Pt/WO3 Nanoparticle-Dispersed Polydimethylsiloxane Membranes for Transparent and Flexible Hydrogen Gas Leakage Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pt-Particle-Dispersed Tungsten Trioxide (Pt/WO3) Nanoparticles
2.3. Preparation of Pt/WO3 Nanoparticle-Dispersed Polydimethylsiloxane Membranes
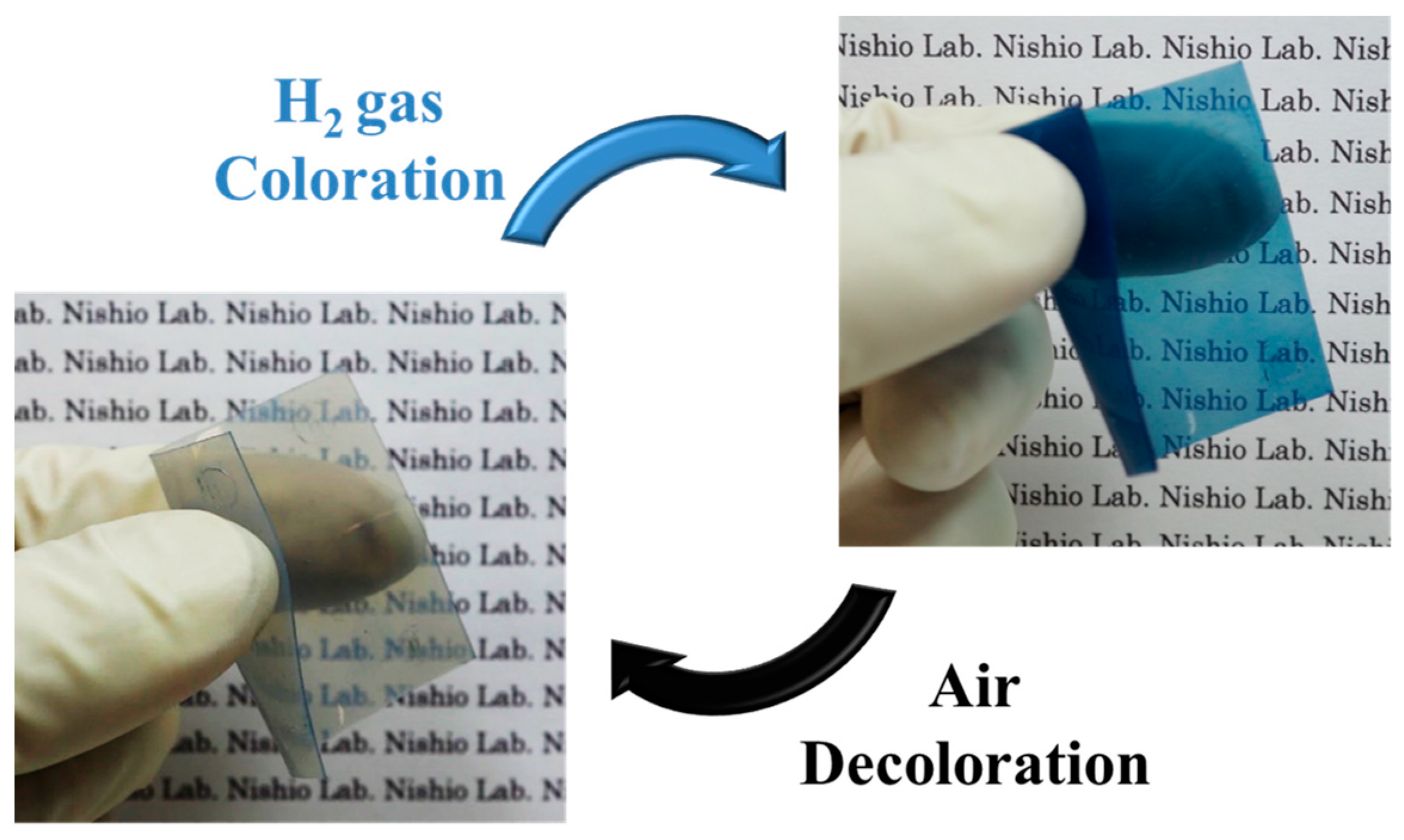
2.4. Evaluation of Hydrogen Gas Response of Pt/WO3 Composite Membranes
3. Results and Discussion
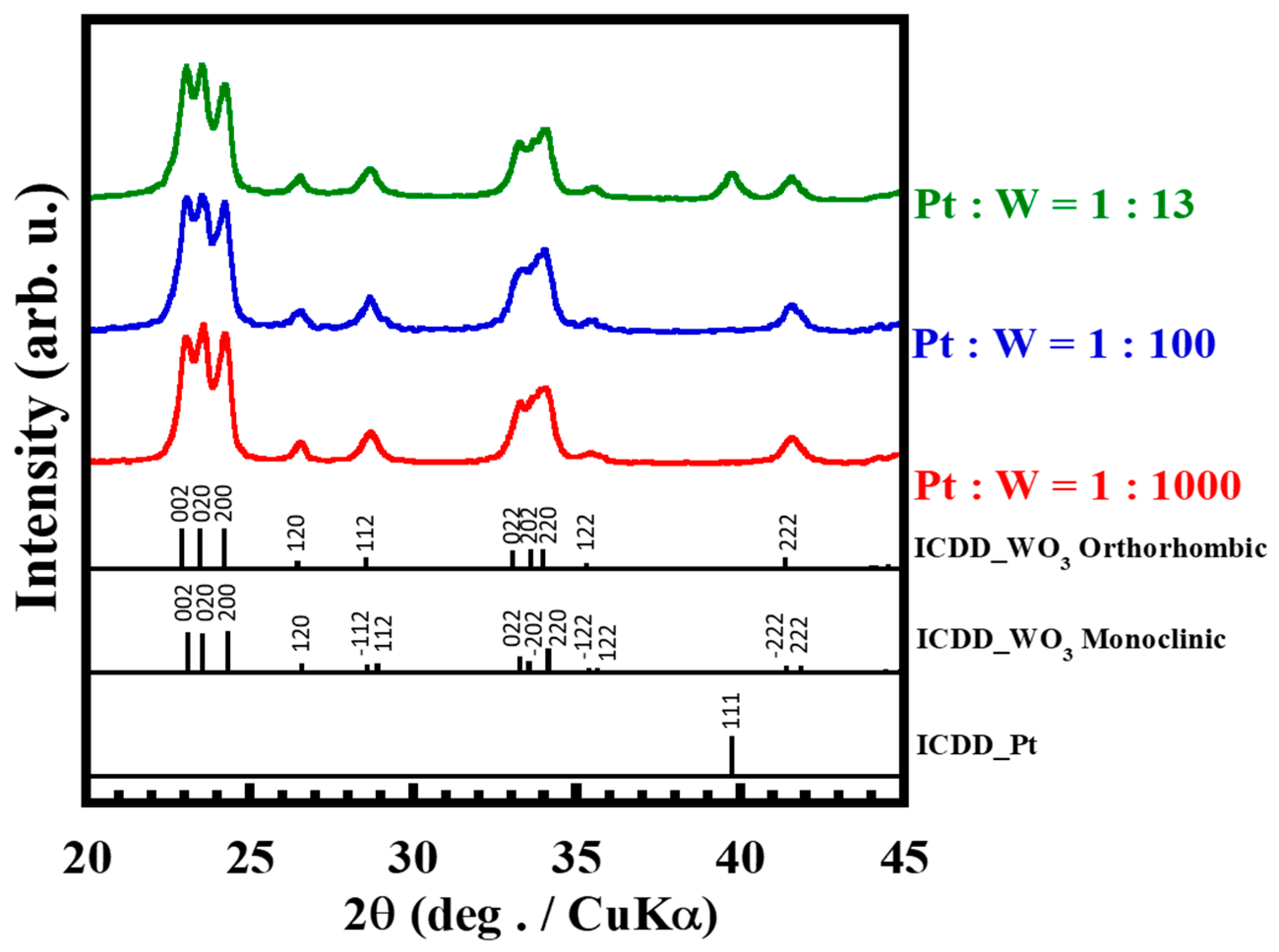
3.1. Effect of Compositional Ratio of Platinum and Tungsten Trioxide
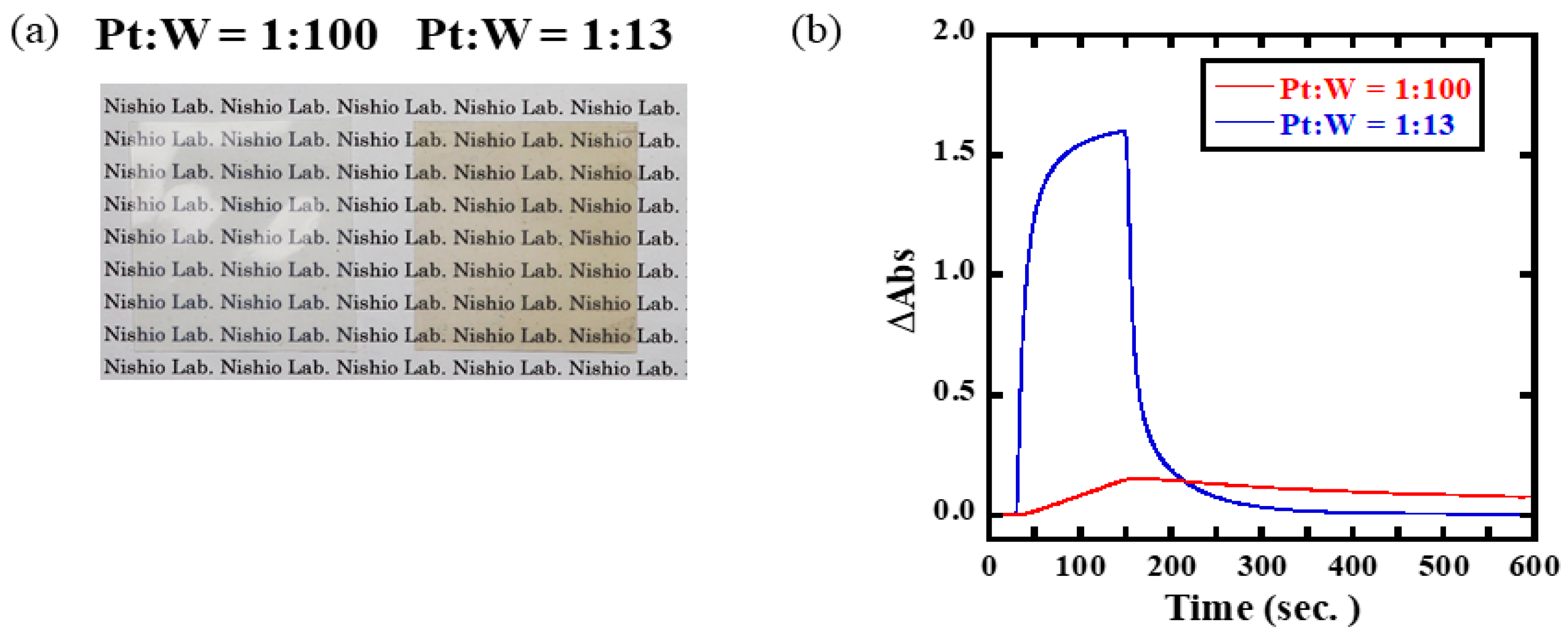
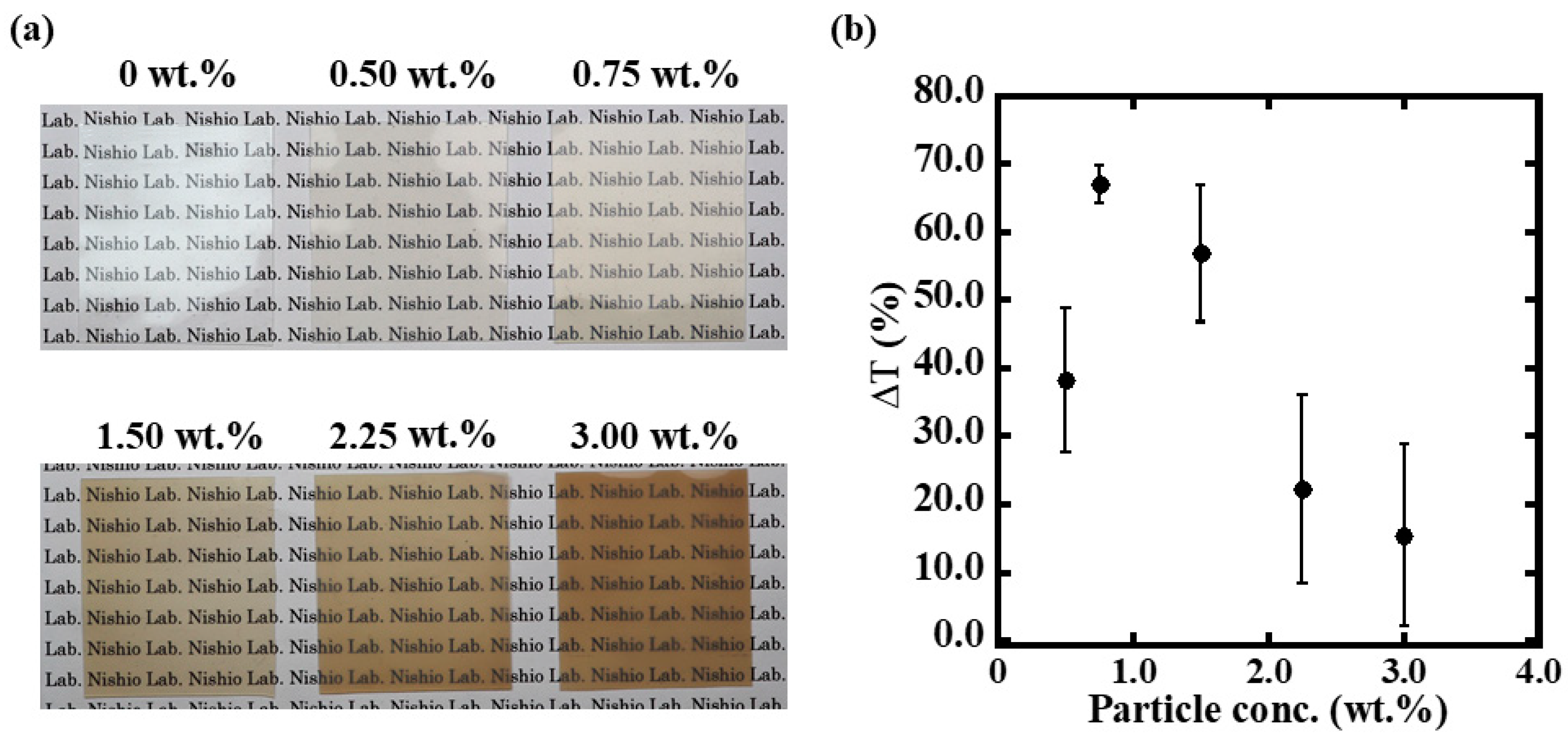
3.2. Optimization of Pt/WO3 Nanoparticle Content
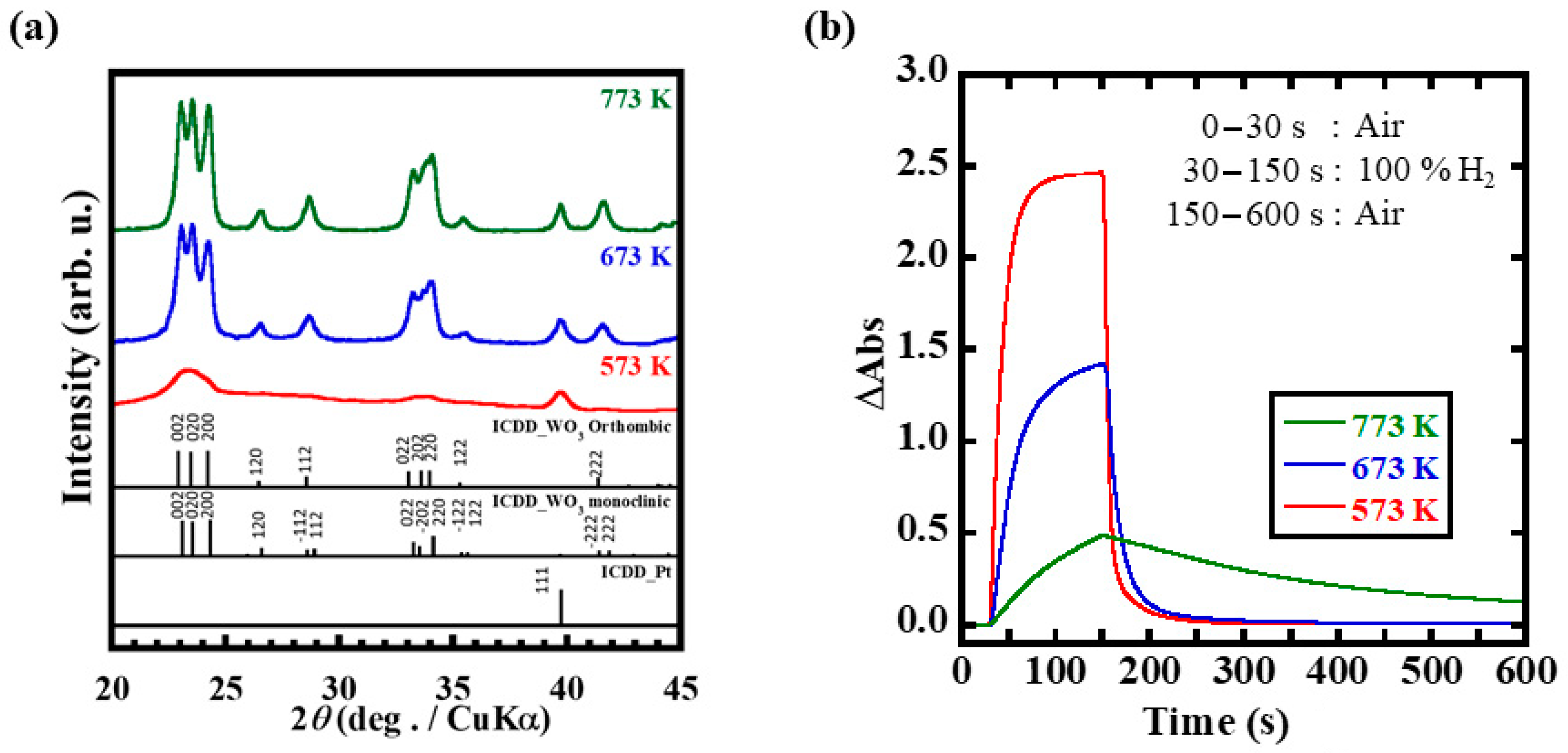
3.3. Optimization of Heat Treatment Temperature on Hydrogen Gas Response Time
3.4. Hydrogen Gas Detection Performance of Pt/WO3 Composite Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sinigaglia, T.; Lewiski, F.; Santos Martins, M.E.; Mairesse Siluk, J.C. Production, storage, fuel stations of hydrogen and its utilization in automotive applications-a review. Int. J. Hydrogen Energy 2017, 42, 24597–24611. [Google Scholar] [CrossRef]
