Silicon Nitride-Based Micro-Apertures Coated with Parylene for the Investigation of Pore Proteins Fused in Free-Standing Lipid Bilayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
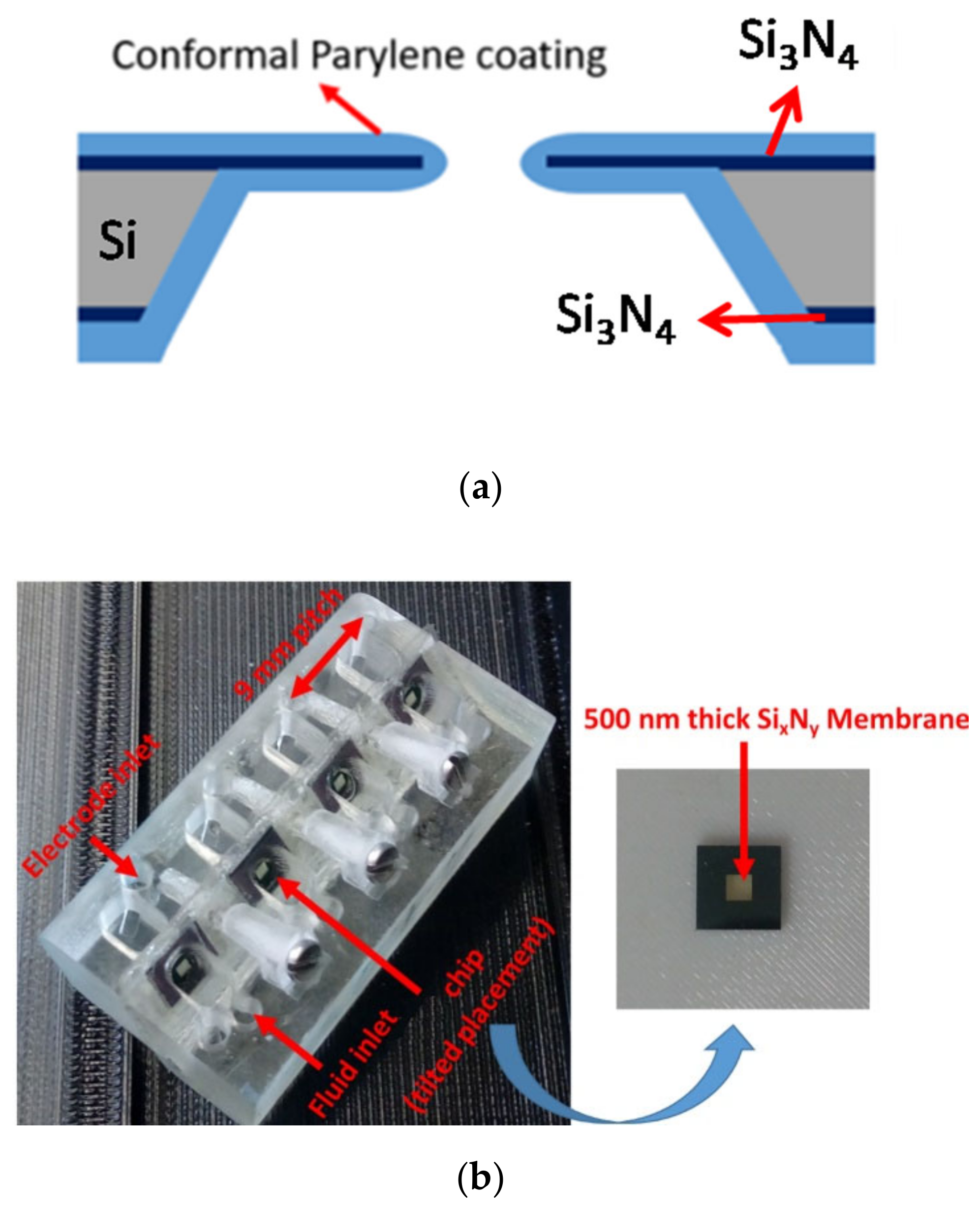
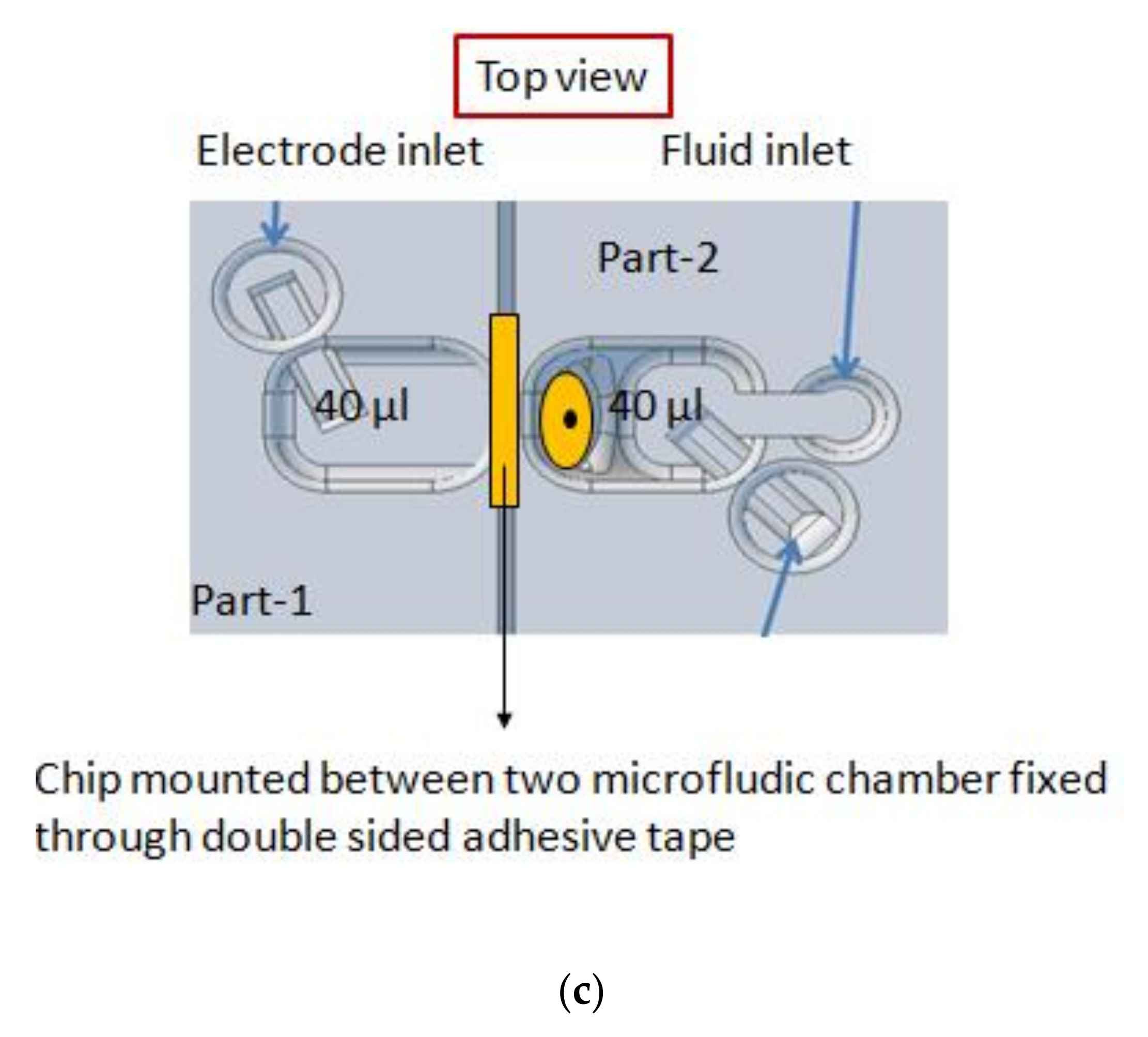
2.2. Micro-Aperture Fabrication and 3d-Printed Platform
2.3. Formation of Free-Standing Bilayers
2.4. Electrophysiological Measurement Method
3. Results and Discussion
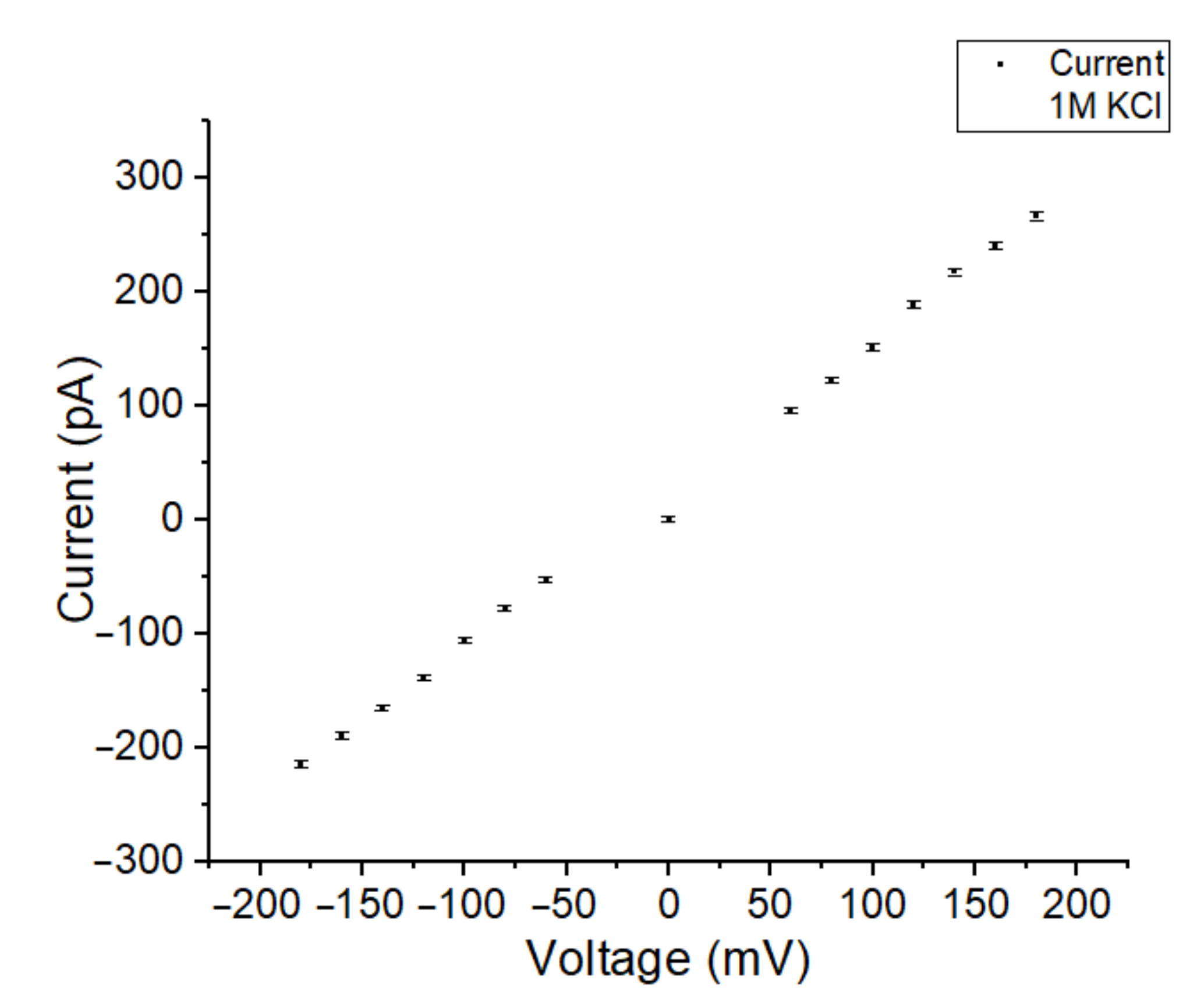
3.1. Parylene versus Teflon
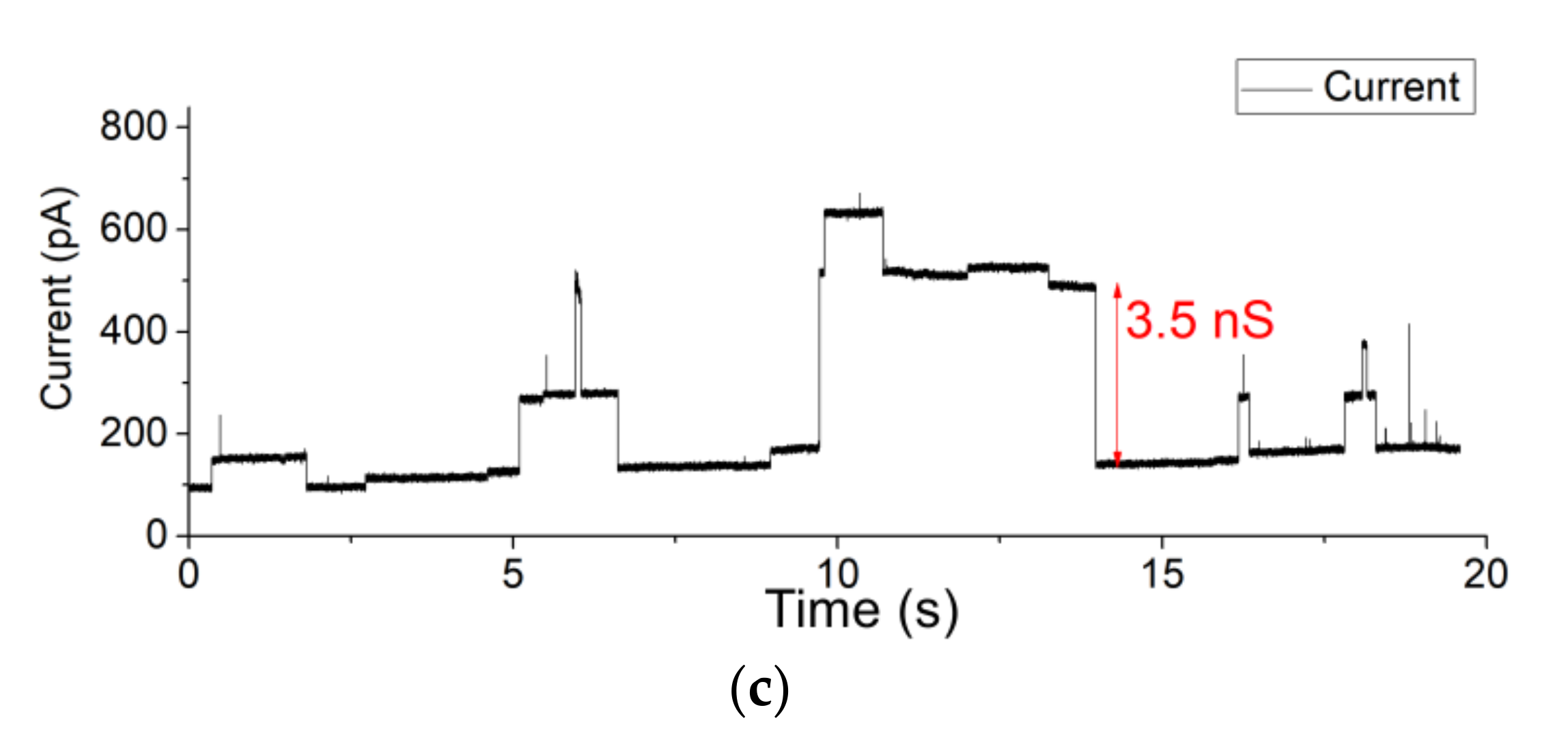
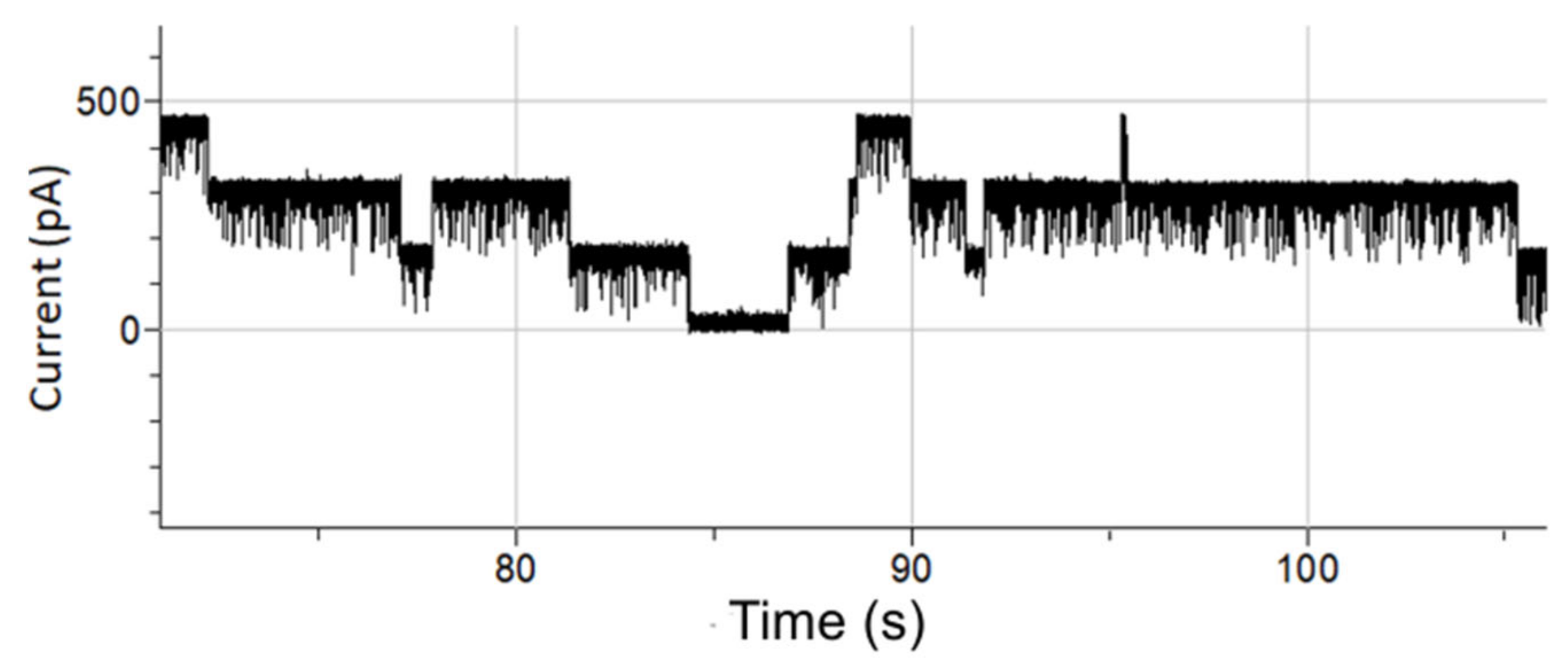
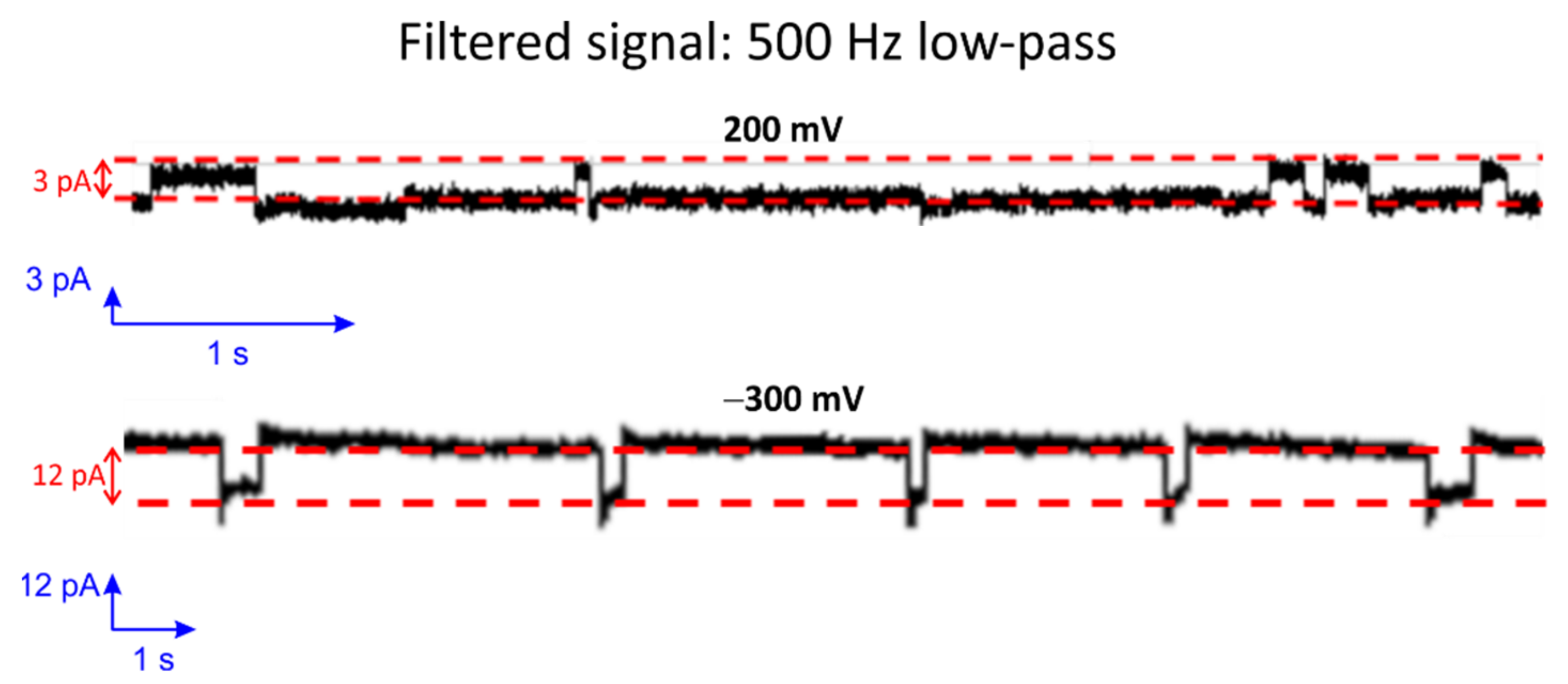
3.2. Fast Gating Measurements of OmpF
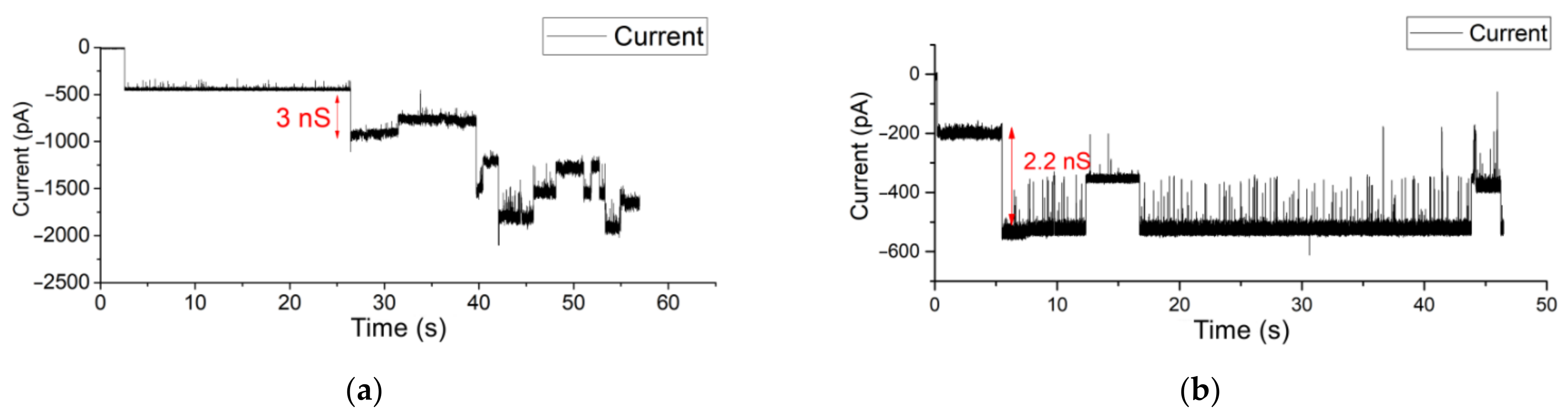
3.3. OMV Fusion in Free-Standing Lipid Bilayer Membranes
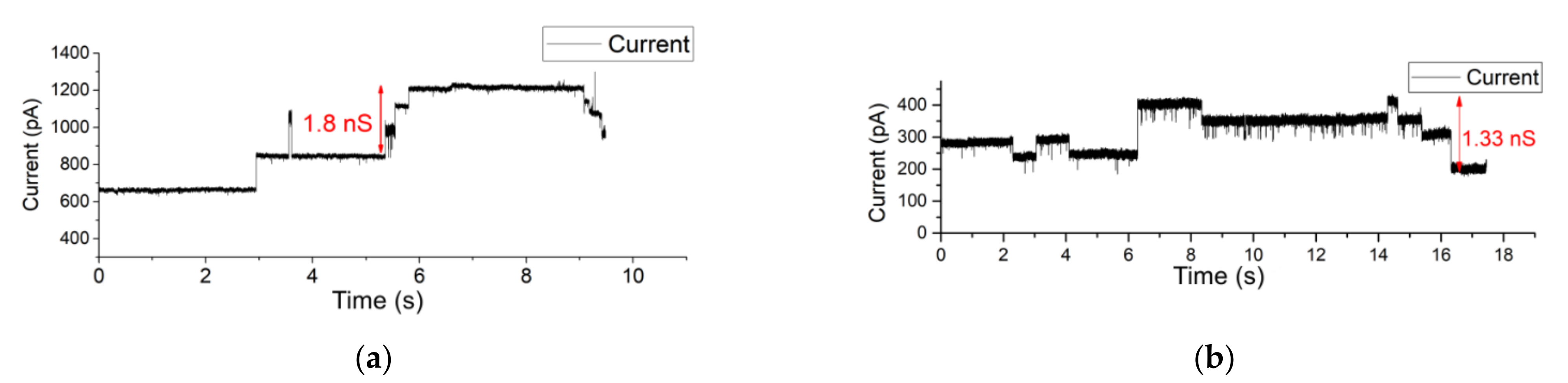
3.4. Single-Channel Detection of Gramicidin-D Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagès, J.-M.; James, C.E.; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008, 6, 893–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forst, S.; Delgado, J.; Ramakrishnan, G.; Inouye, M. Regulation of ompC and ompF expression in Escherichia coli in the absence of envZ. J. Bacteriol. 1988, 170, 5080–5085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestorovich, E.M.; Danelon, C.; Winterhalter, M.; Bezrukov, S.M. Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl. Acad. Sci. USA 2002, 99, 9789–9794. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, K.R.; Chimerel, C.; Mach, T.; Winterhalter, M. Antibiotic translocation through membrane channels: Temperature-dependent ion current fluctuation for catching the fast events. Eur. Biophys. J. 2009, 38, 1141–1145. [Google Scholar] [CrossRef]
