Recent Development and Environmental Applications of Nanocellulose-Based Membranes
Abstract
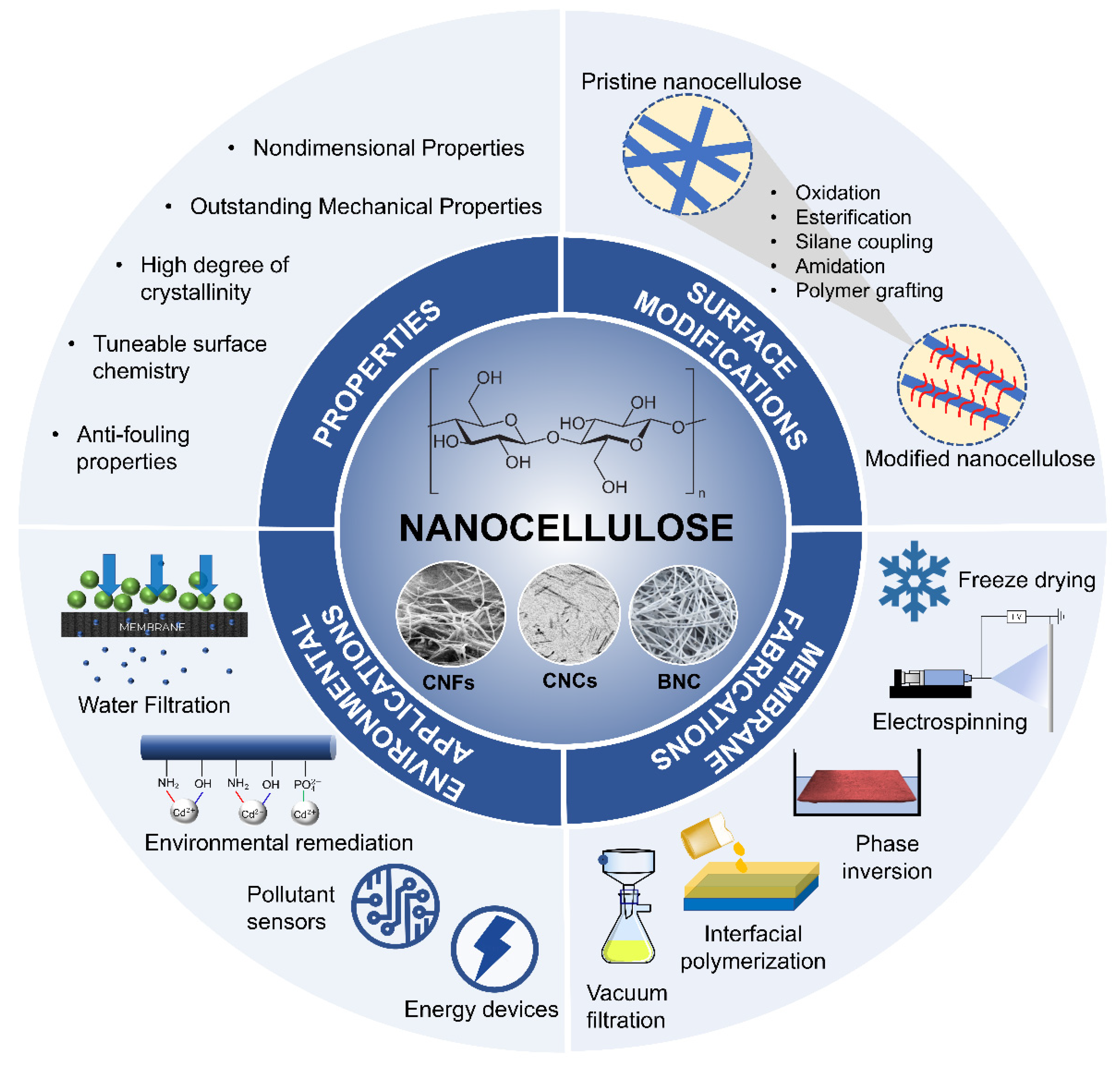
:1. Introduction
2. Desirable Features of Nanocellulose from Membrane Technology Perspective
2.1. Nano-Dimensional Properties
2.2. Outstanding Mechanical Properties
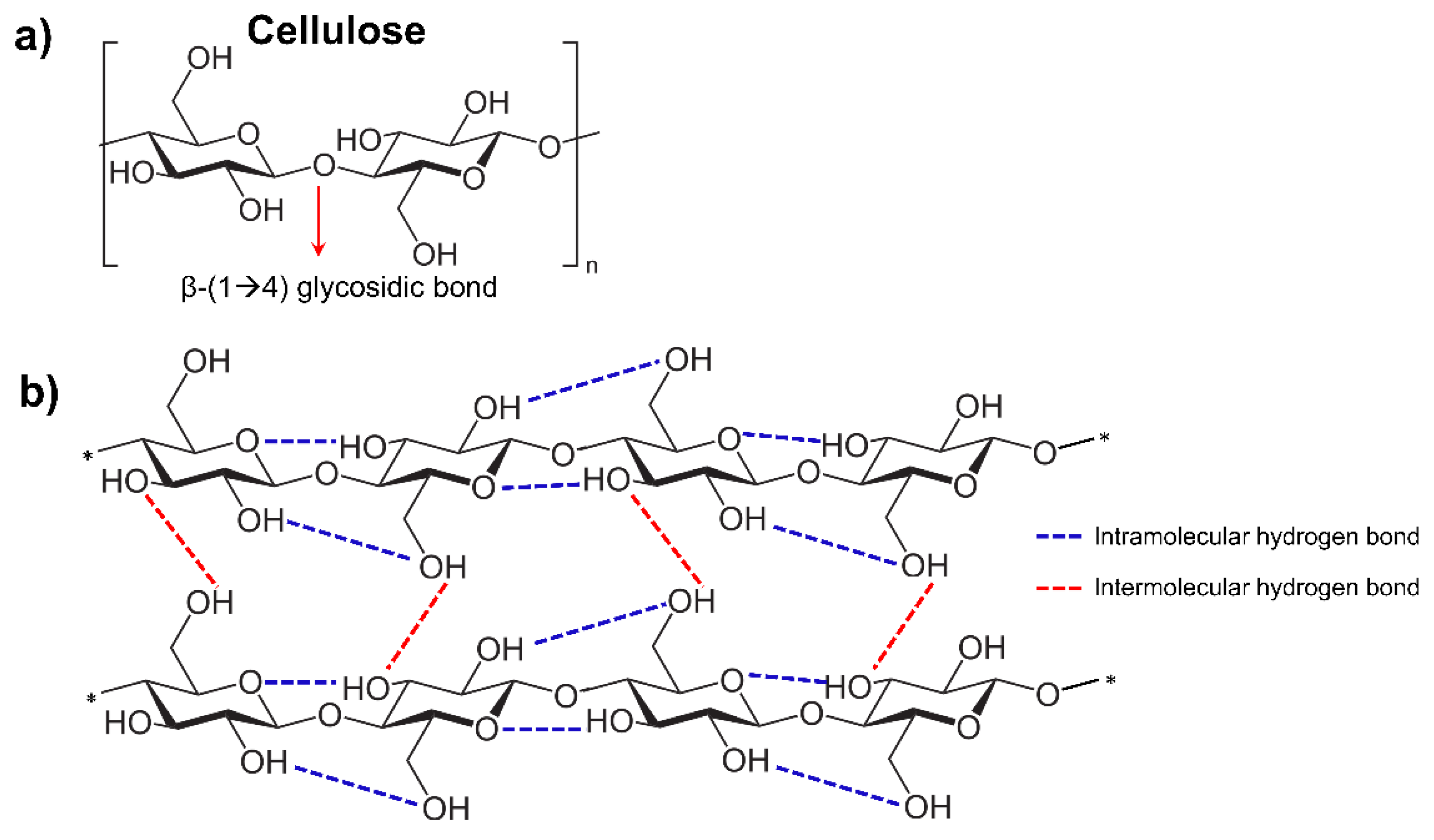
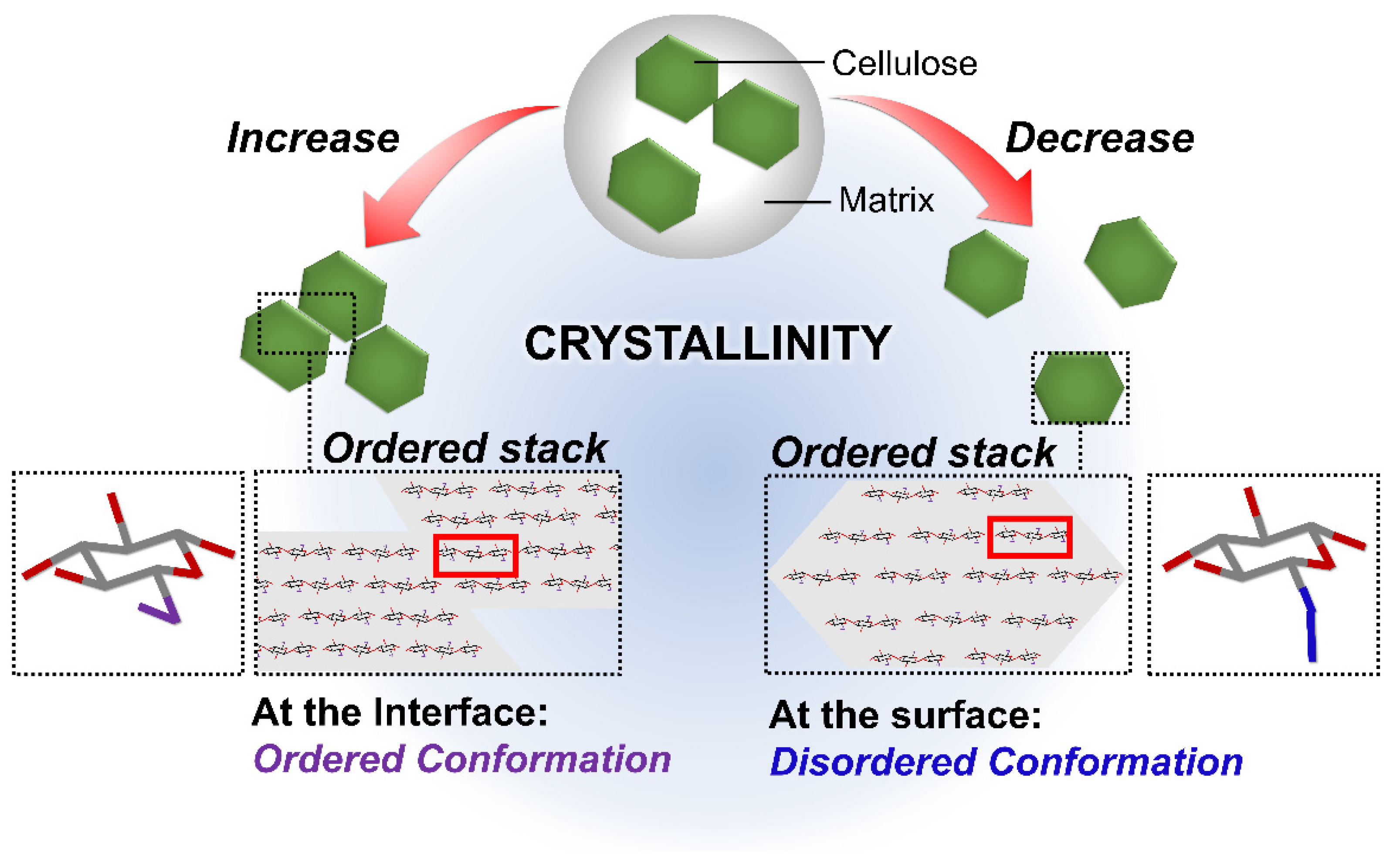
2.3. High Degree of Crystallinity
2.4. Tuneable Surface Chemistry
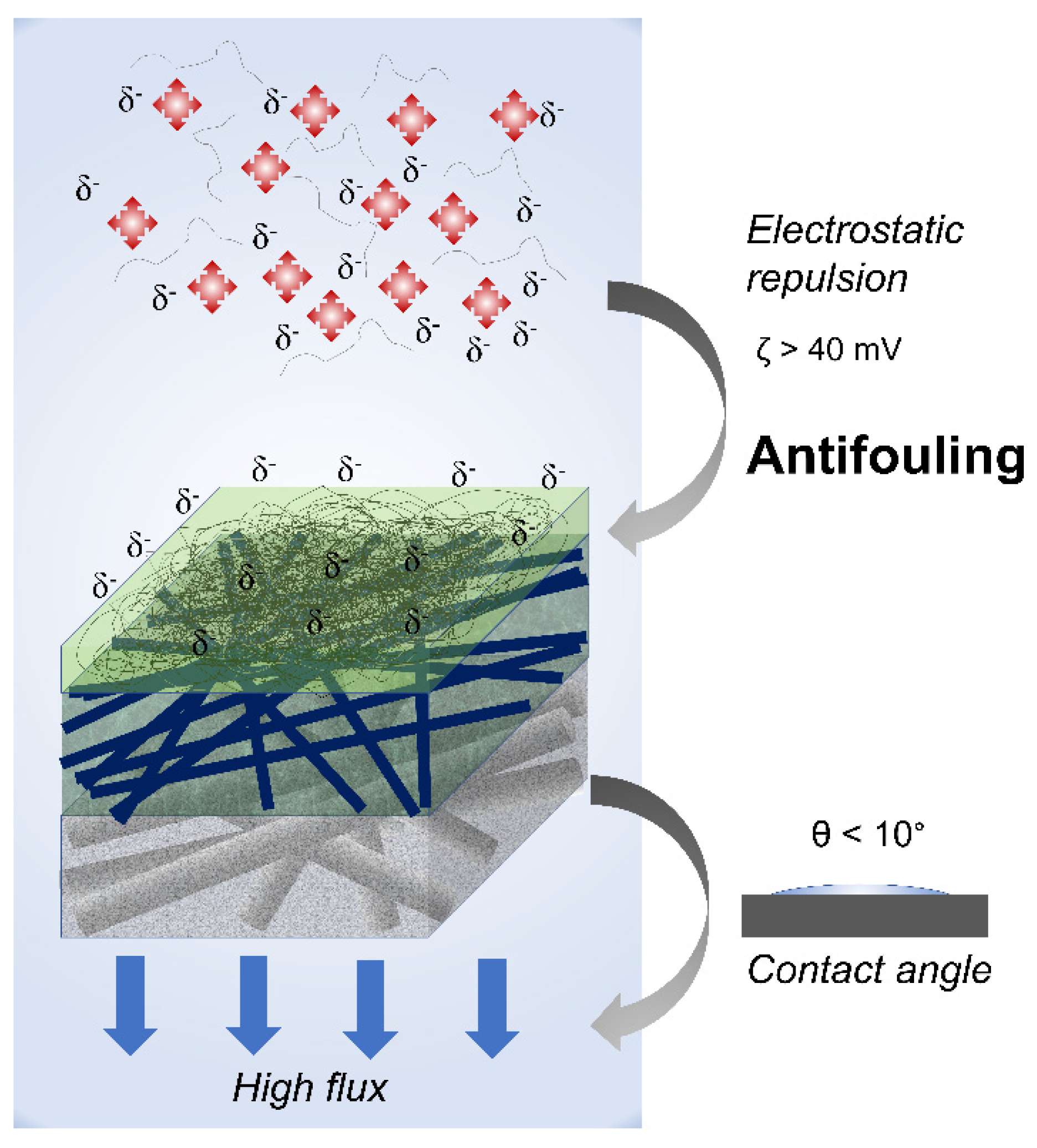
2.5. Anti-Fouling Properties
3. Recent Development in Nanocellulose-Based Membranes
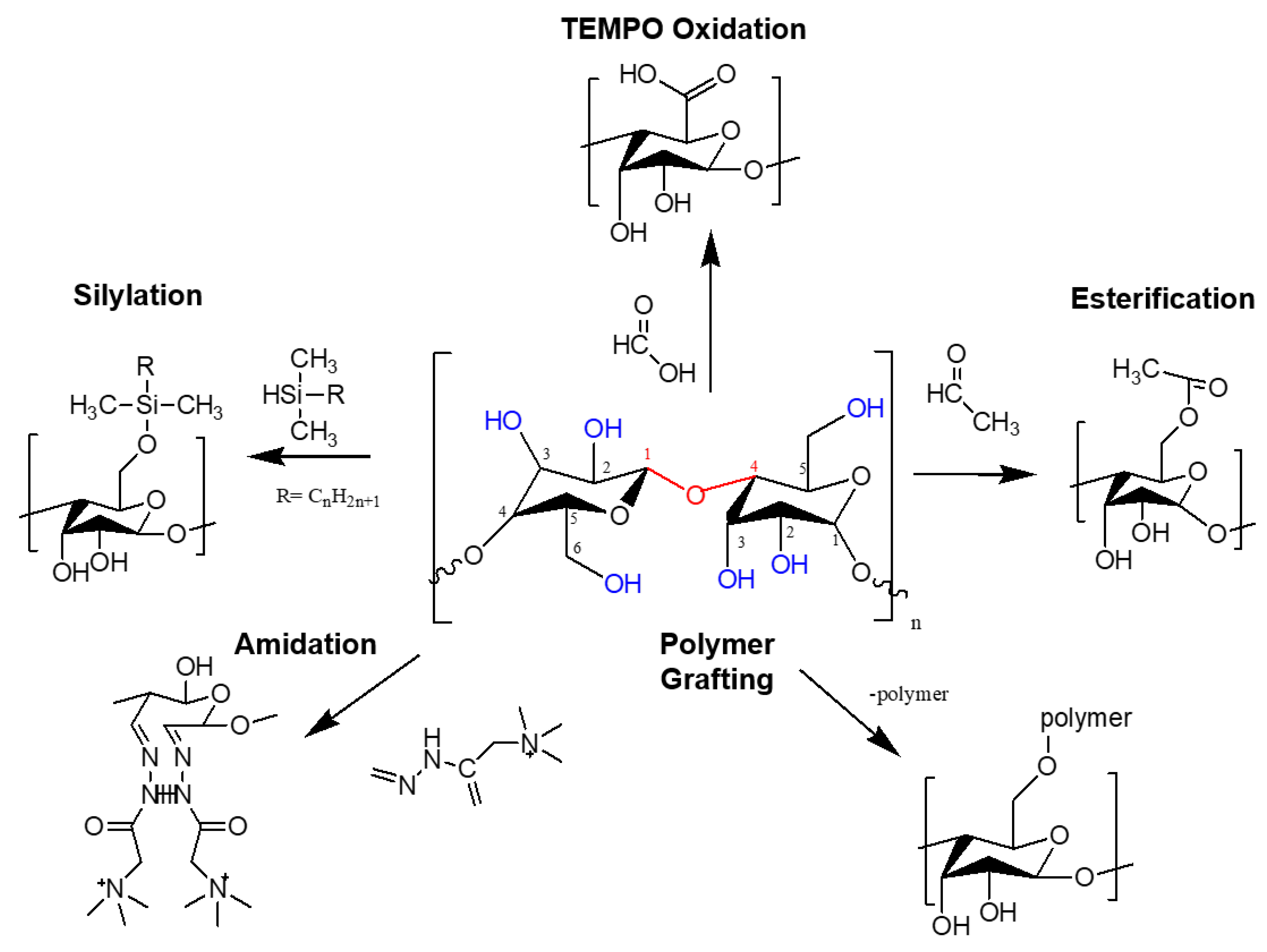
3.1. Surface Modification Strategies of Nanocellulose for Membrane Development
3.1.1. Chemical Modification via Oxidation
3.1.2. Chemical Modification via Esterification
3.1.3. Chemical Modification via Silyation
3.1.4. Chemical Modification via Amidation
3.1.5. Chemical Modification via Polymer Grafting
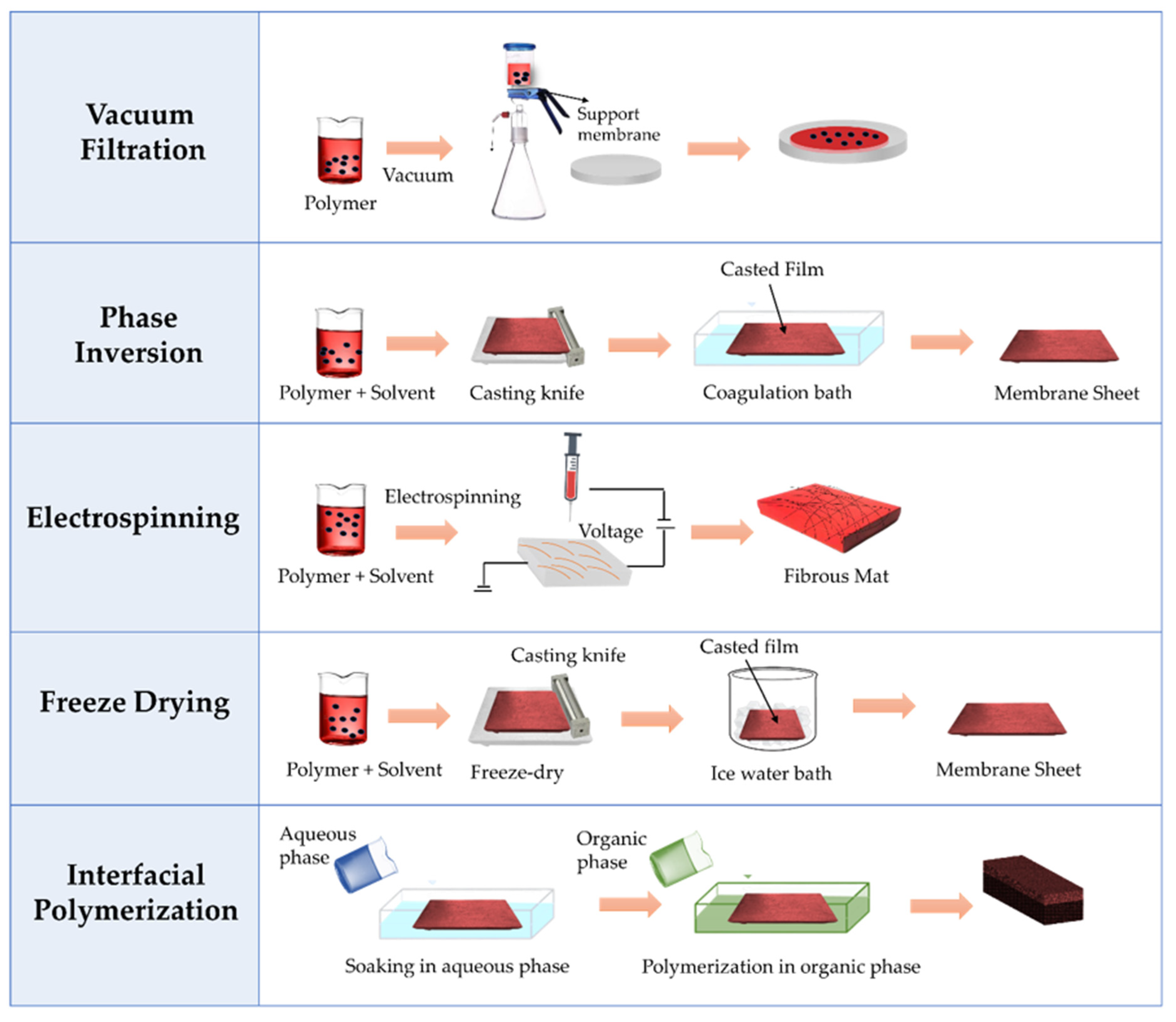
3.2. Preparation Techniques of Nanocellulose-Based Membranes
3.2.1. Phase Inversion
3.2.2. Vacuum Filtration
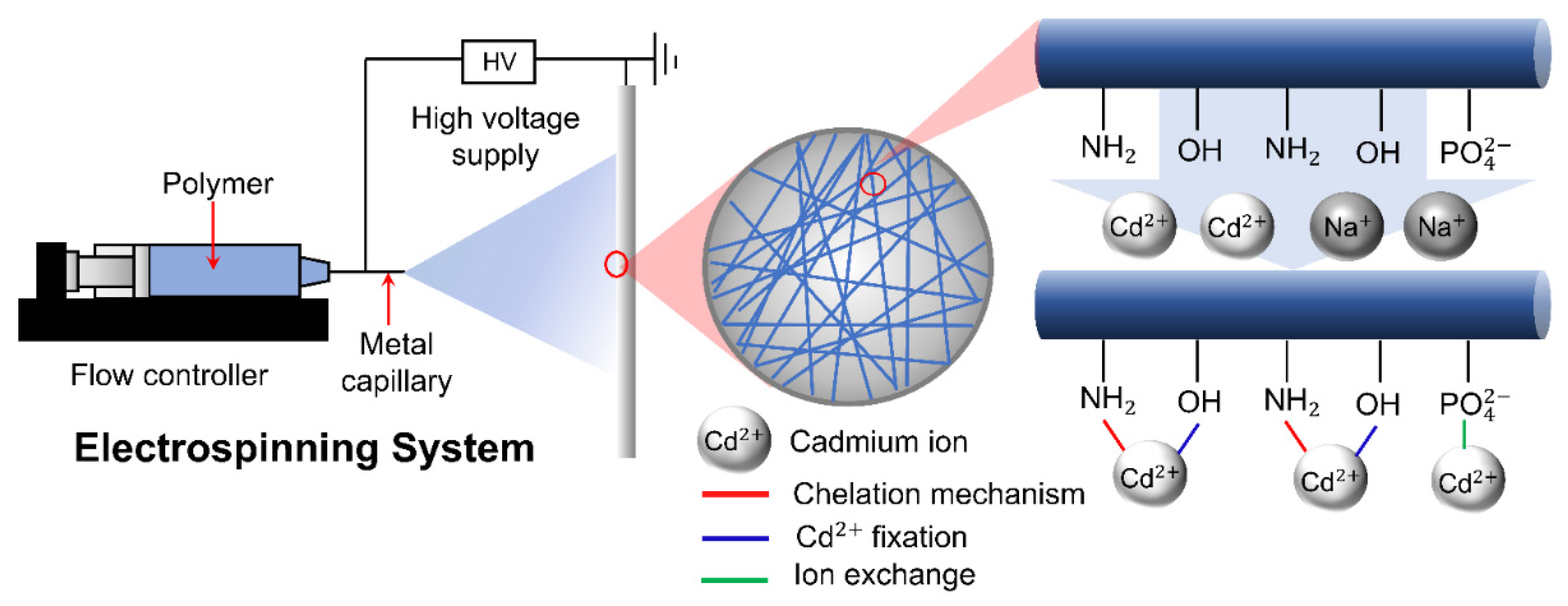
3.2.3. Electrospinning
3.2.4. Interfacial Polymerization
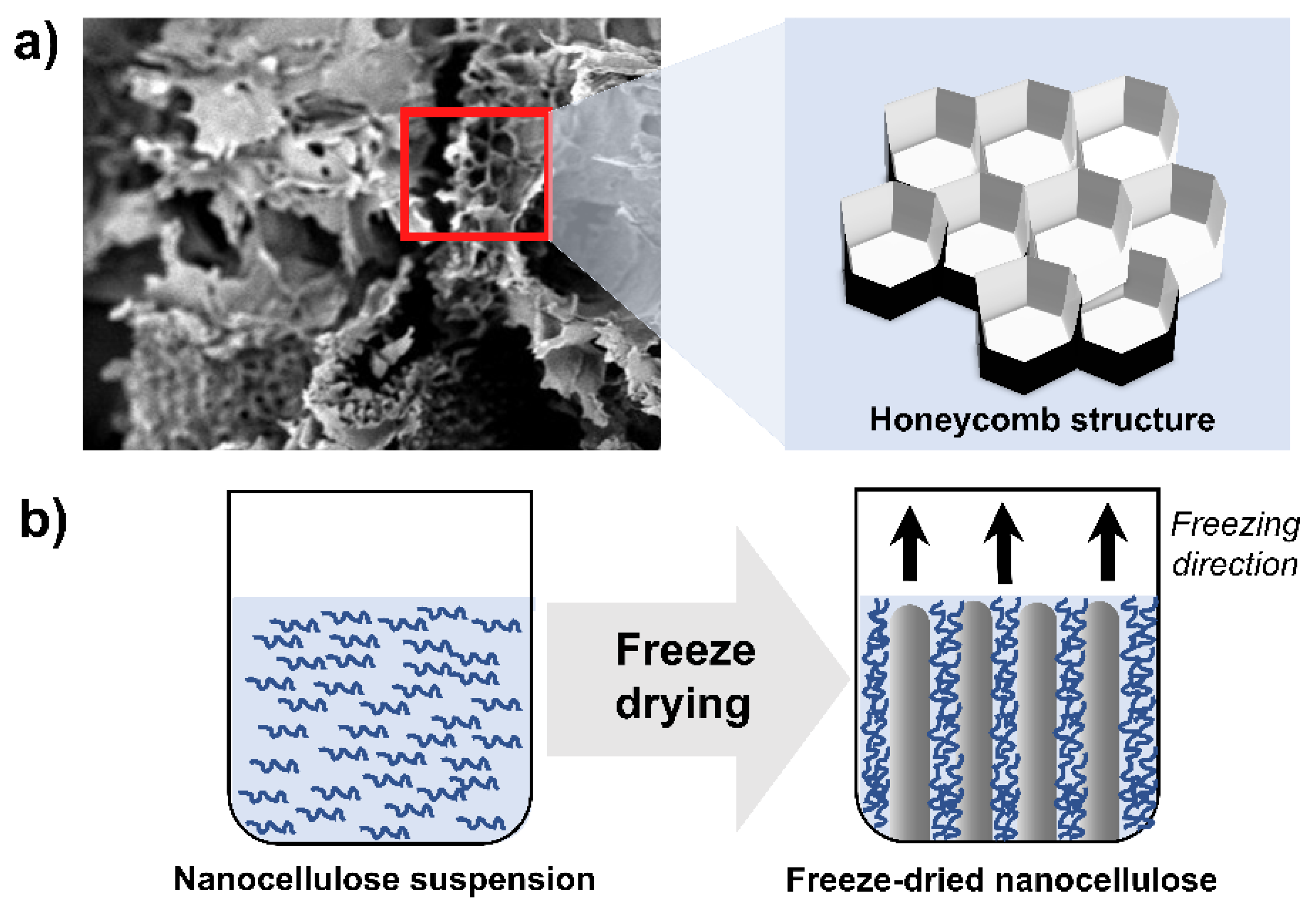
3.2.5. Freeze Drying
4. Environmental Applications of Nanocellulose-Based Membranes
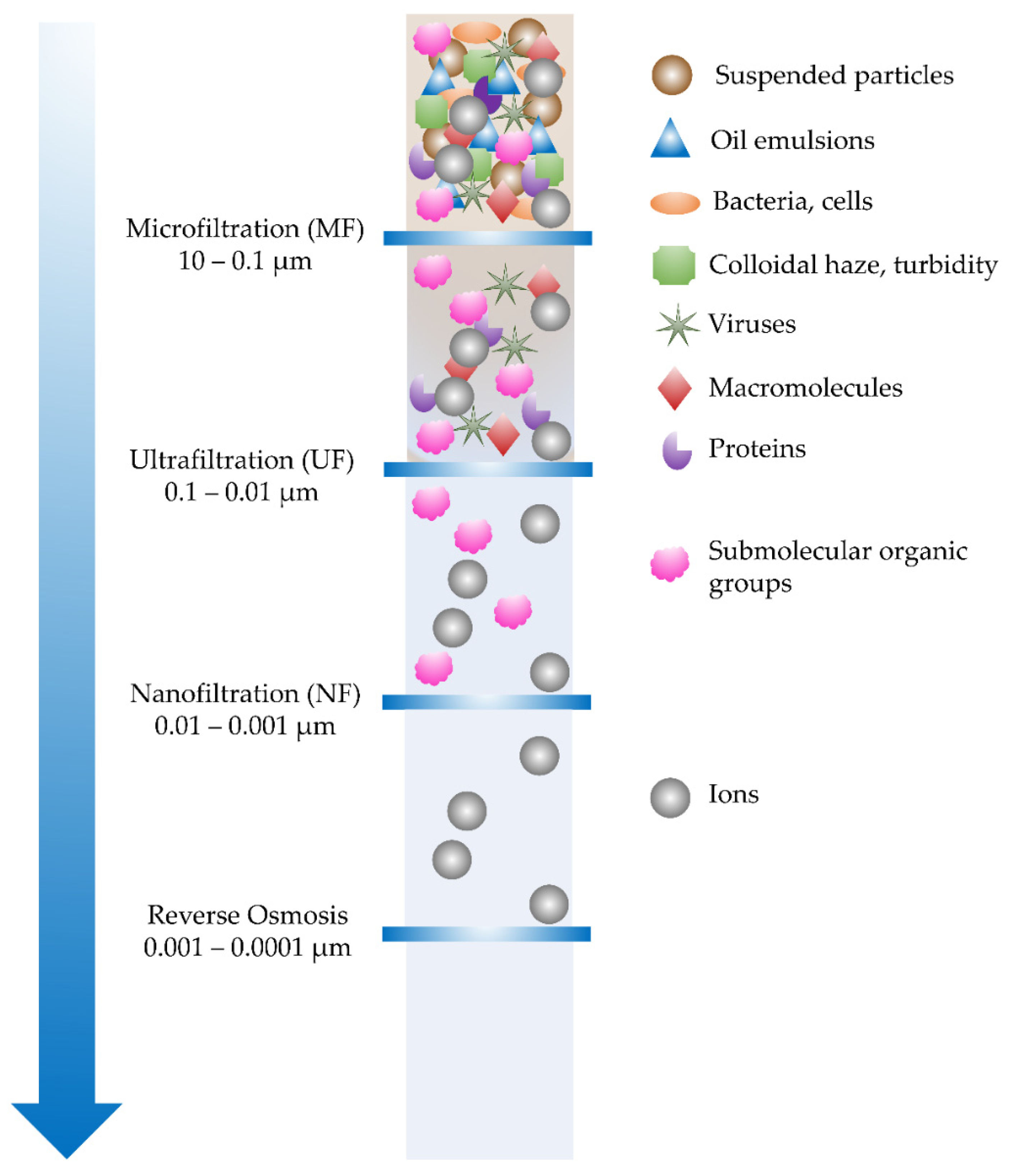
4.1. Water Filtration
| Materials | Method of Preparation | Filtration Process | Sample | Performance | Reference |
|---|---|---|---|---|---|
| Nanocellulose/filter paper (NC/FP) composite membrane | Vacuum filtration | UF | Oily wastewater | Up to 97.14% retention rate; 46,279 L m−2 h−1 flux | [62] |
| Metalized nanocellulose (silver and platinum as additive) | Vacuum filtration | FO | Nanopure water, urea, and wastewater | High water flux and solute rejection with wastewater sample | [84] |
| Cellulose acetate membrane | Phase separation | UF | Wastewater | 207.32 L m−2 h−1 pure water permeability; 90.56% flux recovery ratio | [86] |
| Cellulose acetate/copper oxide nanoparticles | Wet precipitation | UF | Wastewater | Improved hydrophilicity, water permeation, BSA separation, and antifouling performance | [87] |