- Kohn, H.W.; Boudart, M. Reaction of Hydrogen with Oxygen Adsorbed on a Platinum Catalyst. Science 1964, 145, 149–150. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, Y.; Liu, F.; Yang, M. Highly Sensitive and Rapid FBG Hydrogen Sensor Using Pt-WO3 with Different Morphologies. IEEE Sens. J. 2018, 18, 2652–2658. [Google Scholar] [CrossRef]
- Castillero, P.; Rico-Gavira, V.; López-Santos, C.; Barranco, A.; Pérez-Dieste, V.; Escudero, C.; Espinós, J.P.; González-Elipe, A.R. Formation of Subsurface W5+ Species in Gasochromic Pt/WO3 Thin Films Exposed to Hydrogen. J. Phys. Chem. C 2017, 121, 15719–15727. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Emoto, Y.; Kineri, T.; Fujimoto, M.; Mae, H.; Yasumori, A.; Nishio, K. Hydrogen gas-sensing properties of Pt/WO3 thin film in various measurement conditions. Ionics 2012, 18, 449–453. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chang, C.-C.; Tseng, C.-M.; Chan, C.-C.; Chao, W.-H.; Wu, Y.-R.; Wen, M.-H.; Hsieh, Y.-T.; Wang, Y.-C.; Chen, C.-L.; et al. An ultra-fast response gasochromic device for hydrogen gas detection. Sens. Actuators B Chem. 2013, 186, 193–198. [Google Scholar] [CrossRef]
