Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication
Abstract
:1. Introduction
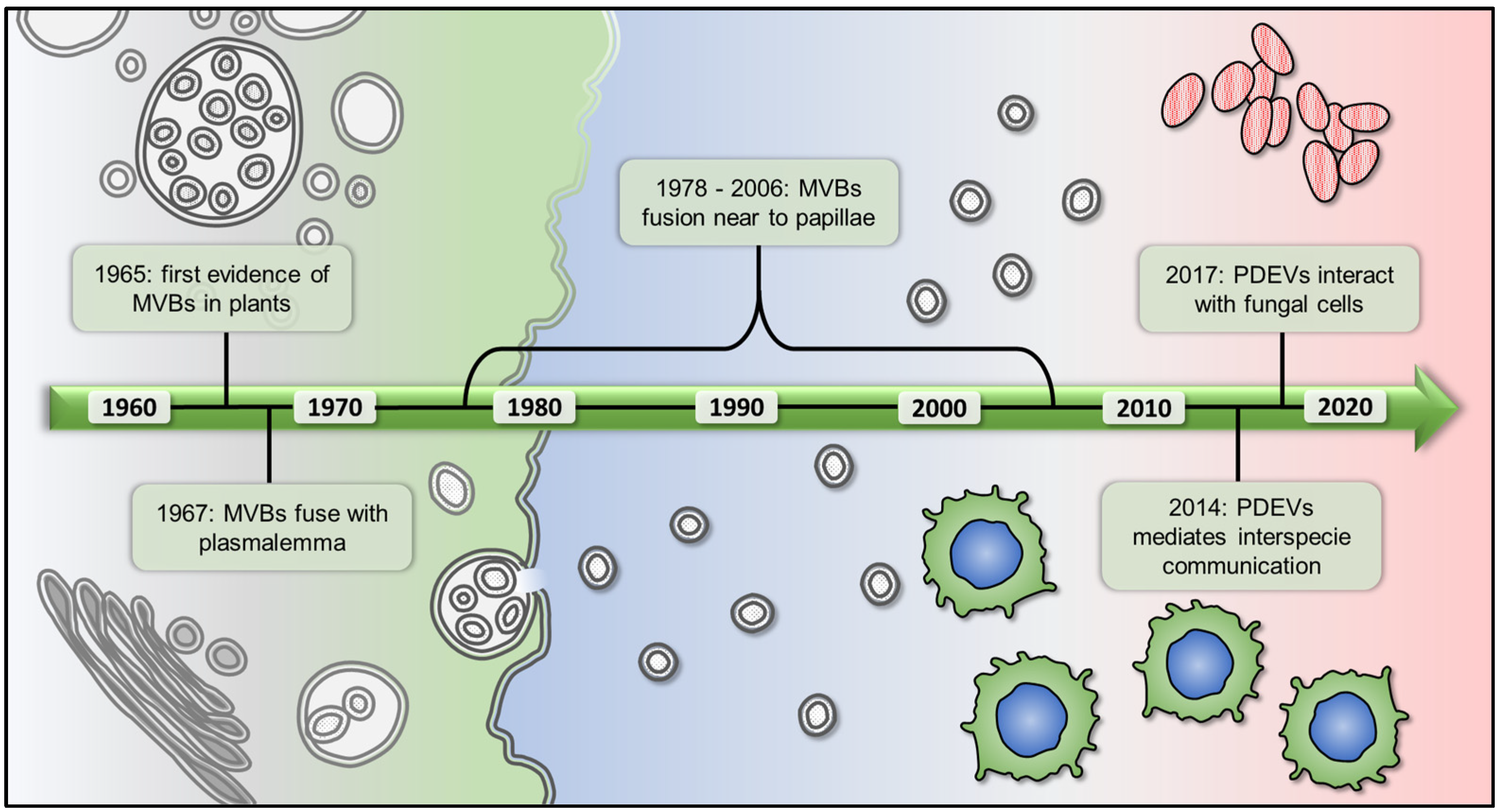
2. The Story of Plant EVs, from Their Discovery to the Purification from Different Matrices
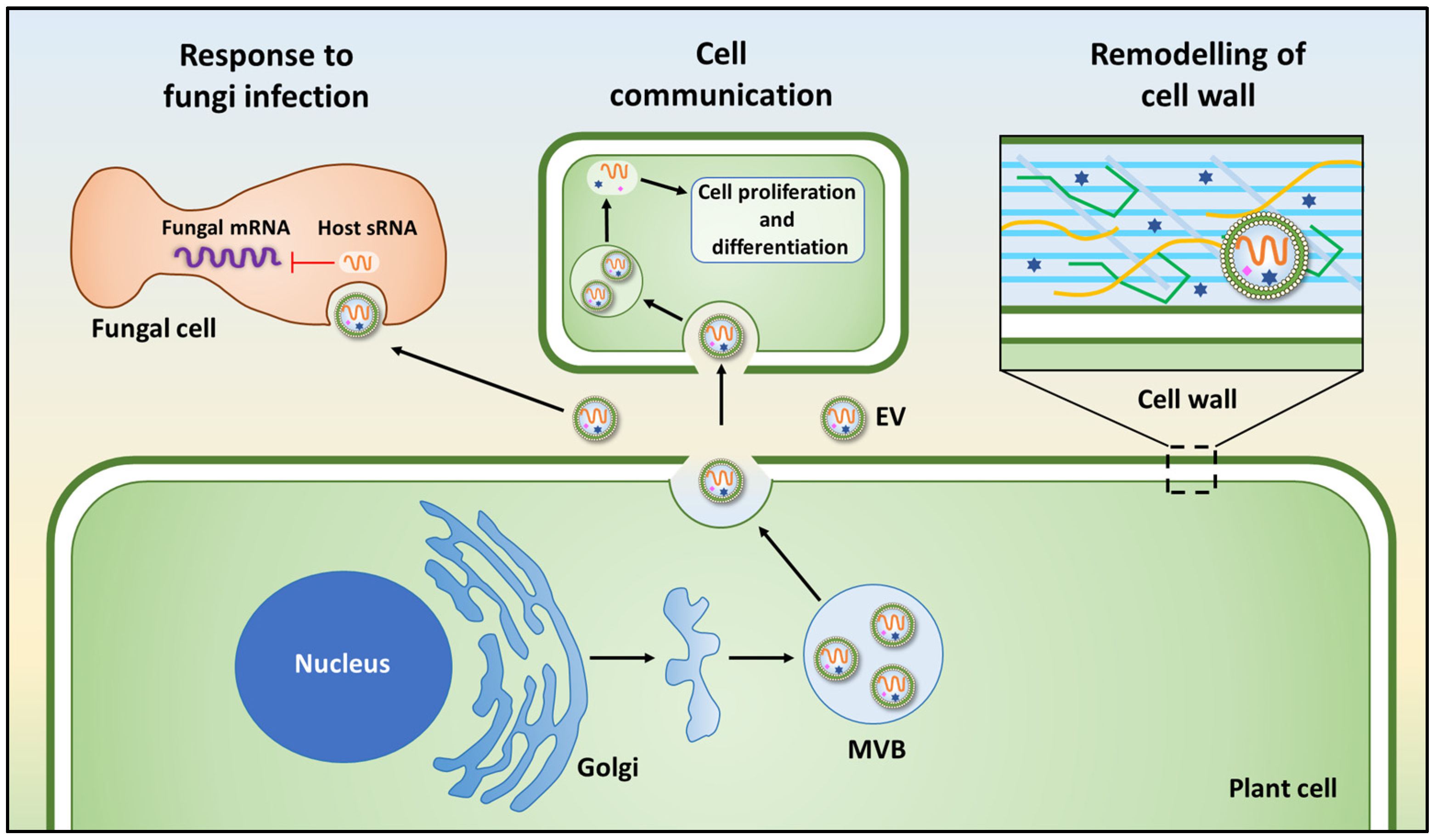
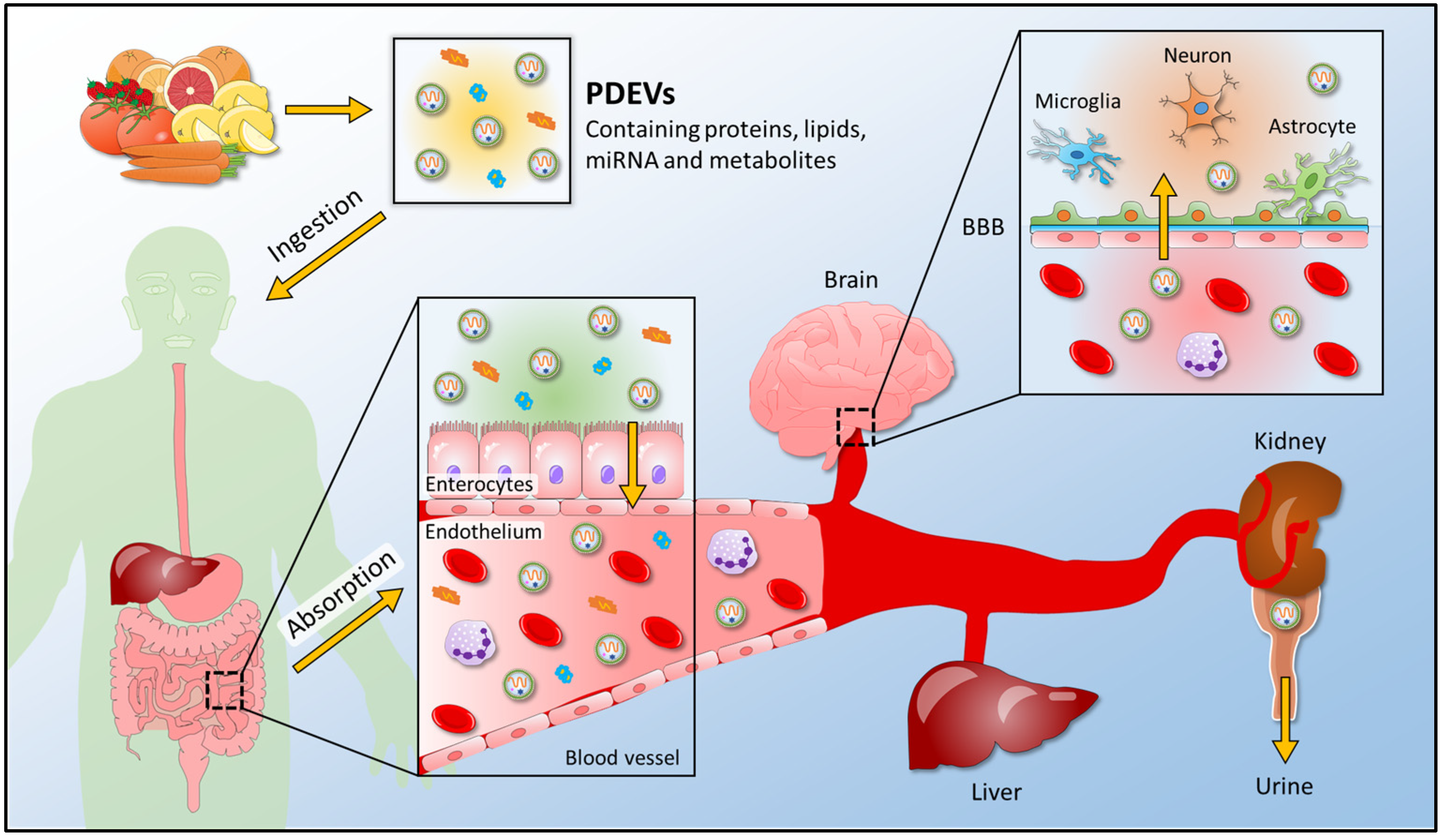
3. Functional Role of PDEV-RNAs in Cross-Kingdom Interactions
3.1. Functional Roles of Plant Extracellular Vesicles in Plant-Mammalian Communication
3.2. Plant Extracellular Vesicles in Plant-Microbe Interaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondo, S.; Urzi, O.; Conigliaro, A.; Bosco, G.L.; Parisi, S.; Carlisi, M.; Siragusa, S.; Raimondi, L.; Luca, A.; Giavaresi, G.; et al. Extracellular vesicle micrornas contribute to the osteogenic inhibition of mesenchymal stem cells in multiple myeloma. Cancers 2020, 12, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Ikeda, G.; Tada, Y.; von Bornstadt, D.; Santoso, M.R.; Wahlquist, C.; Rhee, S.; Jeon, Y.J.; Yu, A.C.; O’Brien, C.G.; et al. Mir-106a-363 cluster in extracellular vesicles promotes endogenous myocardial repair via notch3 pathway in ischemic heart injury. Basic Res. Cardiol. 2021, 116, 19. [Google Scholar] [CrossRef]
- Byun, J.S.; Lee, H.Y.; Tian, J.; Moon, J.S.; Choi, J.; Lee, S.H.; Kim, Y.G.; Yi, H.S. Effect of salivary exosomal mir-25-3p on periodontitis with insulin resistance. Front. Immunol. 2021, 12, 775046. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, A.; Costa, V.; Lo Dico, A.; Saieva, L.; Buccheri, S.; Dieli, F.; Manno, M.; Raccosta, S.; Mancone, C.; Tripodi, M.; et al. Cd90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing h19 lncrna. Mol. Cancer 2015, 14, 155. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F.; et al. M2 microglial small extracellular vesicles reduce glial scar formation via the mir-124/stat3 pathway after ischemic stroke in mice. Theranostics 2021, 11, 1232–1248. [Google Scholar] [CrossRef]
- Pinedo, M.; de la Canal, L.; de Marcos Lousa, C. A call for rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress cml xenograft growth by inducing trail-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.; Leone, A.; Ambrosone, A. Plant-derived nano and microvesicles for human health and therapeutic potential in nanomedicine. Pharmaceutics 2021, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Yuan, T.J.; Dad, H.A.; Shi, M.Y.; Huang, Y.Y.; Jiang, Z.H.; Peng, L.H. Plant exosomes as novel nanoplatforms for microrna transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021, 21, 8151–8159. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal micrornas inhibit lung inflammation induced by exosomes SARS-CoV-2 nsp12. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Jensen, W.A. The composition and ultrastructure of the nucellus in cotton. J. Ultrastruct. Res. 1965, 13, 112–128. [Google Scholar] [CrossRef]