| Cellulose membrane | Thermally inducedphase separation | MF | Oily wastewater | 99% rejections to peanut oil and pump oil nanoemulsion | [88] |
| Nanocellulose as modifer for hollow fiber | Addition of nanocellulose to internal coagulant | UF | Dye | Permeability increased 1.5 times; rejection increased from 96 to 99% | [89] |
| Biocellulose nanofibers membrane | Biosynthetic process followed by a purification step involving alkali treatment | NF | Emulsified oily wastewater | 99% separation efficiency; permeate flux recovery ratio >94% | [90] |
| Carbon nanofiber (CNF)/cellulosic membranes | Carboxylic and amine functionalized CNFs | FO | Desalination | 15 L m−2 h−1 water flux | [91] |
| Cellulose triacetate (CTA) and novel thin film composite | Calcium alginate as a model foulant. | FO | Desalination | Physical cleaning was more efficient | [92] |
| CNC and TOCNF coated polyethersulfone (PES) membrane | Layer-by-layer deposition | MF | Water | Improved antifouling and antibacterial properties | [93] |
4.2. Environmental Remediation
4.2.1. Nanocellulose as Adsorbent
4.2.2. Nanocellulose as Photocatalyst
4.2.3. Nanocellulose for Gas Separation
4.3. Pollutant Sensors
4.4. Energy Devices
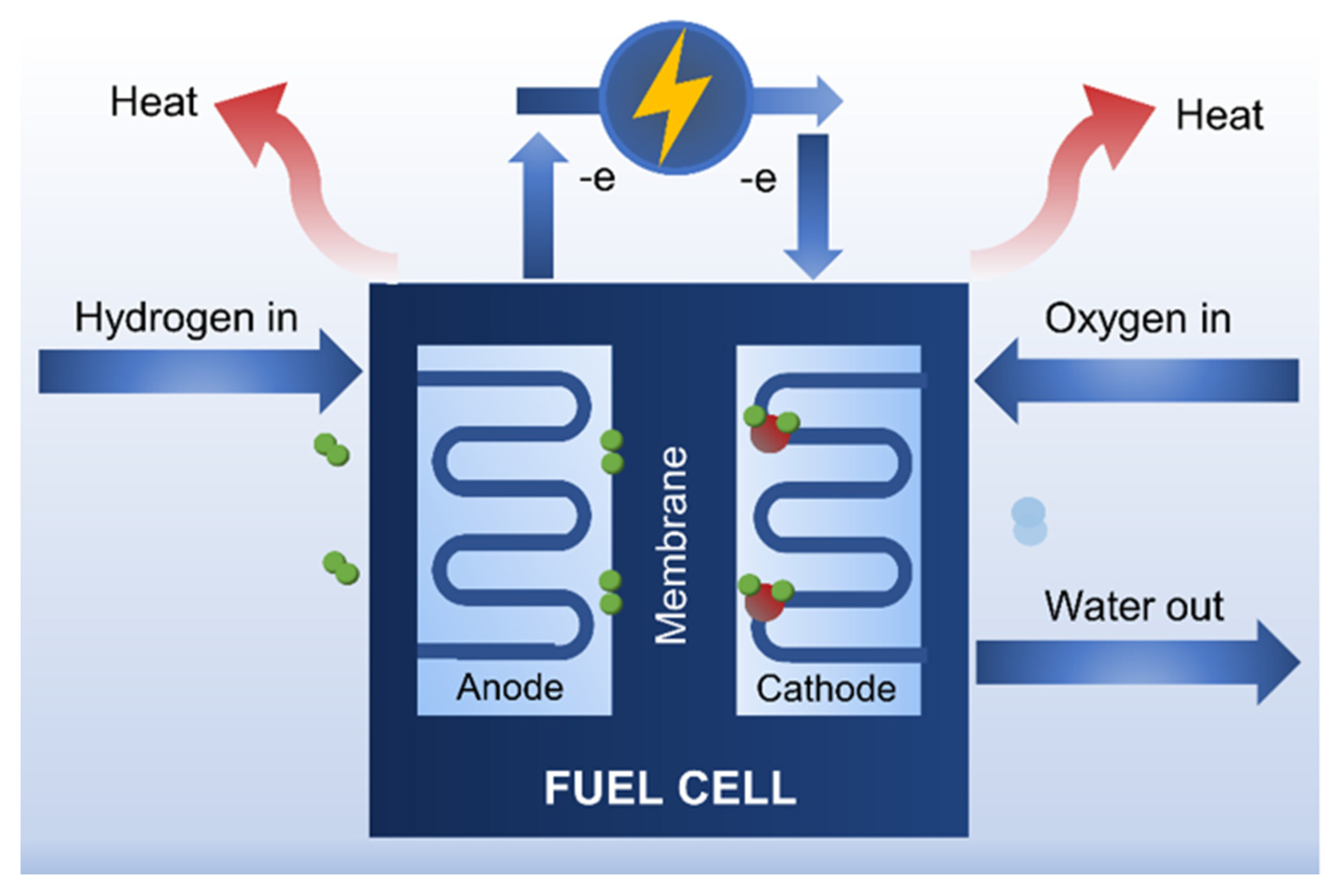
4.4.1. Fuel Cells
4.4.2. Solar Cells
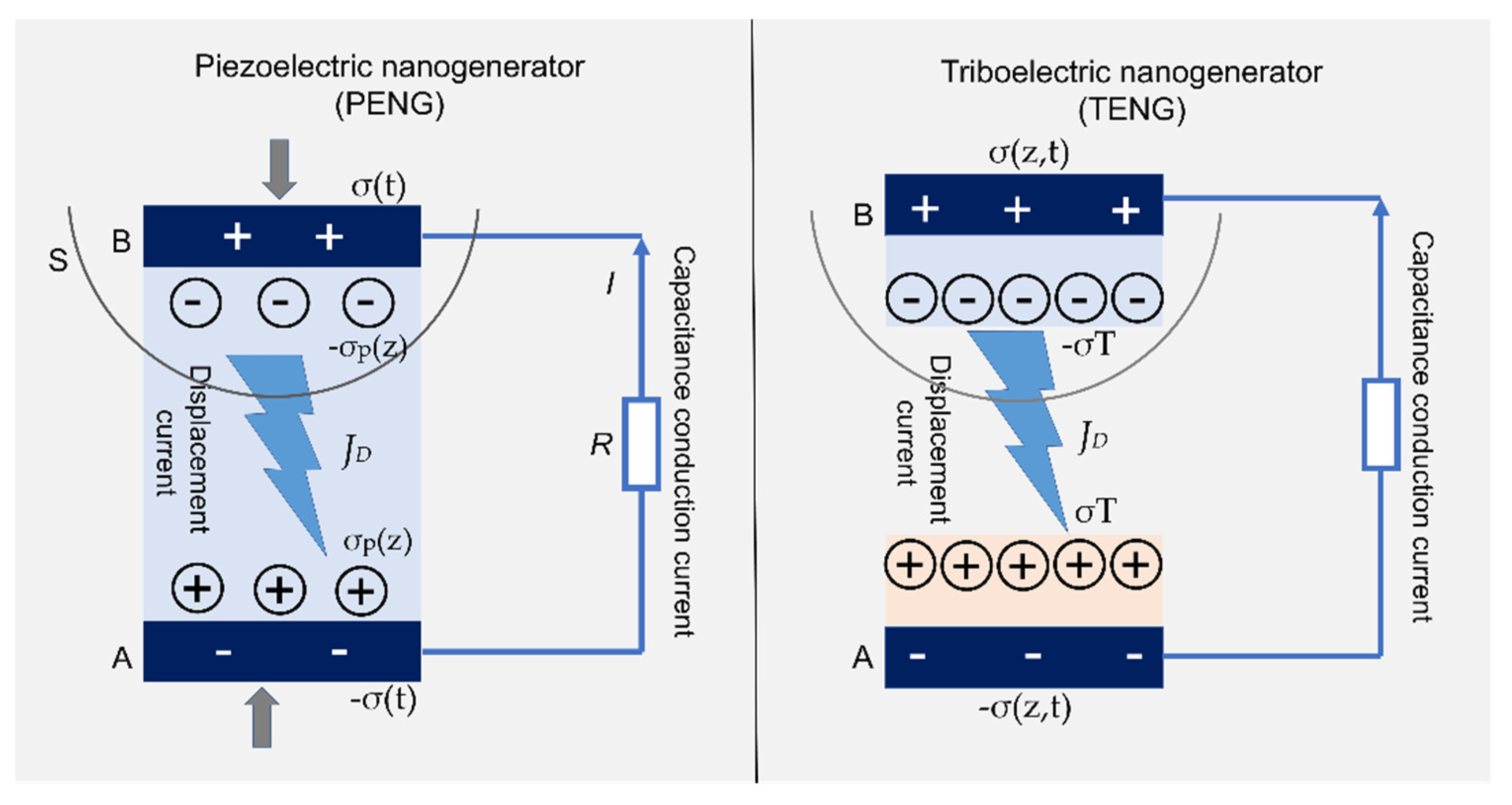
4.4.3. Nanogenerators
5. Challenges and Opportunities
Challenges in Nanocellulose Production and Application
- i.
- Cost-Effective Production and Upscaling
- ii.
- Complex Preparation Process
- iii.
- Dispersion of Nanocellulose
- iv.
- High Tendency of Clustering
- v.
- Homogeneity of Nanocellulose Mixture
6. Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, H.; Sazali, N.; Salleh, W.N.W.; Abidin, M.N.Z. A short review on recent utilization of nanocellulose for wastewater remediation and gas separation. Mater. Today Proc. 2019, 42, 45–49. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Rajini, N.; Reddy, K.O.; Rajulu, A.V.; Siengchin, S.; Ayrilmis, N. All-cellulose composite films with cellulose matrix and napier grass cellulose fibril fillers. Int. J. Biol. Macromol. 