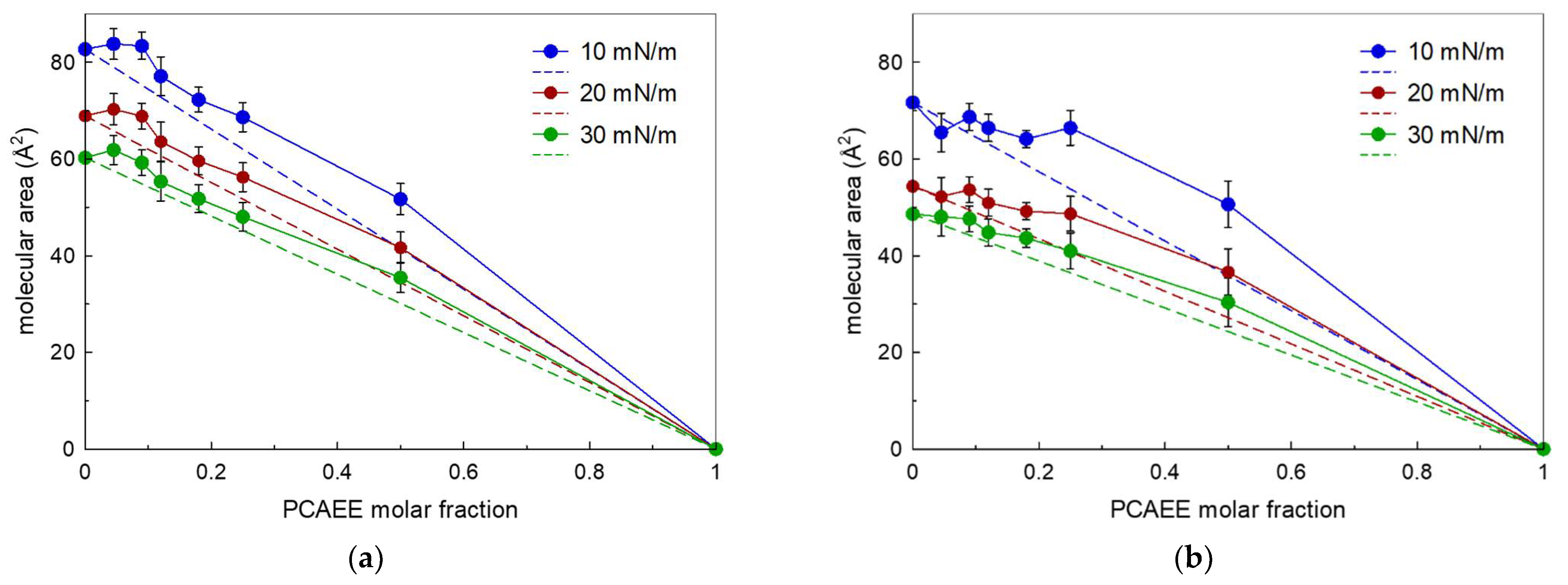
2.6. Surface Tension Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wisniewska-Becker, A.; Gruszecki, W.I. Biomembrane models. In Drug-Biomebrane Interaction Studies: The Application of Calorimetric Techniques; Pignatello, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 46–95. [Google Scholar]
- Torrisi, C.; Morgante, A.; Malfa, G.; Acquaviva, R.; Castelli, F.; Pignatello, R.; Sarpietro, M.G. Sinapic Acid Release at the Cell Level by Incorporation into Nanoparticles: Experimental Evidence Using Biomembrane Models. Micro 2021, 1, 120–128. [Google Scholar] [CrossRef]
- Masella, R.; Santangelo, C.; D’Archivio, M.; LiVolti, G.; Giovannini, C.; Galvano, F. Protocatechuic Acid and Human Disease Prevention: Biological Activities and Molecular Mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.M. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.; Adams, L.; Hardy, M.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Bùsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Acquaviva, R.; Tomasello, B.; Di Giacomo, C.; Santangelo, R.; La Mantia, S.; Naletova, I.; Sarpietro, M.G.; Castelli, F.; Malfa, G.A. Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells. Biomolecules 2021, 11, 1485. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Gliszczyńska, A.; Skonieczna, M.; Poła, A.; Wesołowska, O.; Środa-Pomianek, K. conjugation with phospholipids as a modification increasing anticancer activity of phenolic acids in metastatic melanoma—In Vitro and In Silico Studies. Int. J. Mol. Sci. 2021, 22, 8397. [Google Scholar] [CrossRef]
- Reis, B.; Martins, M.; Barreto, B.; Milhazes, N.; Garrido, E.M.; Silva, P.; Garrido, J.; Borges, F. Structure—Property—Activity Relationship of Phenolic Acids and Derivatives. Protocatechuic Acid Alkyl Esters. J. Agric. Food Chem. 2010, 58, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and Antioxidant Properties of Phenolic Acids Alkyl Esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- de Faria, C.M.Q.G.; Nazaré, A.C.; Petrônio, M.S.; Paracatu, L.C.; Zeraik, M.L.; Regasini, L.O.; Silva, D.H.S.; da Fonseca, L.M.; Ximenes, V.F. Protocatechuic acid alkyl esters: Hydrophobicity as a determinant factor for inhibition of NADPH oxidase. Curr. Med. Chem. 2012, 19, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Cater, B.R.; Chapman, D.; Hawes, S.M.; Saville, J. Lipid phase transitions and drug interactions. Biochim. Biophys. Acta 1974, 363, 54–69. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Jacobson, K.; Poste, G.; Shepherd, G. Effects of local anesthetics on membrane properties I. Changes in the fluidity of phospholipid bilayers. Biochim. Biophys. Acta 1975, 394, 504–519. [Google Scholar] [CrossRef]