- Halperin, W.; Jensen, W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967, 18, 428–443. [Google Scholar] [CrossRef]
- Politis, D.J.G.; Goodman, R.N. Localized cell wall appositions: Incompatibility response of tobacco leaf cells to pseudomonas pisi. Phytopathology 1978, 68, 309–316. [Google Scholar] [CrossRef]
- An, Q.; Ehlers, K.; Kogel, K.H.; van Bel, A.J.; Huckelhoven, R. Multivesicular compartments proliferate in susceptible and resistant mla12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 2006, 172, 563–576. [Google Scholar] [CrossRef] [Green Version]
- Zeyen, R.J.; Bushnel, W.R. Papilla response of barley epidermal cells caused by erysiphe graminis: Rate and method of deposition determined by microcinematography and transmission electron microscopy. Can. J. Bot. 1979, 57, 898–913. [Google Scholar] [CrossRef]
- An, Q.; Huckelhoven, R.; Kogel, K.H.; van Bel, A.J. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006, 8, 1009–1019. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzon, G.; Maldonado, A.M.; Pinedo, M.; Jorrin, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-derived exosomal micrornas shape the gut microbiota. Cell Host. Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef] [Green Version]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant. Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived micrornas from 11 edible fruits and vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. Rna-binding proteins contribute to small rna loading in plant extracellular vesicles. Nat. Plants 2021, 7, 342–352. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiak, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vekey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant. Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiak, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vekey, K. Physiochemical and protein datasets related to citrus juice sac cells-derived nanovesicles and microvesicles. Data Brief 2019, 22, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Nikolic, D.; Conigliaro, A.; Giavaresi, G.; Lo Sasso, B.; Giglio, R.V.; Chianetta, R.; Manno, M.; Raccosta, S.; Corleone, V.; et al. Preliminary results of citraves effects on low density lipoprotein cholesterol and waist circumference in healthy subjects after 12 weeks: A pilot open-label study. Metabolites 2021, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Saieva, L.; Cristaldi, M.; Monteleone, F.; Fontana, S.; Alessandro, R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-coa carboxylase alpha as antitumor target of citrus limon-derived nanovesicles. J. Proteom. 2018, 173, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef] [PubMed]