2018, 112, 1310–1315. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Lim, K.-T. Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 2019, 9, 19143–19162. [Google Scholar] [CrossRef] [Green Version]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Sundaram, J.; Mani, S. Production of cellulose nanofibrils and their application to food: A review. Nanotechnol. Food Environ. Paradig. 2017, 1–33. [Google Scholar] [CrossRef]
- Moreno, G.; Ramirez, K.; Esquivel, M.; Jimenez, G. Isolation and characterization of nanocellulose obtained from industrial crop waste resources by using mild acid hydrolysis. J. Renew. Mater. 2018, 6, 362–369. [Google Scholar] [CrossRef]
- Maciel, M.M.A.D.; de Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H. Obtainment and characterization of nanocellulose from an unwoven industrial textile cotton waste: Effect of acid hydrolysis conditions. Int. J. Biol. Macromol. 2019, 126, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Marakana, P.G.; Dey, A.; Saini, B. Isolation of nanocellulose from lignocellulosic biomass: Synthesis, characterization, modification, and potential applications. J. Environ. Chem. Eng. 2021, 9, 106606. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Tiwari, G.; Sharma, A.; Kumar, A.; Sharma, S. Assessment of microwave-assisted alkali pretreatment for the production of sugars from banana fruit peel waste. Biofuels 2019, 10, 3–10. [Google Scholar] [CrossRef]
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr. Polym. 2018, 201, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Hasanulbasori, M.A.; Chiat, P.F.; Lee, H.V. Pyrus pyrifolia fruit peel as sustainable source for spherical and porous network based nanocellulose synthesis via one-pot hydrolysis system. Int. J. Biol. Macromol. 2019, 123, 1305–1319. [Google Scholar] [CrossRef]
- Qiao, A.; Cui, M.; Huang, R.; Ding, G.; Qi, W.; He, Z.; Klemes, J.J.; Su, R. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydr. Polym. 2021, 272, 118471. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. Recent advances on nanocellulose biomaterials for environmental health photoremediation: An overview. Environ. Res. 2022, 204, 111964. [Google Scholar] [CrossRef]