- Wang, J.; Fertig, N.; Ying, Y.-L. Real-time monitoring β-lactam/β-lactamase inhibitor (BL/BLI) mixture towards the bacteria porin pathway at single molecule level. Anal. Bioanal. Chem. 2019, 411, 4831–4837. [Google Scholar] [CrossRef]
- Wang, J.; Bafna, J.A.; Bhamidimarri, S.P.; Winterhalter, M. Small-Molecule Permeation across Membrane Channels: Chemical Modification to Quantify Transport across OmpF. Angew. Chem. Int. Ed. 2019, 58, 4737–4741. [Google Scholar] [CrossRef]
- Wang, J.; Terrasse, R.; Bafna, J.A.; Benier, L.; Winterhalter, M. Electrophysiological Characterization of Transport Across Outer-Membrane Channels from Gram-Negative Bacteria in Presence of Lipopolysaccharides. Angew. Chem. Int. Ed. 2020, 59, 8517–8521. [Google Scholar] [CrossRef] [Green Version]
- Clifton, L.A.; Skoda, M.W.A.; Daulton, E.L.; Hughes, A.V.; Le Brun, A.P.; Lakey, J.H.; Holt, S.A. Asymmetric phospholipid: Lipopolysaccharide bilayers; a Gram-negative bacterial outer membrane mimic. J. R. Soc. Interface 2013, 10, 20130810. [Google Scholar] [CrossRef]
- Brauser, A.; Schroeder, I.; Gutsmann, T.; Cosentino, C.; Moroni, A.; Hansen, U.-P.; Winterhalter, M. Modulation of enrofloxacin binding in OmpF by Mg2+ as revealed by the analysis of fast flickering single-porin current. J. Gen. Physiol. 2012, 140, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Sousa, C.F.; Gameiro, P. Fluoroquinolone Metalloantibiotics to Bypass Antimicrobial Resistance Mechanisms: Decreased Permeation through Porins. Membranes 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Kongsuphol, P.; Fang, K.B.; Ding, Z. Lipid bilayer technologies in ion channel recordings and their potential in drug screening assay. Sens. Actuators B Chem. 2013, 185, 530–542. [Google Scholar] [CrossRef]
- Zagnoni, M. Miniaturised technologies for the development of artificial lipid bilayer systems. Lab Chip 2012, 12, 1026. [Google Scholar] [CrossRef]