- O’Learly, T.J.; Ross, P.D.; Levin, I.W. Effects of anaesthetic tetradecenol on phosphatidylcholine phase transitions. Implications for the mechanism of the bilayer pretransition. Biophys. J. 1986, 50, 1053. [Google Scholar]
- Jain, M.K. (Ed.) Introduction to Biological Membranes; Wiley and Sons: New York, NY, USA, 1998. [Google Scholar]
- Sahu, A.K.; Mishra, A.K. Interaction of Dopamine with zwitterionic DMPC and anionic DMPS multilamellar vesicle membranes. Langmuir 2021, 37, 13430–13443. [Google Scholar] [CrossRef] [PubMed]
- Sarpietro, M.G.; Torrisi, C.; Di Sotto, A.; Castelli, F. Interaction of limonene, terpineol, and 1,8 cineol with a model of biomembrane: A DSC study. Thermochim. Acta 2021, 700, 178938. [Google Scholar] [CrossRef]
- Sturtevant, J.M. A scanning calorimetric study of small molecule-lipid bilayer mixtures. Proc. Natl. Acad. Sci. USA 1982, 79, 3963–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suezaki, Y.; Tatara, T.; Kaminoh, K.; Kamaya, H.; Ueda, I. Solid-solution theoryofanesthetic interaction with lipid membranes: Temperature span of the main phasetransition. Biochim. Biophys. Acta 1999, 1029, 143–148. [Google Scholar] [CrossRef]
- Guggenheim, E.A. Thermodynamics; Interscience Publisher: Amsterdam, The Netherlands, 1949. [Google Scholar]
- Jorgensen, K.; Ipsen, J.H.; Mouritsen, O.G.; Bennet, D.; Zuckermann, M.J. The effects of density fluctuations on the partitioning of foreign molecules into lipid bilayers: Application to anaesthetics and insecticides. Biochim. Biophys. Acta 1991, 1062, 227–238. [Google Scholar] [CrossRef]
- Brezesinski, G.; Mohwald, H. Langmuir monolayers to study interactions at model membrane surfaces. Adv. Colloid. Interface Sci. Rev. 2003, 100, 563–584. [Google Scholar] [CrossRef]
- Brockman, H. Lipid monolayers: Why use half a membrane to characterize protein-membrane interactions? Curr. Opin. Struct. Biol. 1999, 9, 438–443. [Google Scholar] [CrossRef]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers. Modern. Phys. 1999, 71, 779–819. [Google Scholar] [CrossRef] [Green Version]
- Torrisi, C.; Di Guardia, M.; Castelli, F.; Sarpietro, M.G. Naringenin release to biomembrane models by incorporation into nanoparticles. Experimental evidence using differential scanning calorimetry. Surfaces 2021, 4, 295–305. [Google Scholar] [CrossRef]
- Marrazzo, A.; Torrisi, C.; Barbaraci, C.; Amata, E.; Castelli, F.; Sarpietro, M.G. Interaction of new sigma ligands with biomembrane models evaluated by differential scanning calorimetry and Langmuir-Blodgett studies. Colloids Surf. B Biointerfaces 2021, 201, 111643. [Google Scholar] [CrossRef]
- Banghman, A.D. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238. [Google Scholar] [CrossRef]