- Ho, N.A.D.; Leo, C.P. A review on the emerging applications of cellulose, cellulose derivatives and nanocellulose in carbon capture. Environ. Res. 2021, 197, 111100. [Google Scholar] [CrossRef]
- Rana, A.K.; Gupta, V.K.; Saini, A.K.; Voicu, S.I.; Abdellattifaand, M.H.; Thakur, V.K. Water desalination using nanocelluloses/cellulose derivatives based membranes for sustainable future. Desalination 2021, 520, 115359. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Shen, Z. Nanocellulose based filtration membrane in industrial waste water treatment: A review. Materials 2021, 14, 5398. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A review of the surface modification of cellulose. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced nanocellulose-based composites for flexible functional energy storage devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Mbakop, S.; Nthunya, L.N.; Onyango, M.S. Recent advances in the synthesis of nanocellulose functionalized-hybrid membranes and application in water quality improvement. Processes 2021, 9, 611. [Google Scholar] [CrossRef]
- Mautner, A.; Kwaw, Y.; Weiland, K.; Mvubu, M.; Botha, A.; John, M.J.; Mtibe, A.; Siqueira, G.; Bismarck, A. Natural fibre-nanocellulose composite filters for the removal of heavy metal ions from water. Ind. Crop. Prod. 2019, 133, 325–332. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Ullah, M.W.; Manan, S.; Ul-Islam, M.; Revin, V.V.; Thomas, S.; Yang, G. Introduction to nanocellulose. Nanocellulose 2021, 1–50. [Google Scholar] [CrossRef]
- Borjesson, M.; Westman, G. Crystalline nanocellulose—Preparation, modification, and properties. Cellul.-Fundam. Asp. Curr. Trends 2016, 13, 159–191. [Google Scholar] [CrossRef] [Green Version]
- MatWeb Material Property Data. 2020. Available online: http://www.matweb.com/search/datasheet.aspx?matguid=706f16a3a8be468284571dd36bbdea35 (accessed on 30 January 2022).