- Kalarikkal, S.P.; Sundaram, G.M. Edible plant-derived exosomal micrornas: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021, 414, 115425. [Google Scholar] [CrossRef]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the a375 human melanoma cell line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef]
- Savci, Y.; Kirbas, O.K.; Bozkurt, B.T.; Abdik, E.A.; Tasli, P.N.; Sahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiak, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant roots release small extracellular vesicles with antifungal activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Mammadova, R.; Fiume, I.; Bokka, R.; Kralj-Iglic, V.; Bozic, D.; Kisovec, M.; Podobnik, M.; Zavec, A.B.; Hocevar, M.; Gellen, G.; et al. Identification of tomato infecting viruses that co-isolate with nanovesicles using a combined proteomics and electron-microscopic approach. Nanomaterials 2021, 11, 1922. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Corrigendum: Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2016, 7, 11347. [Google Scholar] [CrossRef] [PubMed]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiak, L.; Sugar, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of tomato-derived nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small rnas in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.J.; Bao, J.J.; Wang, L.J.; Chen, X.Y. Arabidopsis leaf extracellular vesicles in wound-induced jasmonate accumulation. Plant. Signal. Behav. 2020, 15, 1833142. [Google Scholar] [CrossRef]
- Liu, N.J.; Wang, N.; Bao, J.J.; Zhu, H.X.; Wang, L.J.; Chen, X.Y. Lipidomic analysis reveals the importance of gipcs in arabidopsis leaf extracellular vesicles. Mol. Plant 2020, 13, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.R.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102271. [Google Scholar] [CrossRef] [PubMed]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant extracellular vesicles contain diverse small rna species and are enriched in 10- to 17-nucleotide “tiny” RNAs. Plant. Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Rutter, B.D.; Rutter, K.L.; Innes, R.W. Isolation and quantification of plant extracellular vesicles. Bio Protoc. 2017, 7, e2533. [Google Scholar] [CrossRef]