- Saha, S.C.; Thei, F.; de Planque, M.R.R.; Morgan, H. Scalable micro-cavity bilayer lipid membrane arrays for parallel ion channel recording. Sens. Actuators B Chem. 2014, 199, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.R.; Clark, R.B.; Banderali, U.; Giles, W.R. Measurement of the membrane potential in small cells using patch clamp methods. Channels 2011, 5, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [Green Version]
- Ellis, T.N.; Kuehn, M.J. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-S.; Gedi, V.; Kim, J.-K.; Lee, D. Detection of Gram-negative bacterial outer membrane vesicles using DNA aptamers. Sci. Rep. 2019, 9, 13167. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Daniel, O.; Nyč, O.; Mellmann, A. In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure. Membranes 2021, 11, 806. [Google Scholar] [CrossRef]
- El-Beyrouthy, J.; Freeman, E. Characterizing the Structure and Interactions of Model Lipid Membranes Using Electrophysiology. Membranes 2021, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; van den Driesche, S.; Bafna, J.A.; Oellers, M.; Hemmler, R.; Gall, K.; Wagner, R.; Winterhalter, M.; Vellekoop, M.J. Rapid lipid bilayer membrane formation on Parylene coated apertures to perform ion channel analyses. Biomed. Microdevices 2020, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Krishnarjuna, B.; Ravula, T.; Ramamoorthy, A. Detergent-free extraction, reconstitution and characterization of membrane-anchored cytochrome-b5 in native lipids. Chem. Commun. 2020, 56, 6511–6514. [Google Scholar] [CrossRef]
- Karanth, S.; Meesaragandla, B.; Delcea, M. Changing surface properties of artificial lipid membranes at the interface with biopolymer coated gold nanoparticles under normal and redox conditions. Biophys. Chem. 2020, 267, 106465. [Google Scholar] [CrossRef]