- AZO Materials. 2021. Available online: https://www.azom.com/article.aspx?ArticleID=965 (accessed on 30 January 2022).
- Hinestroza, H.P.; Urena-Saborio, H.; Zurita, F.; de Leon, A.A.G.; Sundaram, G.; Sulbaran-Rangel, B. Composite membranes and their potential for the removal of pollutants from water. Molecules 2020, 25, 683. [Google Scholar] [CrossRef] [Green Version]
- TORAY, Intermediate Modulus T1100S. 2018. Available online: https://www.toraycma.com/products/carbon-fiber/ (accessed on 30 January 2022).
- Liu, P.; Zhu, C.; Mathew, A.P. Mechanically robust high flux graphene oxide—Nanocellulose membranes for dye removal from water. J. Hazard. Mater. 2019, 371, 484–493. [Google Scholar] [CrossRef]
- Eom, J.; Park, S.; Jin, H.J.; Kwak, H.W. Multiscale hybridization of natural silk–nanocellulose fibrous composites with exceptional mechanical properties. Front. Mater. 2020, 7, 98. [Google Scholar] [CrossRef]
- Ram, F.; Velayutham, P.; Sahu, A.K.; Lele, A.K.; Shanmuganathan, K. Enhancing thermomechanical and chemical stability of polymer electrolyte membranes using polydopamine coated nanocellulose. ACS Appl. Energy Mater. 2020, 3, 1988–1999. [Google Scholar] [CrossRef]
- Luo, J.; Huang, K.; Zhou, X.; Xu, Y. Hybrid films based on holistic celery nanocellulose and lignin/hemicellulose with enhanced mechanical properties and dye removal. Int. J. Biol. Macromol. 2020, 147, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.; Maghe, M.; Zohdi, N.; Amiralian, N.; Naebe, M.; Laycock, B.; Fox, B.L.; Martin, D.; Annamalai, P.K. Influence of Different Nanocellulose Additives on Processing and Performance of PAN-Based Carbon Fibers. ACS Omega 2019, 4, 9720–9730. [Google Scholar] [CrossRef] [PubMed]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Jannatyha, N.; Shojaee-Aliabadi, S.; Moslehishad, M.; Moradi, E. Comparing mechanical, barrier and antimicrobial properties of nanocellulose/CMC and nanochitosan/CMC composite films. Int. J. Biol. Macromol. 2020, 164, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, D.; Checchetto, R.; Tarter, S.; Caretti, D.; Rizzuto, M.; Fambri, L.; Pegoretti, A. Polylactic acid-lauryl functionalized nanocellulose nanocomposites: Microstructural, thermo-mechanical and gas transport properties. Express Polym. Lett. 2019, 13, 858–876. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- De France, K.; Zeng, Z.; Wu, T.; Nystrom, G. Functional materials from nanocellulose: Utilizing structure–property relationships in bottom-up fabrication. Adv. Mater. 2021, 33, 657. [Google Scholar] [CrossRef] [Green Version]
- Santmarti, A.; Lee, K.-Y. Crystallinity and thermal stability of nanocellulose. Nanocellul. Sustain. Prod. Prop. Appl. Case Stud. 2018, 67–86. [Google Scholar] [CrossRef]
- Costa, L.A.; Fonseca, A.F.; Pereira, F.V.; Druzian, J.I. Extraction and characterization of nanocellulose from corn stover. Mater. Today Proc. 2015, 2, 287–294. [Google Scholar] [CrossRef]
- Kunaver, M.; Anzlovar, A.; Zagar, E. The fast and effective isolation of nanocellulose from selected cellulosic feedstocks. Carbohydr. Polym. 2016, 148, 251–258. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.H.; Ismadji, S. Nanocelluloses: Sources, pretreatment, isolations, modification, and its application as the drug carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in advanced functional material applications of nanocellulose. Processes 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A. The Crystallinity of nanocellulose: Dispersion-induced disordering of the grain boundary in biologically structured cellulose. ACS Appl. Nano Mater. 2018, 1, 5774–5785. [Google Scholar] [CrossRef] [Green Version]
- Helberg, R.M.L.; Torstensen, J.O.; Dai, Z.; Janakiram, S.; Chinga-Carrasco, G.; Gregersen, O.W.; Syverud, K.; Deng, L. Nanocomposite membranes with high-charge and size-screened phosphorylated nanocellulose fibrils for CO2 separation. Green Energy Environ. 2021, 6, 585–596. [Google Scholar] [CrossRef]
- Tortorella, S.; Buratti, V.V.; Maturi, M.; Sambri, L.; Franchini, M.C.; Locatelli, E. Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review. Int. J. Nanomed. 2020, 15, 9909–9937. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hadi, P.; Yin, X.; Yu, J.; Huang, X.; Ma, H.; Walker, H.; Hsiao, B.S. Antifouling nanocellulose membranes: How subtle adjustment of surface charge lead to self-cleaning property. J. Membr. Sci. 2020, 618, 118739. [Google Scholar] [CrossRef]
- Hadi, P.; Yang, M.; Ma, H.; Huang, X.; Walker, H.; Hsiao, B.S. Biofouling-resistant nanocellulose layer in hierarchical polymeric membranes: Synthesis, characterization and performance. J. Membr. Sci. 2019, 579, 162–171. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, H.; Taha, A.A.; Hsiao, B.S. High-flux anti-fouling nanofibrous composite ultrafiltration membranes containing negatively charged water channels. J. Membr. Sci. 2020, 612, 118382. [Google Scholar] [CrossRef]
- Moeinzadeh, R.; Ghadam, A.G.J.; Lau, W.J.; Emadzadeh, D. Synthesis of nanocomposite membrane incorporated with amino-functionalized nanocrystalline cellulose for refinery wastewater treatment. Carbohydr. Polym. 2019, 225, 115212. [Google Scholar] [CrossRef]
- Gopakumar, D.A. Nanocellulose Based Functional Constructs for Clean Water and Microwave Suppression; Mahatma Gandhi University: Kerala, India, 2017. [Google Scholar]
- Barbash, V.A.; Yashchenko, O.V.; Gondovska, A.S.; Deykun, I.M. Preparation and characterization of nanocellulose obtained by TEMPO-mediated oxidation of organosolv pulp from reed stalks. Appl. Nanosci. 2021, 1–14. [Google Scholar] [CrossRef]
- Al-Rawi, A.S.; Al-Khateeb, I.K.I.; Zaidan, T.A. Nanocellulose acetate membranes: Preparation and application. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100529. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.; Alam, Z.; Moniruzzaman, M. Ionic liquids as a sustainable platform for nanocellulose processing from bioresources: Overview and current status. ACS Sustain. Chem. Eng. 2021, 9, 1008–1034. [Google Scholar] [CrossRef]
- Ke, W.-T.; Chiu, H.-L.; Liao, Y.-C. Multifunctionalized cellulose nanofiber for water-repellent and wash-sustainable coatings on fabrics. Langmuir 2020, 36, 8144–8151. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ottesen, V.; Deng, J.; Helberg, R.M.L.; Deng, L. A Brief Review of Nanocellulose Based Hybrid Membranes for CO2 Separation. Fibers 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Gao, Y.; Bai, H.; Zhang, L.; Qu, P.; Bai, L. Polysulfone membrane with NCC. BioResources 2011, 6, 1670–1680. [Google Scholar]
- Li, X.-L.; Zhu, L.-P.; Zhu, B.-K.; Xu, Y.-Y. High-flux and anti-fouling cellulose nanofiltration membranes prepared via phase inversion with ionic liquid as solvent. Sep. Purif. Technol. 2011, 83, 66–73. [Google Scholar] [CrossRef]
- Priyangga, A.; Pambudi, A.B.; Atmaja, L.; Jaafar, J. Synthesis of nanocellulose composite membrane and its properties for direct methanol fuel cell. Mater. Today Proc. 2020, 46, 1998–2003. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Yu, J.; Zhang, L.; Liu, L.; Zhou, X.; Huang, C.; Fan, Y. Preparation of nanocellulose/filter paper (NC/FP) composite membranes for high-performance filtration. Cellulose 2019, 26, 1183–1194. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-enabled membranes for water purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Derami, H.G.; Jiang, Q.; Ghim, D.; Cao, S.; Chandar, Y.J.; Morrissey, J.J.; Jun, Y.-S.; Singamaneni, S. A Robust and scalable polydopamine/bacterial nanocellulose hybrid membrane for efficient wastewater treatment. ACS Appl. Nano Mater. 2019, 2, 1092–1101. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun core–shell nanofibrous membranes with nanocellulose-stabilized carbon nanotubes for use as high-performance flexible supercapacitor electrodes with enhanced water resistance, thermal stability, and mechanical toughness. ACS Appl. Mater. Interfaces 2019, 11, 44624–44635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Wang, Y. Recent progress in cellulose-based electrospun nanofibers as multifunctional materials. Nanoscale Adv. 2021, 3, 6040–6047. [Google Scholar] [CrossRef]
- Goetz, L.A.; Naseri, N.; Nair, S.S.; Karim, Z.; Mathew, A.P. All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 2018, 25, 3011–3023. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-incorporated PCL/gelatin-derived coaxial electrospinning nanocellulose membranes for periodontal tissue regeneration. Front. Bioeng. Biotechnol. 2021, 9, 216. [Google Scholar] [CrossRef]
- Saallah, S.; Naim, M.N.; Mokhtar, M.N.; Bakar, N.F.A.; Gen, M.; Lenggoro, I.W. Preparation and characterisation of cyclodextrin glucanotransferase enzyme immobilised in electrospun nanofibrous membrane. J. Fiber Sci. Technol. 2017, 73, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Brandes, R.; Belosinschi, D.; Brouillette, F.; Chabot, B. A new electrospun chitosan/phosphorylated nanocellulose biosorbent for the removal of cadmium ions from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103477. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.B.; Wang, S. Interfacial Polymerization: From Chemistry to Functional Materials. Angew. Chem. Int. Ed. 2020, 59, 21840–21856. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-F.; Ooi, B.; Leo, C. Future perspectives of nanocellulose-based membrane for water treatment. J. Water Process. Eng. 2020, 37, 101502. [Google Scholar] [CrossRef]