- Kirbas, O.K.; Bozkurt, B.T.; Asutay, A.B.; Mat, B.; Ozdemir, B.; Ozturkoglu, D.; Olmez, H.; Islek, Z.; Sahin, F.; Tasli, P.N. Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci. Rep. 2019, 9, 19159. [Google Scholar] [CrossRef]
- Stanly, C.; Kim, H.; Antonucci, G.; Fiume, I.; Guescini, M.; Kim, K.P.; Ciardiello, M.A.; Giangrieco, I.; Mari, A.; Pocsfalvi, G. Crosstalk between the immune system and plant-derived nanovesicles: A study of allergen transporting. Front. Bioeng. Biotechnol. 2021, 9, 760730. [Google Scholar] [CrossRef]
- Stanly, C.; Fiume, I.; Capasso, G.; Pocsfalvi, G. Isolation of exosome-like vesicles from plants by ultracentrifugation on sucrose/deuterium oxide (d2o) density cushions. Methods Mol. Biol. 2016, 1459, 259–269. [Google Scholar] [PubMed]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiak, L.; Pocsfalvi, G. Membrane transporters in citrus clementina fruit juice-derived nanovesicles. Int. J. Mol. Sci. 2019, 2, 6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timo, S.; Lisa, W.; Patrick, B.; Timo, W.B.; Christian, P.e.; Martin, H.; Anna, M.; Dagmar, B.; Martina, C.; Lukas, J.; et al. Host-induced gene silencing involves arabidopsis escrt-iii pathway for the transfer of dsrna-derived sirna. bioRxiv 2021. [Google Scholar] [CrossRef] [Green Version]
- Movahed, N.; Cabanillas, D.G.; Wan, J.; Vali, H.; Laliberte, J.F.; Zheng, H. Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant. Physiol. 2019, 180, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, N.; Alche Jde, D.; Casado-Vela, J.; Mas, S.; Villalba, M.; Rodriguez, R.; Batanero, E. Nanovesicles are secreted during pollen germination and pollen tube growth: A possible role in fertilization. Mol. Plant. 2014, 7, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from dss-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Woith, E.; Guerriero, G.; Hausman, J.F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant extracellular vesicles and nanovesicles: Focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell amp-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Ye, J.; Cai, X.; Hu, C.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Deng, Z.B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like nanoparticles from mulberry bark prevent dss-induced colitis via the ahr/cops8 pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S.; et al. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics 2022, 12, 1220–1246. [Google Scholar] [CrossRef]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2020, 9, 1703480. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Yoo, H.J.; Jung, J.H.; Lee, R.; Hyun, J.K.; Park, J.H.; Na, D.; Yeon, J.H. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J. Funct. Biomater. 2020, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Trentini, M.; Zanotti, F.; Tiengo, E.; Camponogara, F.; Degasperi, M.; Licastro, D.; Lovatti, L.; Zavan, B. An apple a day keeps the doctor away: Potential role of mirna 146 on macrophages treated with exosomes derived from apples. Biomedicines 2022, 10, 415. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant mir168a specifically targets mammalian ldlrap1: Evidence of cross-kingdom regulation by microrna. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Aquilano, K.; Ceci, V.; Gismondi, A.; De Stefano, S.; Iacovelli, F.; Faraonio, R.; Di Marco, G.; Poerio, N.; Minutolo, A.; Minopoli, G.; et al. Adipocyte metabolism is improved by tnf receptor-targeting small rnas identified from dried nuts. Commun. Biol. 2019, 2, 317. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; Zhang, L.; et al. Honeysuckle-encoded atypical microrna2911 directly targets influenza a viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sang, X.; Hong, Z. Beyond nutrients: Food-derived micrornas provide cross-kingdom regulation. Bioessays 2012, 34, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, A.R.; Fong, M.Y.; Somlo, G.; Wu, J.; Swiderski, P.; Wu, X.; Wang, S.E. Cross-kingdom inhibition of breast cancer growth by plant mir159. Cell Res. 2016, 26, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Zheng, B.; Yang, G.S.; Yang, H.J.; Zhou, J.; Yang, Z.; Zhang, X.H.; Zhao, H.Y.; Shi, J.H.; Wen, J.K. Salvia miltiorrhiza-derived sal-mir-58 induces autophagy and attenuates inflammation in vascular smooth muscle cells. Mol. Ther. Nucleic Acids 2020, 21, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Marzano, F.; Caratozzolo, M.F.; Consiglio, A.; Licciulli, F.; Liuni, S.; Sbisa, E.; D’Elia, D.; Tullo, A.; Catalano, D. Plant mirnas reduce cancer cell proliferation by targeting malat1 and neat1: A beneficial cross-kingdom interaction. Front. Genet. 2020, 11, 552490. [Google Scholar] [CrossRef]
- Snow, J.W.; Hale, A.E.; Isaacs, S.K.; Baggish, A.L.; Chan, S.Y. Ineffective delivery of diet-derived micrornas to recipient animal organisms. RNA Biol. 2013, 10, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; McAlexander, M.A.; Queen, S.E.; Adams, R.J. Real-time quantitative pcr and droplet digital pcr for plant mirnas in mammalian blood provide little evidence for general uptake of dietary mirnas: Limited evidence for general uptake of dietary plant xenomirs. RNA Biol. 2013, 10, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.; Zhang, Y.; Petrick, J.S.; Heck, G.; Ivashuta, S.; Marshall, W.S. Lack of detectable oral bioavailability of plant micrornas after feeding in mice. Nat. Biotechnol. 2013, 31, 965–967. [Google Scholar] [CrossRef]