- Goto, M.; Kazama, A.; Fukuhara, K.; Sato, H.; Tamai, N.; Ito, H.-O.; Matsuki, H. Membrane fusion of phospholipid bilayers under high pressure: Spherical and irreversible growth of giant vesicles. Biophys. Chem. 2021, 277, 106639. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, P.; Harsman, A.; Wagner, R. Single Channel Analysis of Membrane Proteins in Artificial Bilayer Membranes; Humana Press: Totowa, NJ, USA, 2013; pp. 345–361. [Google Scholar]
- Gennis, R.B. Biomembranes; Springer Advanced Texts in Chemistry; Springer: New York, NY, USA, 1989; ISBN 978-1-4757-2067-9. [Google Scholar]
- Iglič, A. (Ed.) Advances in Planar Lipid Bilayers and Liposomes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 9780123810137. [Google Scholar]
- Fujiwara, H.; Fujihara, M.; Ishiwata, T. Dynamics of the spontaneous formation of a planar phospholipid bilayer: A new approach by simultaneous electrical and optical measurements. J. Chem. Phys. 2003, 119, 6768–6775. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanke, W.; Schlue, W.-R. Planar Lipid Bilayers; Elsevier: Amsterdam, The Netherlands, 1993; ISBN 9780123229953. [Google Scholar]
- Braun, C.J.; Baer, T.; Moroni, A.; Thiel, G. Pseudo painting/air bubble technique for planar lipid bilayers. J. Neurosci. Methods 2014, 233, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Bafna, J.A.; van den Driesche, S.; Oellers, M.; Hemmler, R.; Gall, K.; Wagner, R.; Winterhalter, M.; Vellekoop, M.J. Rapid formation of lipid bilayer membranes in Parylene-C coated chips by pseudo-painting of an air bubble for the fusion and detection of outer membrane vesicles (OMVs). In Proceedings of the 23th International Conference on Miniaturized Systems for Chemistry and Life Science, µTAS, Basel, Switzerland, 27–31 October 2019; pp. 360–361. [Google Scholar]