- Walde, P. Preparation of vesicles (liposomes). In ASP Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 9, pp. 43–79. [Google Scholar]
- Heimburg, T. A model for the lipid pretransition: Coupling of ripple formation with the chain-melting transition. Biophys. J. 2000, 78, 1154–1165. [Google Scholar] [CrossRef] [Green Version]
- Gardikis, K.; Hatziantoniou, S.; Viras, K.; Wagner, M.; Demetzos, C. A DSC and Raman spectroscopy study on the effect of PAMAM dendrimer on DPPC model lipid membranes. Int. J. Pharm. 2006, 318, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Lipid phase transitions and phase diagrams II. Mixtures involving lipids. Biochim. Biophys. Acta 1977, 472, 285–344. [Google Scholar] [CrossRef]
- Gaines, G.L., Jr. Insoluble Monolayers at Liquid-Gas Interfaces; Wiley-Interscience: New York, NY, USA, 1966. [Google Scholar]
- Shahgaldian, P.; Coleman, A.W. Miscibility studies on amphiphilic Calix[4]arene—Natural phospholipid mixed films. Langmuir 2003, 19, 5261–5265. [Google Scholar] [CrossRef]
- Goracci, L.; Germani, R.; Rathman, J.F.; Savelli, G. Anomalous behavior of amine oxide surfactants at the air/water interface. Langmuir 2007, 23, 10525–10532. [Google Scholar] [CrossRef] [PubMed]


| Sample | PCAEE Molar Fraction | EE% |
|---|---|---|
| DMPC MLV | 0.0 | - |
| 0.015 | 86.00 ± 5.00 | |
| 0.03 | 87.16 ± 6.21 | |
| 0.045 | 88.79 ± 3.81 | |
| 0.09 | 85.72 ± 7.12 | |
| 0.12 | 86.40 ± 2.80 | |
| DSPC MLV | 0.0 | - |
| 0.015 | 66.70 ± 3.20 | |
| 0.03 | 65.83 ± 4.51 | |
| 0.045 | 65.27 ± 4.32 | |
| 0.09 | 65.79 ± 2.82 | |
| 0.12 | 68.36 ± 3.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrisi, C.; Malfa, G.A.; Acquaviva, R.; Castelli, F.; Sarpietro, M.G. Effect of Protocatechuic Acid Ethyl Ester on Biomembrane Models: Multilamellar Vesicles and Monolayers. Membranes 2022, 12, 283. https://doi.org/10.3390/membranes12030283
Torrisi C, Malfa GA, Acquaviva R, Castelli F, Sarpietro MG. Effect of Protocatechuic Acid Ethyl Ester on Biomembrane Models: Multilamellar Vesicles and Monolayers. Membranes. 2022; 12(3):283. https://doi.org/10.3390/membranes12030283
Chicago/Turabian StyleTorrisi, Cristina, Giuseppe Antonio Malfa, Rosaria Acquaviva, Francesco Castelli, and Maria Grazia Sarpietro. 2022. "Effect of Protocatechuic Acid Ethyl Ester on Biomembrane Models: Multilamellar Vesicles and Monolayers" Membranes 12, no. 3: 283. https://doi.org/10.3390/membranes12030283
APA StyleTorrisi, C., Malfa, G. A., Acquaviva, R., Castelli, F., & Sarpietro, M. G. (2022). Effect of Protocatechuic Acid Ethyl Ester on Biomembrane Models: Multilamellar Vesicles and Monolayers. Membranes, 12(3), 283. https://doi.org/10.3390/membranes12030283