- Ippolito, S.J.; Kandasamy, S.; Kalantar-zadeh, K.; Wlodarski, W. Hydrogen sensing characteristics of WO3 thin film conductometric sensors activated by Pt and Au catalysts. Sens. Actuators B Chem. 2005, 108, 154–158. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Imamura, S.; Ito, S.; Nishio, K.; Fujimoto, K. Influence of oxygen gas concentration on hydrogen sensing of Pt/WO3 thin film prepared by sol–gel process. Sens. Actuators B Chem. 2015, 216, 394–401. [Google Scholar] [CrossRef]
- Okazaki, S.; Johjima, S. Temperature dependence and degradation of gasochromic response behavior in hydrogen sensing with Pt/WO3 thin film. Thin Solid Film. 2014, 558, 411–415. [Google Scholar] [CrossRef]
- Deptula, A.; Olczak, W.L.T.; Sartowska, B.; Giorgi, L.; Moreno, A.; di Bartolomeo, A. Preparation of Pt/WO3 Powders and Thin Films on Porous Carbon Black and Metal Supports by the Complex Sol-Gel Process. J. New Mater. Electrochem. Syst. 2003, 6, 71–74. [Google Scholar]
- Ishihara, R.; Yamaguchi, Y.; Tanabe, K.; Makino, Y.; Nishio, K. Preparation of Pt/WO3-coated polydimethylsiloxane membrane for transparent/flexible hydrogen gas sensors. Mater. Chem. Phys. 2019, 226, 226–229. [Google Scholar] [CrossRef]