- Maglia, G.; Heron, A.J.; Stoddart, D.; Japrung, D.; Bayley, H. Analysis of Single Nucleic Acid Molecules with Protein Nanopores; Academic Press: Cambridge, MA, USA, 2010; pp. 591–623. [Google Scholar]
- Takei, T.; Yaguchi, T.; Fujii, T.; Nomoto, T.; Toyota, T.; Fujinami, M. Measurement of membrane tension of free standing lipid bilayers via laser-induced surface deformation spectroscopy. Soft Matter 2015, 11, 8641–8647. [Google Scholar] [CrossRef]
- Tadaki, D.; Yamaura, D.; Araki, S.; Yoshida, M.; Arata, K.; Ohori, T.; Ishibashi, K.; Kato, M.; Ma, T.; Miyata, R.; et al. Mechanically stable solvent-free lipid bilayers in nano- and micro-tapered apertures for reconstitution of cell-free synthesized hERG channels. Sci. Rep. 2017, 7, 17736. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dosoky, N.S.; Mustafa, G.; Patel, D.; Berdiev, B.; Williams, J.D. Electrophysiology of Epithelial Sodium Channel (ENaC) Embedded in Supported Lipid Bilayer Using a Single Nanopore Chip. Langmuir 2017, 33, 13680–13688. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.; Tsounidi, D.; Petrou, P.S.; Beltsios, K.G.; Kakabakos, S.E. Functionalization of silicon dioxide and silicon nitride surfaces with aminosilanes for optical biosensing applications. Med. Devices Sens. 2020, 3, e10072. [Google Scholar] [CrossRef]
- Tan, S.; Wang, L.; Yu, J.; Hou, C.; Jiang, R.; Li, Y.; Liu, Q. DNA-functionalized silicon nitride nanopores for sequence-specific recognition of DNA biosensor. Nanoscale Res. Lett. 2015, 10, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mescola, A.; Canale, C.; Prato, M.; Diaspro, A.; Berdondini, L.; Maccione, A.; Dante, S. Specific Neuron Placement on Gold and Silicon Nitride-Patterned Substrates through a Two-Step Functionalization Method. Langmuir 2016, 32, 6319–6327. [Google Scholar] [CrossRef] [PubMed]