- Yang, J.; Farmer, L.M.; Agyekum, A.A.; Hirschi, K.D. Detection of dietary plant-based small rnas in animals. Cell Res. 2015, 25, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Farmer, L.M.; Agyekum, A.A.; Elbaz-Younes, I.; Hirschi, K.D. Detection of an abundant plant-based small rna in healthy consumers. PLoS ONE 2015, 10, e0137516. [Google Scholar] [CrossRef] [Green Version]
- Hirschi, K.D.; Pruss, G.J.; Vance, V. Dietary delivery: A new avenue for microrna therapeutics? Trends Biotechnol. 2015, 33, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Potesta, M.; Roglia, V.; Fanelli, M.; Pietrobono, E.; Gismondi, A.; Vumbaca, S.; Nguedia Tsangueu, R.G.; Canini, A.; Colizzi, V.; Grelli, S.; et al. Effect of microvesicles from moringa oleifera containing mirna on proliferation and apoptosis in tumor cell lines. Cell Death Discov. 2020, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, Q.; Feng, X.; Zhang, R.; Li, J.; Chen, F. Nlrp3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer 2018, 18, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Luo, Q.; Wang, H.; Zhang, H.; Chen, F. Microrna-22 suppresses cell proliferation, migration and invasion in oral squamous cell carcinoma by targeting nlrp3. J. Cell Physiol. 2018, 233, 6705–6713. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, Q.; Chen, X.; Chen, F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J. Nanobiotechnol. 2021, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.P.; Paolini, A.; D’Oria, V.; Sarra, A.; Sennato, S.; Bordi, F.; Masotti, A. Extracellular vesicles derived from citrus sinensis modulate inflammatory genes and tight junctions in a human model of intestinal epithelium. Front. Nutr. 2021, 8, 778998. [Google Scholar] [CrossRef] [PubMed]
- Kameli, N.; Dragojlovic-Kerkache, A.; Savelkoul, P.; Stassen, F.R. Plant-derived extracellular vesicles: Current findings, challenges, and future applications. Membranes 2021, 11, 411. [Google Scholar] [CrossRef]
- Cavalieri, D.; Rizzetto, L.; Tocci, N.; Rivero, D.; Asquini, E.; Si-Ammour, A.; Bonechi, E.; Ballerini, C.; Viola, R. Plant micrornas as novel immunomodulatory agents. Sci. Rep. 2016, 6, 25761. [Google Scholar] [CrossRef] [Green Version]