- Saallah, S.; Roslan, J.; Zakaria, N.N.; Pindi, W.; Siddiquee, S.; Misson, M.; Ongkudon, C.M.; Jamil, N.H.A.M.; Lenggoro, W. Isolation of nanocellulose from Saba’ (Musa acuminata x balbisiana) banana peel by one-pot oxidation-hydrolysis system. Adv. Agric. Food Res. J. 2020, 1, 96. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Nissila, T.; Wei, J.; Geng, S.; Teleman, A.; Oksman, K. Ice-Templated Cellulose Nanofiber Filaments as a Reinforcement Material in Epoxy Composites. Nanomaterials 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Tarafdar, A.; et al. Potential of nanocellulose for wastewater treatment. Chemosphere 2021, 281, 130738. [Google Scholar] [CrossRef]
- Mautner, A. Nanocellulose water treatment membranes and filters: A review. Polym. Int. 2020, 69, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, S.; Low, Z.-X.; Batchelor, W. Cellulose nanofibre composite membranes—Biodegradable and recyclable UF membranes. Chem. Eng. J. 2015, 265, 138–146. [Google Scholar] [CrossRef]
- Sijabat, E.K.; Nuruddin, A.; Aditiawati, P.; Purwasasmita, B.S. Synthesis and Characterization of Bacterial Nanocellulose from Banana Peel for Water Filtration Membrane Application. J. Phys. Conf. Ser. 2019, 1230, 012085. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for extraction of nanocellulose from various sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–49. [Google Scholar] [CrossRef]
- Haafiz, M.M.; Hassan, A.; Khalil, H.A.; Khan, I.; Inuwa, I.; Islam, S.; Hossain, S.; Syakir, M.; Fazita, M.N. Bionanocomposite based on cellulose nanowhisker from oil palm biomass-filled poly(lactic acid). Polym. Test. 2015, 48, 133–139. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Mtibe, A.; John, M.J.; Sadiku, E.R.; Sefadi, J.S. Electrospun Alginate Nanofibers Toward Various Applications: A Review. Materials 2020, 13, 934. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Tato, P.; Ortiz-Quiles, E.O.; Vega-Figueroa, K.; Santiago-Martoral, L.; Flynn, M.; Díaz-Vázquez, L.M.; Nicolau, E. Metalized Nanocellulose Composites as a Feasible Material for Membrane Supports: Design and Applications for Water Treatment. Environ. Sci. Technol. 2017, 51, 4585–4595. [Google Scholar] [CrossRef]
- Goswami, R.; Mishra, A.; Bhatt, N.; Mishra, A.; Naithani, P. Potential of chitosan/nanocellulose based composite membrane for the removal of heavy metal (chromium ion). Mater. Today Proc. 2021, 46, 10954–10959. [Google Scholar] [CrossRef]
- Yang, S.; Tang, R.; Dai, Y.; Wang, T.; Zeng, Z.; Zhang, L. Fabrication of cellulose acetate membrane with advanced ultrafiltration performances and antibacterial properties by blending with HKUST-1@LCNFs. Sep. Purif. Technol. 2021, 279, 119524. [Google Scholar] [CrossRef]
- Vetrivel, S.; Saraswathi, M.S.S.A.; Rana, D.; Divya, K.; Nagendran, A. Cellulose acetate ultrafiltration membranes customized with copper oxide nanoparticles for efficient separation with antifouling behavior. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Hu, M.-X.; Niu, H.-M.; Chen, X.-L.; Zhan, H.-B. Natural cellulose microfiltration membranes for oil/water nanoemulsions separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 142–151. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Bazhenov, S.; Borisov, I.L.; Vasilevsky, V.; Vinokurov, V.; Volkov, A. Nanocellulose as Modifier for Hollow Fiber Ultrafiltration PSF Membranes. Key Eng. Mater. 2019, 816, 238–243. [Google Scholar] [CrossRef]
- Zhuang, G.-L.; Wu, S.-Y.; Lo, Y.-C.; Chen, Y.-C.; Tung, K.-L.; Tseng, H.-H. Gluconacetobacter xylinus synthesized biocellulose nanofiber membranes with superhydrophilic and superoleophobic underwater properties for the high-efficiency separation of oil/water emulsions. J. Membr. Sci. 2020, 605, 118091. [Google Scholar] [CrossRef]
- Ahmed, D.; Isawi, H.; Badway, N.; Elbayaa, A.; Shawky, H. Highly porous cellulosic nanocomposite membranes with enhanced performance for forward osmosis desalination. Iran. Polym. J. 2021, 30, 423–444. [Google Scholar] [CrossRef]
- Mazlan, N.M.; Marchetti, P.; Maples, H.; Gu, B.; Karan, S.; Bismarck, A.; Livingston, A. Organic fouling behaviour of structurally and chemically different forward osmosis membranes—A study of cellulose triacetate and thin film composite membranes. J. Membr. Sci. 2016, 520, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Sanchez, A.; Jalvo, B.; Mautner, A.; Nameer, S.; Pöhler, T.; Tammelin, T.; Mathew, A.P. Waterborne nanocellulose coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. J. Membr. Sci. 2021, 620, 118842. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, D.; Shao, Z.; Han, B.; Lv, Y.; Gao, K.; Peng, X. Superior effect of TEMPO-oxidized cellulose nanofibrils (TOCNs) on the performance of cellulose triacetate (CTA) ultrafiltration membrane. Desalination 2014, 332, 117–125. [Google Scholar] [CrossRef]
- Tshikovhi, A.; Mishra, S.B.; Mishra, A.K. Nanocellulose-based composites for the removal of contaminants from wastewater. Int. J. Biol. Macromol. 2020, 152, 616–632. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Yusuf, M. Application of nanocellulose composites in the environmental engineering as a catalyst, flocculants, and energy storages: A review. J. Compos. Compd. 2021, 3, 114–128. [Google Scholar]
- Reshmy, R.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sindhu, R.; Sirohi, R.; Awasthi, M.K.; Pandey, A.; Binod, P. Nanocellulose as green material for remediation of hazardous heavy metal contaminants. J. Hazard. Mater. 2022, 424, 127516. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, Y.; Zelekew, O.A.; Abdeta, A.B.; Kuo, D.-H.; Wu, Q.; Zhang, J.; Yuan, Z.; Lin, J.; Chen, X. Biological renewable nanocellulose templated CeO2/TiO2 synthesis and its photocatalytic removal efficiency of pollutants. J. Mol. Liq. 2021, 336, 116873. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Mammadli, N.; Yildiz, U. Bioinspired modified nanocellulose adsorbent for enhanced boron recovery from aqueous media: Optimization, kinetics, thermodynamics and reusability study. J. Environ. Chem. Eng. 2019, 7, 103281. [Google Scholar] [CrossRef]
- Septevani, A.A.; Rifathin, A.; Sari, A.A.; Sampora, Y.; Ariani, G.N.; Sudiyarmanto; Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohydr. Polym. 2020, 229, 115433. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Sganappa, A.; Melone, L.; Punta, C.; Cametti, M. Naked-Eye Heterogeneous Sensing of Fluoride Ions by Co-Polymeric Nanosponge Systems Comprising Aromatic-Imide-Functionalized Nanocellulose and Branched Polyethyleneimine. ChemPlusChem 2019, 84, 1512–1518. [Google Scholar] [CrossRef]
- Mo, L.; Pang, H.; Lu, Y.; Li, Z.; Kang, H.; Wang, M.; Zhang, S.; Li, J. Wood-inspired nanocellulose aerogel adsorbents with excellent selective pollutants capture, superfast adsorption, and easy regeneration. J. Hazard. Mater. 2021, 415, 125612. [Google Scholar] [CrossRef]
- Geng, B.; Xu, Z.; Liang, P.; Zhang, J.; Christie, P.; Liu, H.; Wu, S.; Liu, X. Three-dimensional macroscopic aminosilylated nanocellulose aerogels as sustainable bio-adsorbents for the effective removal of heavy metal ions. Int. J. Biol. Macromol. 2021, 190, 170–177. [Google Scholar] [CrossRef]
- Shahnaz, T.; Priyan, V.V.; Jayakumar, A.; Narayanasamy, S. Magnetic nanocellulose from Cyperus rotundas grass in the absorptive removal of rare earth element cerium (III): Toxicity studies and interpretation. Chemosphere 2022, 287, 131912. [Google Scholar] [CrossRef]
- Mutar, H.R.; Jasim, K.K. Adsorption study of disperse yellow dye on nanocellulose surface. Mater. Today Proc. 2021, 1–7. [Google Scholar] [CrossRef]
- Nematollahi, R.; Ziyadi, H.; Ghasemi, E.; Taheri, H. Cinnamon nanocellulose as a novel catalyst to remove methyl orange from aqueous solution. Inorg. Chem. Commun. 2022, 137, 109222. [Google Scholar] [CrossRef]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and Conductive Nanocellulose-Based Films for Active and Intelligent Food Packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soon, C.Y.; Rahman, N.A.; Tee, Y.B.; Talib, R.A.; Tan, C.H.; Abdan, K.; Chan, E.W.C. Electrospun biocomposite: Nanocellulose and chitosan entrapped within a poly(hydroxyalkanoate) matrix for Congo red removal. J. Mater. Res. Technol. 2019, 8, 5091–5102. [Google Scholar] [CrossRef]
- Shahnaz, T.; Bedadeep, D.; Narayanasamy, S. Investigation of the adsorptive removal of methylene blue using modified nanocellulose. Int. J. Biol. Macromol. 2022, 200, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, Z.; Li, X.; Li, P.; Wei, J.; Hu, M.; Geng, L.; Peng, X. Constructing Acid-Resistant Chitosan/Cellulose Nanofibrils Composite Membrane for the Adsorption of Methylene Blue. Available online: https://ssrn.com/abstract=4020850 (accessed on 30 January 2022).