- Oshima, A.; Hirano-Iwata, A.; Nasu, T.; Kimura, Y.; Niwano, M. Mechanically Stable Lipid Bilayers in Teflon-Coated Silicon Chips for Single-Channel Recordings. Micro Nanosyst. 2012, 4, 2–7. [Google Scholar] [CrossRef]
- Suzuki, H.; Tabata, K.; Kato-Yamada, Y.; Noji, H.; Takeuchi, S. Planar lipid bilayer reconstitution with a micro-fluidic system. Lab Chip 2004, 4, 502. [Google Scholar] [CrossRef]
- Gardiner, J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem. 2015, 68, 13. [Google Scholar] [CrossRef]
- Baker, C.A.; Bright, L.K.; Aspinwall, C.A. Photolithographic Fabrication of Microapertures with Well-Defined, Three-Dimensional Geometries for Suspended Lipid Membrane Studies. Anal. Chem. 2013, 85, 9078–9086. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Pioufle, B.L.; Takeuhci, S. Ninety-six-well planar lipid bilayer chip for ion channel recording Fabricated by hybrid stereolithography. Biomed. Microdevices 2009, 11, 17–22. [Google Scholar] [CrossRef]
- Ahmed, T.; van den Driesche, S.; Arun Bafna, J.; Oellers, M.; Hemmler, R.; Gall, K.; Wagner, R.; Winterhalter, M.; Vellekoop, M.J. Parylene-C coated micro-apertures with painted synthetic lipid bilayer membranes for the investigation of outer-membrane-vesicle fusion. In 2019 IEEE SENSORS; IEEE: Piscataway, NJ, USA, 2019; pp. 1–4. [Google Scholar]
- Bafna, J.A.; Sans-Serramitjana, E.; Acosta-Gutiérrez, S.; Bodrenko, I.V.; Hörömpöli, D.; Berscheid, A.; Brötz-Oesterhelt, H.; Winterhalter, M.; Ceccarelli, M. Kanamycin Uptake into Escherichia coli Is Facilitated by OmpF and OmpC Porin Channels Located in the Outer Membrane. ACS Infect. Dis. 2020, 6, 1855–1865. [Google Scholar] [CrossRef]
