Wearable Crop Sensor Based on Nano-Graphene Oxide for Noninvasive Real-Time Monitoring of Plant Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO
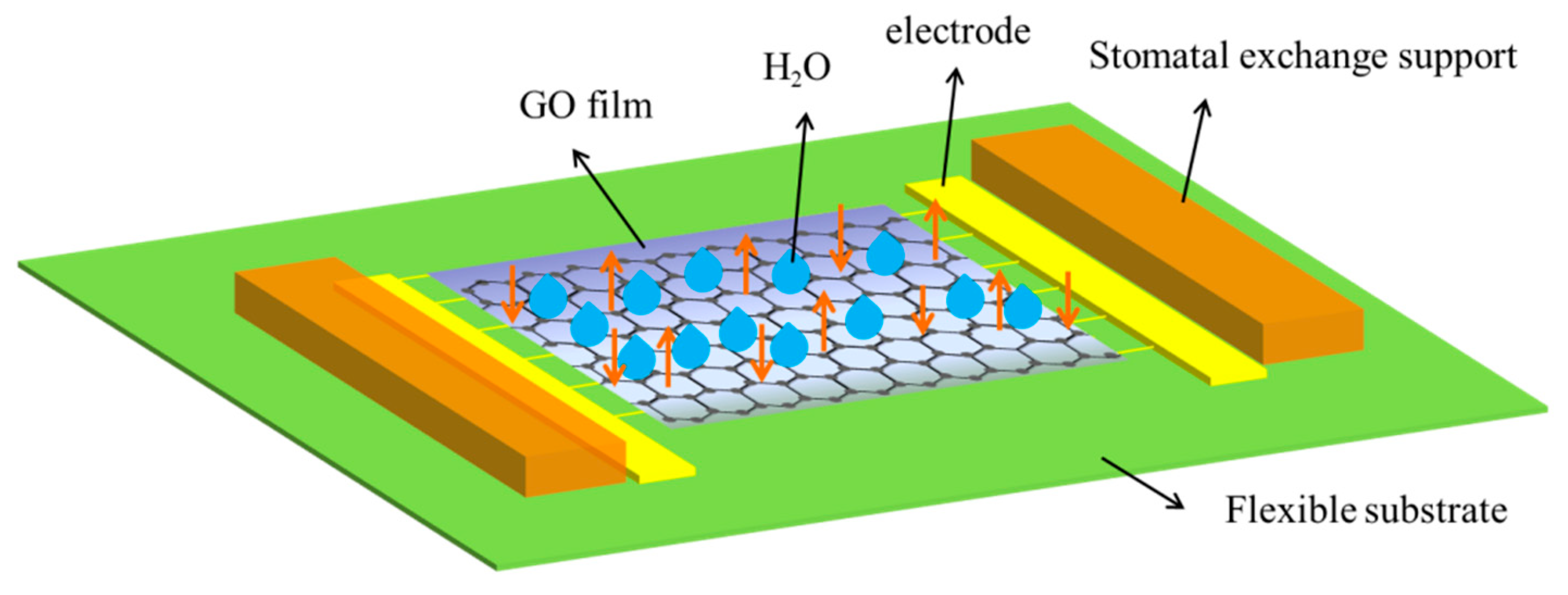
2.3. Preparation of Polyimide (PI) Flexible Substrate Sensor
2.4. Characterizations and Testing
3. Results and Discussion