- Link, J.; Thon, C.; Schanze, D.; Steponaitiene, R.; Kupcinskas, J.; Zenker, M.; Canbay, A.; Malfertheiner, P.; Link, A. Food-derived xeno-micrornas: Influence of diet and detectability in gastrointestinal tract-proof-of-principle study. Mol. Nutr. Food Res. 2019, 63, e1800076. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef]
- Alshehri, B. Plant-derived xenomirs and cancer: Cross-kingdom gene regulation. Saudi J. Biol. Sci. 2021, 28, 2408–2422. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small rnas—Big players in plant-microbe interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Zhai, Y.; Feng, L.; Karimi, H.Z.; Rutter, B.D.; Zeng, L.; Choi, D.S.; Zhang, B.; Gu, W.; Chen, X.; et al. A phytophthora effector suppresses trans-kingdom rnai to promote disease susceptibility. Cell Host Microbe 2019, 25, 153–165.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P.R.J.; Jin, H. Message in a bubble: Shuttling small rnas and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. Plant. Biol. 2021, 72, 497–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular vesicles: Emerging players in plant defense against pathogens. Front. Plant. Sci. 2021, 12, 757925. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Pei, R.; Mao, B.; Zhao, Z.; Li, H.; Lin, Y.; Lu, K. The SARS-CoV-2 protein orf3a inhibits fusion of autophagosomes with lysosomes. Cell Discov. 2021, 7, 31. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, Y.; Huang, F.; Luo, B.; Yuan, Y.; Xia, B.; Ma, X.; Yang, T.; Yu, F.; et al. The orf8 protein of sars-cov-2 mediates immune evasion through down-regulating mhc-iota. Proc. Natl. Acad. Sci. USA 2021, 118, e2024202118. [Google Scholar] [CrossRef]

| PDEV Origin | microRNA | Functional Role | References |
|---|---|---|---|
| Bitter melon | miR-156 d, miR-162, miR-166 5p, miR-167, miR-172,miR-390, miR-394, miR-396 3p, miR-399, miR-529, miR-2111 5p | Potential role in the regulation of NLRP3 mRNA | [84] |
| Soybean | miR-5781, miR-4996, miR-5671a | Regulatation of interleukin 17A, interleukin 10, interleukin 33 | [27] |
| gma-miR-6300 | Targeting gene ORF3a of SARS-CoV-2 | [35] | |
| mtr-miR-156a | Targeting gene ORF1ab of SARS-CoV-2 | ||
| Hami melon | miR-164a | Regulatation of interleukin 16 | [27] |
| ath-miR-164b-5p, zma-miR-398b-5p, cme-miR-530b, cme-miR-399d | Targeting gene ORF1ab of SARS-CoV-2 | [35] | |
| Orange | miR-398b | Regulatation of interleukin 1, alpha | [27] |
| Ginger | miR-1078 | Regulatation of interleukin 6 | |
| miR-7267-3p | Suppression of Lactobacillus rhamnosus monooxygenase ycnE mRNA, in the gut microbiome | [23] | |