- Nishizawa, K.; Yamada, Y.; Yoshimura, K. Low-temperature chemical fabrication of Pt-WO3 gasochromic switchable films using UV irradiation. Sol. Energy Mater. Sol. Cells 2017, 170, 21–26. [Google Scholar] [CrossRef]
- Takács, M.; Zámbó, D.; Deák, A.; Pap, A.E.; Dücső, C. WO3 nano-rods sensitized with noble metal nano-particles for H2S sensing in the ppb range. Mater. Res. Bull. 2016, 84, 480–485. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.; Lee, J.S.; Yoon, S.; Lee, J.-H. Biphenyl-based covalent triazine framework-incorporated polydimethylsiloxane membranes with high pervaporation performance for n-butanol recovery. J. Membr. Sci. 2020, 598, 117654. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Fouda, A.E.; Matsuura, T. A study of pervaporation of aqueous benzyl alcohol solution by polydimethylsiloxane membrane. J. Membr. Sci. 1992, 70, 249–255. [Google Scholar] [CrossRef]
- Ishihara, R.; Tanabe, K.; Inomata, S.; Matsui, R.; Kitane, R.; Hosokawa, K.; Maeda, M.; Kikuchi, A. Fabrication of Storable Surface-Functionalized Power-Free Microfluidic Chip for Sensitive MicroRNA Detection Utilizing Ultraviolet Grafting. Ind. Eng. Chem. Res. 2020, 59, 10464–10468. [Google Scholar] [CrossRef]