- Bello, J.; Kim, Y.-R.; Kim, S.M.; Jeon, T.-J.; Shim, J. Lipid bilayer membrane technologies: A review on single-molecule studies of DNA sequencing by using membrane nanopores. Microchim. Acta 2017, 184, 1883–1897. [Google Scholar] [CrossRef]
- Thoma, J.; Manioglu, S.; Kalbermatter, D.; Bosshart, P.D.; Fotiadis, D.; Müller, D.J. Protein-enriched outer membrane vesicles as a native platform for outer membrane protein studies. Commun. Biol. 2018, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Prilipov, A.; Phale, P.S.; Gelder, P.; Rosenbusch, J.P.; Koebnik, R. Coupling site-directed mutagenesis with high-level expression: Large scale production of mutant porins from E. coli. FEMS Microbiol. Lett. 1998, 163, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; van den Driesche, S.; Oellers, M.; Hemmler, R.; Gall, K.; Bhamidimarri, S.P.; Winterhalter, M.; Wagner, R.; Vellekoop, M.J. Fast Formation of Lipid Bilayer Membranes for Simultaneous Analysis of Molecular Transport Using Parylene Coated Chips. Proceedings 2018, 2, 920. [Google Scholar] [CrossRef] [Green Version]
- Van den Driesche, S.; Lucklum, F.; Bunge, F.; Vellekoop, M.J. 3D printing solutions for microfluidic chip-to-world connections. Micromachines 2018, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Kara, S.; Afonin, S.; Babii, O.; Tkachenko, A.N.; Komarov, I.V.; Ulrich, A.S. Diphytanoyl lipids as model systems for studying membrane-active peptides. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1828–1837. [Google Scholar] [CrossRef]
- Dondapati, S.K.; Wüstenhagen, D.A.; Kubick, S. Functional Analysis of Membrane Proteins Produced by Cell-Free Translation; Humana Press: Totowa, NJ, USA, 2018; pp. 171–186. [Google Scholar]
- Noise in Electronics Engineering. Available online: https://www.allaboutcircuits.com/technical-articles/noise-in-electronics-engineering-distribution-noise-rms-peak-to-peak-value-PSD (accessed on 12 January 2021).