| aly-miR396a-5p | Inhibition of the expression of inflammatory cytokines induced by Nsp12 of SARS-CoV-2; suppression of the SARS-CoV-2 cytopathic effect by inhibiting the expression of the viral S and Nsp12 | [14] | |
| rlcv-miR-rL1-28-3p | Suppression of the SARS-CoV-2 cytopathic effect by inhibiting the expression of the viral S and Nsp12 | ||
| gma-miR-6300 | Targeting gene ORF3a of SARS-CoV-2 | [35] | |
| aqc-miR-159 | Targeting gene M of SARS-CoV-2 | ||
| Tomato | miR-4995 | Regulatation of interleukin 5 | [27] |
| gma-miR-6300 | Targeting gene ORF3a of SARS-CoV-2 | [35] | |
| gma-miR-4375, zma-miR-398b-5p, bdi-miR-5059, osa-miR-5077 | Targeting gene ORF1ab of SARS-CoV-2 | ||
| sly-miR-1919a | Targeting gene ORF10 of SARS-CoV-2 | ||
| Fragaria | miR-166g | Disruption of the morphogenesis of leaves | [89] |
| Moringa oleifera | mol-miR160h, mol-mir482b, mol-mir166, mol-mir 159c, mol-mir2118a, mol-mir167f-3p, mol-mir156e, mol-mir395d, mol-mir393a, mol-mir397a, mol-mir858b, mol-mir396a | Potential regulation of proapoptotic and antiapoptotic targets | [81] |
| Walnuts | miR-156c, miR-159a | Regulation of mammalian TNF-α signaling pathway in adipocytes and regulate inflammation | [69] |
| Coconut | gma-miR-4995 | Targeting gene SPIKE of SARS-CoV-2 | [35] |
| mtr-miR-156a | Targeting gene ORF1ab of SARS-CoV-2 | ||
| Pear | mdm-miR-1511 | Targeting gene SPIKE of SARS-CoV-2 | |
| zma-miR-164b-3p | Targeting gene N of SARS-CoV-2 | ||
| Pea | pvu-miR-482-5p | Targeting gene ORF8 of SARS-CoV-2 | |
| gma-miR-156f | Targeting gene ORF1ab of SARS-CoV-2 | ||
| Blueberry | zma-miR-398b-5p | ||
| Grapefruit | bdi-miR-5059, osa-miR-5077 | ||
| Kiwifruit | osa-miR-530-5p | ||
| Grapes | vvi-miR-3630, vvi-miR-156a/n, vvi-miR-169r/u |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urzì, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R.; Raimondo, S. Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication. Membranes 2022, 12, 352. https://doi.org/10.3390/membranes12040352
Urzì O, Gasparro R, Ganji NR, Alessandro R, Raimondo S. Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication. Membranes. 2022; 12(4):352. https://doi.org/10.3390/membranes12040352
Chicago/Turabian StyleUrzì, Ornella, Roberta Gasparro, Nima Rabienezhad Ganji, Riccardo Alessandro, and Stefania Raimondo. 2022. "Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication" Membranes 12, no. 4: 352. https://doi.org/10.3390/membranes12040352
APA StyleUrzì, O., Gasparro, R., Ganji, N. R., Alessandro, R., & Raimondo, S. (2022). Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication. Membranes, 12(4), 352. https://doi.org/10.3390/membranes12040352