- Zeng, J.; Wang, T.; Cheng, Z.; Liu, L.; Hu, F. Ultrahigh Adsorption of Toxic Substances from Cigarette Smoke Using Nanocellulose-SiO2 Hybrid Aerogels. ACS Appl. Polym. Mater. 2022, 4, 1173–1182. [Google Scholar] [CrossRef]
- Lebogang, L.; Bosigo, R.; Lefatshe, K.; Muiva, C. Ag3PO4/nanocellulose composite for effective sunlight driven photodegradation of organic dyes in wastewater. Mater. Chem. Phys. 2019, 236, 121756. [Google Scholar] [CrossRef]
- Voisin, H.; Falourd, X.; Rivard, C.; Capron, I. Versatile nanocellulose-anatase TiO2 hybrid nanoparticles in Pickering emulsions for the photocatalytic degradation of organic and aqueous dyes. JCIS Open 2021, 3, 100014. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Pan, X.-J.; Li, J.; Li, C.; Ji, C.-L. Facile preparation and characterization of anatase TiO2/nanocellulose composite for photocatalytic degradation of methyl orange. J. Saudi Chem. Soc. 2021, 25, 101383. [Google Scholar] [CrossRef]
- Nahi, J.; Radhakrishnan, A.; Beena, B. Green synthesis of zinc oxide incorporated nanocellulose with visible light photocatalytic activity and application for the removal of antibiotic enrofloxacin from aqueousmedia. Mater. Today Proc. 2020, 41, 583–589. [Google Scholar] [CrossRef]
- Saeed, M.; Farahani, V.; Ghorbani, M. Fabrication of fe-doped ZnO/nanocellulose nanocomposite as an efficient photocatalyst for degradation of methylene blue under visible light. Res. Sq. 2022, 1–28. [Google Scholar] [CrossRef]
- Helmiyati, H.; Fitriana, N.; Chaerani, M.L.; Dini, F.W. Green hybrid photocatalyst containing cellulose and γ–Fe2O3–ZrO2 heterojunction for improved visible-light driven degradation of Congo red. Opt. Mater. 2022, 124, 111982. [Google Scholar] [CrossRef]
- Moodley, V.; Maddila, S.; Jonnalagadda, S.B.; van Zyl, W.E. Degradation of o-Chloranil Using Nanocrystalline-Cellulose/TiO2 Composites via a Solar Photocatalytic Route. S. Afr. J. Chem. 2021, 75, 91–97. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Yus, J.; Bravo, Y.; Sanchez-Herencia, A.J.; Ferrari, B. Exploitation of Lignocellulose Fiber-Based Biotemplates to Improve the Performance of an Immobilized TiO2 Photocatalyst. Catalysts 2021, 11, 156. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, Y.; van Bochove, B.; Makila, E.; Seppala, J.; Xu, W.; Willfor, S.; Xu, C. Robust shape-retaining nanocellulose-based aerogels decorated with silver nanoparticles for fast continuous catalytic discoloration of organic dyes. Sep. Purif. Technol. 2020, 242, 116523. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.; Hagg, M.-B.; Gregersen, W. Decoupling the effect of membrane thickness and CNC concentration in PVA based nanocomposite membranes for CO2/CH4 separation. Sep. Purif. Technol. 2018, 204, 220–225. [Google Scholar] [CrossRef]
- Torstensen, J.; Helberg, R.M.; Deng, L.; Gregersen, W.; Syverud, K. PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int. J. Greenh. Gas Control 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Zou, X.; Chen, Z.; Liu, H.; Ni, Y. Ultrasensitive Physical, Bio, and Chemical Sensors Derived from 1-, 2-, and 3-D Nanocellulosic Materials. Small 2020, 16, e1906567. [Google Scholar] [CrossRef]
- Taheri, M.; Ahour, F.; Keshipour, S. Sensitive and selective determination of Cu 2+ at d -penicillamine functionalized nano-cellulose modified pencil graphite electrode. J. Phys. Chem. Solids 2018, 117, 180–187. [Google Scholar] [CrossRef]
- Shahi, N.; Lee, E.; Min, B.; Kim, D.-J. Rice Husk-Derived Cellulose Nanofibers: A Potential Sensor for Water-Soluble Gases. Sensors 2021, 21, 4415. [Google Scholar] [CrossRef]
- Pouzesh, M.; Nekouei, S.; Zadeh, M.A.F.; Keshtpour, F.; Wang, S.; Nekouei, F. Fabrication of stable copper nanoparticles embedded in nanocellulose film as a bionanocomposite plasmonic sensor and thereof for optical sensing of cyanide ion in water samples. Cellulose 2019, 26, 4945–4956. [Google Scholar] [CrossRef]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Kämpf, M.M.; Zimmermann, T.; Maniura, K.; Faccio, G. A Protein-Nanocellulose Paper for Sensing Copper Ions at the Nano- to Micromolar Level. Adv. Funct. Mater. 2017, 27, 1604291. [Google Scholar] [CrossRef]
- Copur, F.; Bekar, N.; Zor, E.; Alpaydin, S.; Bingol, H. Nanopaper-based photoluminescent enantioselective sensing of L-Lysine by L-Cysteine modified carbon quantum dots. Sens. Actuators B Chem. 2019, 279, 305–312. [Google Scholar] [CrossRef]
- Zor, E.; Alpaydin, S.; Arici, A.; Saglam, M.E.; Bingol, H. Photoluminescent nanopaper-based microcuvette for iodide detection in seawater. Sens. Actuators B Chem. 2018, 254, 1216–1224. [Google Scholar] [CrossRef]
- Abbasi-Moayed, S.; Golmohammadi, H.; Hormozi-Nezhad, M.R. A nanopaper-based artificial tongue: A ratiometric fluorescent sensor array on bacterial nanocellulose for chemical discrimination applications. Nanoscale 2018, 10, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Wua, J.; Fenga, Y.; Zhangb, L.; Wuac, W. Nanocellulose-based Surface-enhanced Raman spectroscopy sensor for highly sensitive detection of TNT. Carbohydr. Polym. 2020, 248, 116766. [Google Scholar] [CrossRef]
- Jung, J.-W.; Jang, J.-S. D-space-controlled graphene oxide hybrid membrane-loaded SnO2 nanosheets for selective H2 detection. J. Sens. Sci. Technol. 2021, 30, 376–380. [Google Scholar] [CrossRef]
- Zor, E. Silver nanoparticles-embedded nanopaper as a colorimetric chiral sensing platform. Talanta 2018, 184, 149–155. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.-J.; Zhang, D.; Li, H.-Y.; Ma, Y.; Qi, L.; Zhou, Y.-L.; Zhang, X.-X. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 2011, 84, 71–77. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhang, D.; Zhang, X.; Ma, Y.; Zhou, Y.; Qi, L. Biotemplated Synthesis of Gold Nanoparticle-Bacteria Cellulose Nanofiber Nanocomposites and Their Application in Biosensing. Adv. Funct. Mater. 2010, 20, 1152–1160. [Google Scholar] [CrossRef]
- Park, M.; Chang, H.; Jeong, D.H.; Hyun, J. Spatial deformation of nanocellulose hydrogel enhances SERS. BioChip J. 2013, 7, 234–241. [Google Scholar] [CrossRef]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Calle-Gil, R.; Castillo-Martínez, E.; Carretero-González, J. Cellulose Nanocrystals in Sustainable Energy Systems. Adv. Sustain. Syst. 2022, 2100395. [Google Scholar] [CrossRef]
- Rogalsky, S.; Bardeau, J.-F.; Makhno, S.; Babkina, N.; Tarasyuk, O.; Cherniavska, T.; Orlovska, I.; Kozyrovska, N.; Brovko, O. New proton conducting membrane based on bacterial cellulose/polyaniline nanocomposite film impregnated with guanidinium-based ionic liquid. Polymer 2018, 142, 183–195. [Google Scholar] [CrossRef]
- Sriruangrungkamol, A.; Chonkaew, W. Modification of nanocellulose membrane by impregnation method with sulfosuccinic acid for direct methanol fuel cell applications. Polym. Bull. 2021, 78, 3705–3728. [Google Scholar] [CrossRef]
- Muhmed, S.; Jaafar, J.; Daud, S.; Hanifah, M.F.R.; Purwanto, M.; Othman, M.; Rahman, M.; Ismail, A. Improvement in properties of nanocrystalline cellulose/poly (vinylidene fluoride) nanocomposite membrane for direct methanol fuel cell application. J. Environ. Chem. Eng. 2021, 9, 105577. [Google Scholar] [CrossRef]
- Ni, C.; Wei, Y.; Zhao, Q.; Liu, B.; Sun, Z.; Gu, Y.; Zhang, M.; Hu, W. Novel proton exchange membranes based on structure-optimized poly(ether ether ketone ketone)s and nanocrystalline cellulose. Appl. Surf. Sci. 2018, 434, 163–175. [Google Scholar] [CrossRef]
- Aguas, H.; Mateus, T.; Vicente, A.; Gaspar, D.; Mendes, M.J.; Schmidt, W.A.; Pereira, L.; Fortunato, E.; Martins, R. Thin Film Silicon Photovoltaic Cells on Paper for Flexible Indoor Applications. Adv. Funct. Mater. 2015, 25, 3592–3598. [Google Scholar] [CrossRef]
- Nogi, M.; Karakawa, M.; Komoda, N.; Yagyu, H.; Nge, T.T. Transparent Conductive Nanofiber Paper for Foldable Solar Cells. Sci. Rep. 2015, 5, 17254. [Google Scholar] [CrossRef]
- Jia, C.; Li, T.; Chen, C.; Dai, J.; Kierzewski, I.M.; Song, J.; Li, Y.; Yang, C.; Wang, C.; Hu, L. Scalable, anisotropic transparent paper directly from wood for light management in solar cells. Nano Energy 2017, 36, 366–373. [Google Scholar] [CrossRef]
- Voggu, V.R.; Sham, J.; Pfeffer, S.; Pate, J.; Fillip, L.; Harvey, T.B.; Brown, J.R.M.; Korgel, B.A. Flexible CuInSe2 Nanocrystal Solar Cells on Paper. ACS Energy Lett. 2017, 2, 574–581. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, Q.; Zhang, G.; Zhang, Z.; Liang, Q.; Liao, X.; Zhang, Y. High output piezoelectric nanocomposite generators composed of oriented BaTiO3 NPs@PVDF. Nano Energy 2015, 11, 719–727. [Google Scholar] [CrossRef]
- Yao, C.; Hernandez, A.; Yu, Y.Y.; Cai, Z.; Wang, X. Triboelectric nanogenerators and power-boards from cellulose nanofibrils and recycled materials. Nano Energy 2016, 30, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Yin, X.; Yu, Y.; Cai, Z.; Wang, X. Chemically Functionalized Natural Cellulose Materials for Effective Triboelectric Nanogenerator Development. Adv. Funct. Mater. 2017, 27, 794. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yim, E.-C.; Kim, J.-H.; Kim, S.-J.; Park, J.-Y.; Oh, I. Bacterial Nano-Cellulose Triboelectric Nanogenerator. Nano Energy 2017, 33, 130–137. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Vayer, M.; Foulon, L.; Pernes, M.; Devers, T.; Bigarré, J.; Aguie-Beghin, V. Nafion membranes reinforced by cellulose nanocrystals for fuel cell applications: Aspect ratio and heat treatment effects on physical properties. J. Mater. Sci. 2022, 57, 4684–4703. [Google Scholar] [CrossRef]