- Plasma-Parylene Systems GmbH. Available online: http://www.plasmaparylene.de/ (accessed on 29 May 2020).
- Specialty Coating Systems. Available online: http://scscoatings.com/ (accessed on 29 May 2020).
- Schroeder, I. How to resolve microsecond current fluctuations in single ion channels: The power of beta distributions. Channels 2015, 9, 262–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, M.; Rautenbach, M.; Vosloo, J.A.; Siersma, T.; Aisenbrey, C.H.M.; Zaitseva, E.; Laubscher, W.E.; van Rensburg, W.; Behrends, J.C.; Bechinger, B.; et al. The Multifaceted Antibacterial Mechanisms of the Pioneering Peptide Antibiotics Tyrocidine and Gramicidin S. MBio 2018, 9, e00802-18. [Google Scholar] [CrossRef] [Green Version]
- Basu, I.; Chattopadhyay, A.; Mukhopadhyay, C. Ion channel stability of Gramicidin A in lipid bilayers: Effect of hydrophobic mismatch. Biochim. Biophys. Acta Biomembr. 2014, 1838, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, J.A.; Levitt, D.G. Water transport and ion-water interaction in the gramicidin channel. Biophys. J. 1981, 35, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Urry, D.W.; Long, M.M.; Jacobs, M.; Harris, R.D. Conformation and molecular mechanisms of carriers and channels fn1. Ann. N. Y. Acad. Sci. 1975, 264, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Koeppe, R.E.; Berg, J.M.; Hodgson, K.O.; Stryer, L. Gramicidin A crystals contain two cation binding sites per channel. Nature 1979, 279, 723–725. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, H.; Iwata, S.; Choi, S.; Ki Kim, M.; Kim, Y.-R.; Maruta, S.; Min Kim, S.; Jeon, T.-J. Investigation of Ion Channel Activities of Gramicidin A in the Presence of Ionic Liquids Using Model Cell Membranes. Sci. Rep. 2015, 5, 11935. [Google Scholar] [CrossRef] [Green Version]
- Kleinzeller, A.; Fambrough, D. (Eds.) Chloride Channels; Academic Press: Cambridge, MA, USA, 1994; ISBN 9780080585130. [Google Scholar]
- Schulze Greiving, V.C.; de Boer, H.L.; Bomer, J.G.; van den Berg, A.; Le Gac, S. Integrated microfluidic biosensing platform for simultaneous confocal microscopy and electrophysiological measurements on bilayer lipid membranes and ion channels. Electrophoresis 2018, 39, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Kondrashov, O.V.; Galimzyanov, T.R.; Molotkovsky, R.J.; Batishchev, O.V.; Akimov, S.A. Membrane-Mediated Lateral Interactions Regulate the Lifetime of Gramicidin Channels. Membranes 2020, 10, 368. [Google Scholar] [CrossRef]
- Setiadi, J.; Kuyucak, S. Computational Investigation of the Effect of Lipid Membranes on Ion Permeation in Gramicidin A. Membranes 2016, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Ionera. Available online: https://www.ionera.de/applications.htm (accessed on 12 January 2021).
- Hille, B. Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys. J. 1978, 22, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Ridi, A.; Scalas, E.; Robello, M.; Gliozzi, A. Linear response of a fluctuating lipid bilayer. Thin Solid Films 1998, 327–329, 796–799. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.; Bafna, J.A.; Hemmler, R.; Gall, K.; Wagner, R.; Winterhalter, M.; Vellekoop, M.J.; van den Driesche, S. Silicon Nitride-Based Micro-Apertures Coated with Parylene for the Investigation of Pore Proteins Fused in Free-Standing Lipid Bilayers. Membranes 2022, 12, 309. https://doi.org/10.3390/membranes12030309
Ahmed T, Bafna JA, Hemmler R, Gall K, Wagner R, Winterhalter M, Vellekoop MJ, van den Driesche S. Silicon Nitride-Based Micro-Apertures Coated with Parylene for the Investigation of Pore Proteins Fused in Free-Standing Lipid Bilayers. Membranes. 2022; 12(3):309. https://doi.org/10.3390/membranes12030309
Chicago/Turabian StyleAhmed, Tanzir, Jayesh Arun Bafna, Roland Hemmler, Karsten Gall, Richard Wagner, Mathias Winterhalter, Michael J. Vellekoop, and Sander van den Driesche. 2022. "Silicon Nitride-Based Micro-Apertures Coated with Parylene for the Investigation of Pore Proteins Fused in Free-Standing Lipid Bilayers" Membranes 12, no. 3: 309. https://doi.org/10.3390/membranes12030309
APA StyleAhmed, T., Bafna, J. A., Hemmler, R., Gall, K., Wagner, R., Winterhalter, M., Vellekoop, M. J., & van den Driesche, S. (2022). Silicon Nitride-Based Micro-Apertures Coated with Parylene for the Investigation of Pore Proteins Fused in Free-Standing Lipid Bilayers. Membranes, 12(3), 309. https://doi.org/10.3390/membranes12030309