- Ishihara, R.; Katagiri, A.; Nakajima, T.; Matsui, R.; Komatsu, S.; Hosokawa, K.; Maeda, M.; Tomooka, Y.; Kikuchi, A. Design of a surface-functionalized power-free microchip for extracellular vesicle detection utilizing UV grafting. React. Funct. Polym. 2019, 142, 183–188. [Google Scholar] [CrossRef]
- Evenou, F.; Fujii, T.; Sakai, Y. Spontaneous Formation of Highly Functional Three-Dimensional Multilayer from Human Hepatoma Hep G2 Cells Cultured on an Oxygen-Permeable Polydimethylsiloxane Membrane. Tissue Eng. Part C Methods 2009, 16, 311–318. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Nomani, M.W.; Wen, X.; Hsia, T.-Y.; Koley, G. A highly sensitive pressure sensor using a Au-patterned polydimethylsiloxane membrane for biosensing applications. J. Micromech. Microeng. 2013, 23, 025022. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nemoto, C.; Ito, S.; Nishio, K.; Fujimoto, K. Improvement of hydrogen gas sensing property of the sol–gel derived Pt/WO3 thin film by Ti-doping. J. Ceram. Soc. Jpn. 2015, 123, 1102–1105. [Google Scholar] [CrossRef] [Green Version]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Imamura, S.; Nishio, K.; Fujimoto, K. Influence of temperature and humidity on the electrical sensing of Pt/WO3 thin film hydrogen gas sensor. J. Ceram. Soc. Jpn. 2016, 124, 629–633. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, R.; Makino, Y.; Yamaguchi, Y.; Fujimoto, K.; Nishio, K. Pt/WO3 Nanoparticle-Dispersed Polydimethylsiloxane Membranes for Transparent and Flexible Hydrogen Gas Leakage Sensors. Membranes 2022, 12, 291. https://doi.org/10.3390/membranes12030291
Ishihara R, Makino Y, Yamaguchi Y, Fujimoto K, Nishio K. Pt/WO3 Nanoparticle-Dispersed Polydimethylsiloxane Membranes for Transparent and Flexible Hydrogen Gas Leakage Sensors. Membranes. 2022; 12(3):291. https://doi.org/10.3390/membranes12030291
Chicago/Turabian StyleIshihara, Ryo, Yoshihiro Makino, Yuki Yamaguchi, Kenjiro Fujimoto, and Keishi Nishio. 2022. "Pt/WO3 Nanoparticle-Dispersed Polydimethylsiloxane Membranes for Transparent and Flexible Hydrogen Gas Leakage Sensors" Membranes 12, no. 3: 291. https://doi.org/10.3390/membranes12030291