- Ni, C.; Wang, H.; Zhao, Q.; Liu, B.; Sun, Z.; Zhang, M.; Hu, W.; Liang, L. Crosslinking effect in nanocrystalline cellulose reinforced sulfonated poly (aryl ether ketone) proton exchange membranes. Solid State Ion. 2018, 323, 5–15. [Google Scholar] [CrossRef]
- Etuk, S.S.; Lawan, I.; Zhou, W.; Jiang, Y.; Zhang, Q.; Wei, X.; Zhang, M.; Fernando, G.F.; Yuan, Z. Synthesis and characterization of triazole based sulfonated nanocrystalline cellulose proton conductor. Cellulose 2020, 27, 3197–3209. [Google Scholar] [CrossRef]
- Hubler, A.; Trnovec, B.; Zillger, T.; Ali, M.; Wetzold, N.; Mingebach, M.; Wagenpfahl, A.; Deibel, C.; Dyakonov, V. Printed Paper Photovoltaic Cells. Adv. Energy Mater. 2011, 1, 1018–1022. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- Brunetti, F.; Operamolla, A.; Castro-Hermosa, S.; Lucarelli, G.; Manca, V.; Farinola, G.M.; Brown, T.M. Printed Solar Cells and Energy Storage Devices on Paper Substrates. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Chao, L.; Hou, M.; Liang, J.; Chen, Y.; Yu, H.-D.; Huang, W. Flexible, transparent nanocellulose paper-based perovskite solar cells. npj. Flex. Electron. 2019, 3, 4. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Cai, H.; Liu, Q.; Tong, G. Processing nanocellulose foam into high-performance membranes for harvesting energy from nature. Carbohydr. Polym. 2020, 241, 116253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.L.; Fadel, S.M.; Abouzeid, R.E.; Elseoud, W.S.A.; Hassan, E.A.; Berglund, L.; Oksman, K. Water purification ultrafiltration membranes using nanofibers from unbleached and bleached rice straw. Sci. Rep. 2020, 10, 11278. [Google Scholar] [CrossRef]
- Camacho, M.; Ureña, Y.R.C.; Lopretti, M.; Carballo, L.B.; Moreno, G.; Alfaro, B.; Baudrit, J.R.V. Synthesis and Characterization of Nanocrystalline Cellulose Derived from Pineapple Peel Residues. J. Renew. Mater. 2017, 5, 271–279. [Google Scholar] [CrossRef]
- Derami, H.G.; Gupta, P.; Gupta, R.; Rathi, P.; Morrissey, J.J.; Singamaneni, S. Palladium Nanoparticle-Decorated Mesoporous Polydopamine/Bacterial Nanocellulose as a Catalytically Active Universal Dye Removal Ultrafiltration Membrane. ACS Appl. Nano Mater. 2020, 3, 5437–5448. [Google Scholar] [CrossRef]





| Material | Density, ρ (g cm−3) | Tensile Strength, σ (GPa) | Elastic Modulus, E (GPa) | Reference |
|---|---|---|---|---|
| Stainless steel 304 | 8.00 | 0.50–0.70 | 193 | [28] |
| E-glass fiber | 2.54–2.60 | 0.52–3.79 | 72.40 | [27] |
| TORAYCA carbon fiber | 1.79 | 7.00 | 324 | [30] |
| Kevlar 49 Aramid fiber | 1.47 | 3.45 | 179 | [27] |
| Nanocellulose | 1.6 | 2–7.7 | 110–220 | [26] |
| Material | Nanocellulose Composition (%) | Tensile Strength (MPa) | Tensile Strain (%) | Young’s Modulus (GPa) | Reference |
|---|---|---|---|---|---|
| GO/CNF | 0 | 50.2 ± 6.3 | 4.8 ± 1.3 | 2.99 ± 0.31 | [31] |
| 1 | 74.4 ± 2.8 | 4.8 ± 0.9 | 3.90 ± 0.84 | ||
| 4 | 80.0 ± 14.9 | 5.9 ± 2.5 | 4.13 ± 0.73 | ||
| SF/CNF | 0 | 66 ± 18.9 | 18.6 ± 8.5 | 1.2 ± 0.2 | [32] |
| 5 | 111.1 ± 11.7 | 12.7 ± 0.4 | 2.0 ± 0.2 | ||
| 10 | 140.1 ± 14.3 | 12.3 ± 0.6 | 2.7 ± 0.2 | ||
| 15 | 143.5 ± 8.3 | 11.4 ± 1.6 | 3.0 ± 0.1 | ||
| PNC/Nafion | 0 | 11.5 | ~50 | 0.35 | [33] |
| 3 | 15.15 | ~25 | 0.54 | ||
| 7.5 | 13.00 | ~20 | 0.75 | ||
| Celery CNF/Lignin/hemicellulose | 0 | 24.1 ± 0.9 | 0.5 ± 0.1 | 4.83 ± 0.10 | [34] |
| 10 | 39.0 ± 4.1 | 0.6 ± 0.1 | 7.10 ± 0.31 | ||
| 20 | 72.5 ± 0.3 | 1.1 ± 0.2 | 6.42 ± 0.14 | ||
| 30 | 79.3 ± 3.4 | 1.4 ± 0.1 | 5.62 ± 0.24 | ||
| 50 | 85.2 ± 2.6 | 2.6 ± 0.3 | 3.25 ± 0.16 | ||
| PAN | 0 | 150 ± 10 | 44 ± 16 | 5.9 ± 0.4 | [35] |
| PAN/c-CNC | 0.1 | 190 ± 10 | 21 ± 5 | 6.7 ± 0.4 | |
| PAN/s-CNC | 0.1 | 190 ± 10 | 19 ± 6 | 7.0 ± 0.2 | |
| PAN/s-CNF | 0.1 | 150 ± 2 | 22 ± 7 | 6.3 ± 0.4 | |
| CNC/PVA | 0 | 117 | 0.7 | 32 | [36] |
| 0.5 | 98 | 1.4 | 26 | ||
| 1.0 | 105 | 12 | 15 | ||
| 1.5 | 104 | 5 | 20 | ||
| 2.0 | 118 | 7 | 20 | ||
| 4.0 | 132 | 10 | 30 | ||
| 6.0 | 155 | 1.4 | 38 | ||
| CMC/CNC | 0 | 6.10 ± 0.24 | 201.73 ± 0.15 | na | [37] |
| 0.1 | 7.23 ± 0.71 | 101.05 ± 1.32 | |||
| 0.5 | 9.98 ± 0.55 | 70.53 ± 0.23 | |||
| 1 | 12.30 ± 0.30 | 89.53 ± 0.18 | |||
| PLA/LNC | 0 | 40 ± 1 | 70 ± 20 | 1.77 ± 0.10 | [38] |
| 1 | 45 ± 3 | 30 ± 10 | 1.74 ± 0.14 | ||
| 3 | 26 ± 1 | >230 | 1.13 ± 0.10 | ||
| 5 | 27 ± 4 | >130 | 1.06 ± 0.06 | ||
| 10 | 21 ± 2 | 35 ± 10 | 1.01 ± 0.06 | ||
| 20 | 18 ± 2 | 30 ± 10 | 0.95 ± 0.03 | ||
| PEO/CNC | 0 | 14.2 ± 0.9 | 86 ± 14 | 0.76 ± 0.19 | [39] |
| 1 | 15.9 ± 0.1 | 495 ± 43 | 0.82 ± 0.20 | ||
| 4 | 16.0 ± 0.8 | 504 ± 34 | 0.90 ± 0.14 | ||
| 7 | 17.6 ± 0.7 | 526 ± 40 | 0.94 ± 0.15 | ||
| 10 | 15.3 ± 0.2 | 416 ± 43 | 0.76 ± 0.33 | ||
| PEO/CNF | 1 | 17.7 ± 0.9 | 491 ± 21 | 0.90 ± 0.10 | [39] |
| 4 | 20.8 ± 0.7 | 281 ± 56 | 0.99 ± 0.22 | ||
| 7 | 27.3 ± 0.9 | 340 ± 62 | 1.73 ± 0.10 | ||
| 10 | 14.4 ± 0.5 | 89 ± 55 | 1.24 ± 0.10 |
| Membrane Material | Target Compound | Adsorption Capacity (mg/g) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|
| Amino-modified nanocellulose | Boron | 120.9 | 86.73 | [99] |
| (EFB)-based nanocellulose functionalized with activated carbon | Pb2+ | 24.94 | 86 | [101] |
| Electrospun CS/PEO/PNC | Cd2+ | 62.3 | n.a | [70] |
| TOCNF/graphene oxide/trimethylolpropane-tris-(2-methyl-1-aziridine) propionate | Pb2+ | 571 | n.a | [102] |
| Cu2+ | 462 | |||
| Zn2+ | 361 | |||
| Cd2+ | 263 | |||
| Mn2+ | 208 | |||
| TOCNF/Si/NH2 | Cu2+ | 99.0 | 95.6 | [103] |
| Cd2+ | 124.5 | 85.2 | ||
| Hg2+ | 242.1 | 96.9 | ||
| Magnetic grass nanocellulose | Cerium (III) | 353.04 | n.a | [104] |
| Cellulose microcrystalline for TLC | Disperse yellow | n.a | 62.5 | [105] |
| Cinnamon nanocellulose | Methyl orange | n.a | 90.4 ± 2.3 | [106] |
| Cross-linked poly(2-methacryloyloxyethyl phosphorylcholine) and bacterial nanocellulose | Methylene blue | 4.44 ± 0.32 | n.a | [107] |
| Methyl orange | 4.56 ± 0.43 | |||
| Electrospun PHA/CNC/Cs | Congo red | 18.95 | 75.8 | [108] |
| EDTA-embedded nanocellulose | Methylene blue | n.a | 91.14 | [109] |
| Acid-Resistant Chitosan/CNF | Methylene blue | 14.71 | n.a | [110] |
| Nanocellulose/SiO2 | Tar | n.a | 92.23 | [111] |
| Total particulate matter | 90.25 | |||
| Nicotine | 95.02 | |||
| CO | 20.63 |
| Membrane Material | Degraded Compound | Photocatalytic Performance | Reference |
|---|---|---|---|
| Anatase TiO2/CNF | Methyl orange | 99.72% degradation within 30 min, no obvious activity loss after reused for five cycles | [114] |
| CeO2/TiO2-CNC | Rhodamine B Methyl orange Cr(VI) | Complete removal of MO and RhB, and reduction of Cr(VI) solution within 70, 50, and 60 min | [98] |
| ZnO/NC | Enrofloxacin | 97% degradation efficiency within 120 min | [115] |
| Ag3PO4/NC | Methyl orange | 90% degradation efficiency in DI and 70% in wastewater within 80 min | [112] |
| Fe-doped ZnO/NC | Methylene blue | 98.84% degradation efficiency within 90 min, 92% degradation efficiency after reused for 5 cycles | [116] |
| NC/γ–Fe2O3–ZrO2 | Congo red | Increase degradation efficiency from 80.0% to 98.5% in 30 min | [117] |
| TiO2/CNC | o-chloranil | ~90% degradation after 2 h | [118] |
| TiO2/CNC | Methyl orange | 100% degradation in less than 6 h | [119] |
| CNF//PEI/Ag | Methylene blue Congo red | Up to 98% degradation efficiency after 10 times reuse, high water flux (up to 5 × 104 L·m−2 h−1) | [120] |
| Type of Sensor | Material | Target Pollutant | Reference |
|---|---|---|---|
| Electrochemical sensor | d-penicillamine anchored nano-cellulose (DPA-NC) modified pencil graphite electrode | Copper ions | [124] |
| Colorimetric sensor | Aromatic imide functionalized nanocellulose and branched polyethyleneimine | Fluoride | [101] |
| Optical plasmonic chemosensor | Copper nanoparticles embedded with flexible nanocellulose | Cyanide | [126] |
| Biosensor | Cyanobacterial C-phycocyanin (CPC)/TOCNF | Copper ions | [127] |
| Optical sensor | In situ synthesized AgNPs embedded nanopaper | Chiral compounds | [128] |
| Optical sensor | Carbon quantum dots embedded nanopaper | Iodide | [129] |
| Electrochemical sensor | Rice-husk derived CNF and TOCNF/glycerol | Water soluble gases (ammonia, acetone, methane, hydrogen sulfide) | [125] |
| Fluorescent sensor | Carbon Dots-Rhodamine B (CDs-RhB) nanohybrid on nanopaper | Cadmium (Cd), lead (Pb), mercury (Hg), copper (Cu) and iron (Fe) ions | [130] |
| Optical sensor (SERS) | Gold nanorod/Silver nanocubes (AuNRs/AgNCs) embedded on bacterial nanocellulose network | 2,4,6-trinitrotoluene (TNT) | [131] |
| Chemiresistive sensor | Nanocellulose/graphene oxide membrane attached to SnO2 nanosheets (NSs) | Hydrogen gas | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. https://doi.org/10.3390/membranes12030287
Jaffar SS, Saallah S, Misson M, Siddiquee S, Roslan J, Saalah S, Lenggoro W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes. 2022; 12(3):287. https://doi.org/10.3390/membranes12030287
Chicago/Turabian StyleJaffar, Syafiqah Syazwani, Suryani Saallah, Mailin Misson, Shafiquzzaman Siddiquee, Jumardi Roslan, Sariah Saalah, and Wuled Lenggoro. 2022. "Recent Development and Environmental Applications of Nanocellulose-Based Membranes" Membranes 12, no. 3: 287. https://doi.org/10.3390/membranes12030287
APA StyleJaffar, S. S., Saallah, S., Misson, M., Siddiquee, S., Roslan, J., Saalah, S., & Lenggoro, W. (2022). Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes, 12(3), 287. https://doi.org/10.3390/membranes12030287