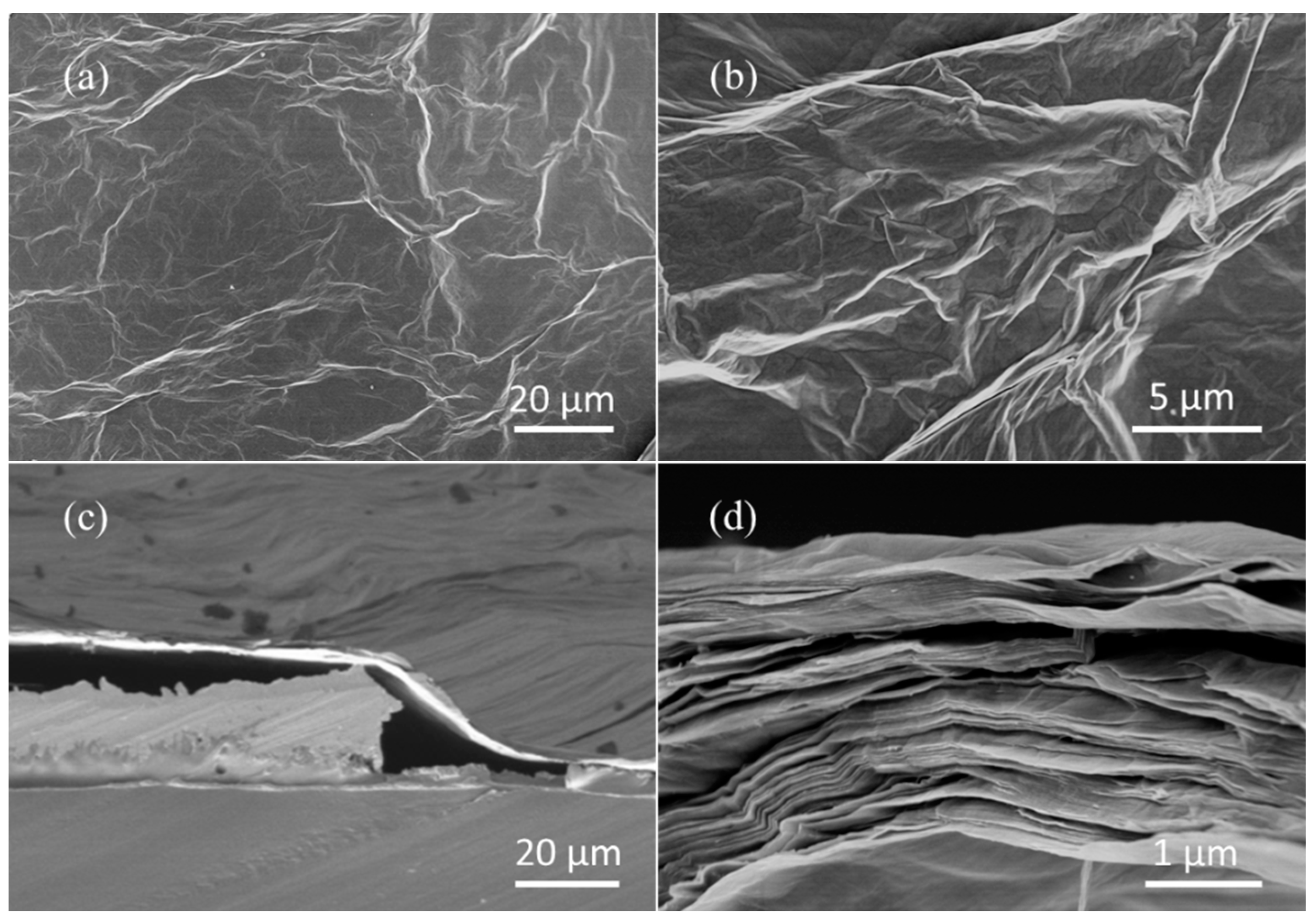
3.1. Characterization of Sensing Films
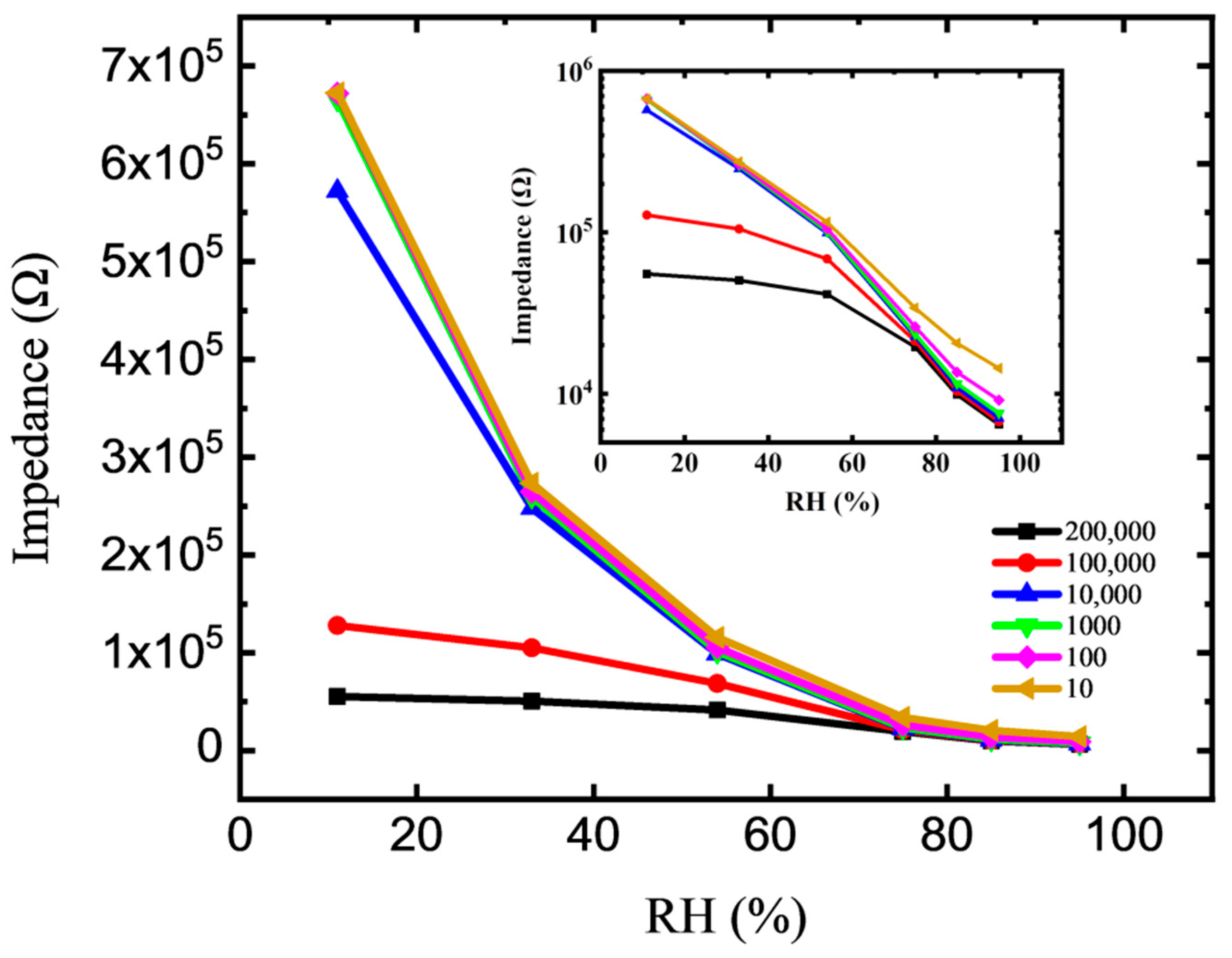
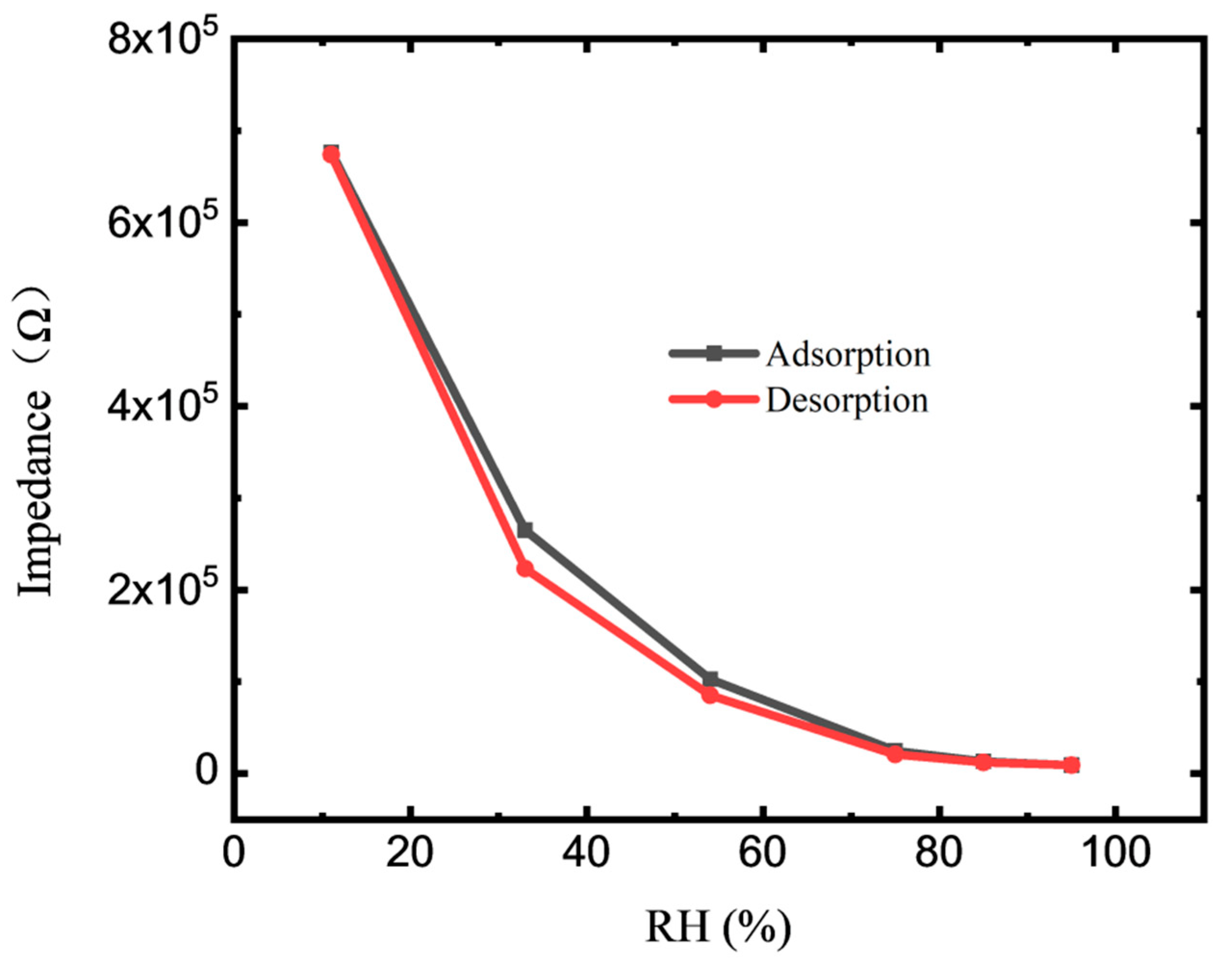
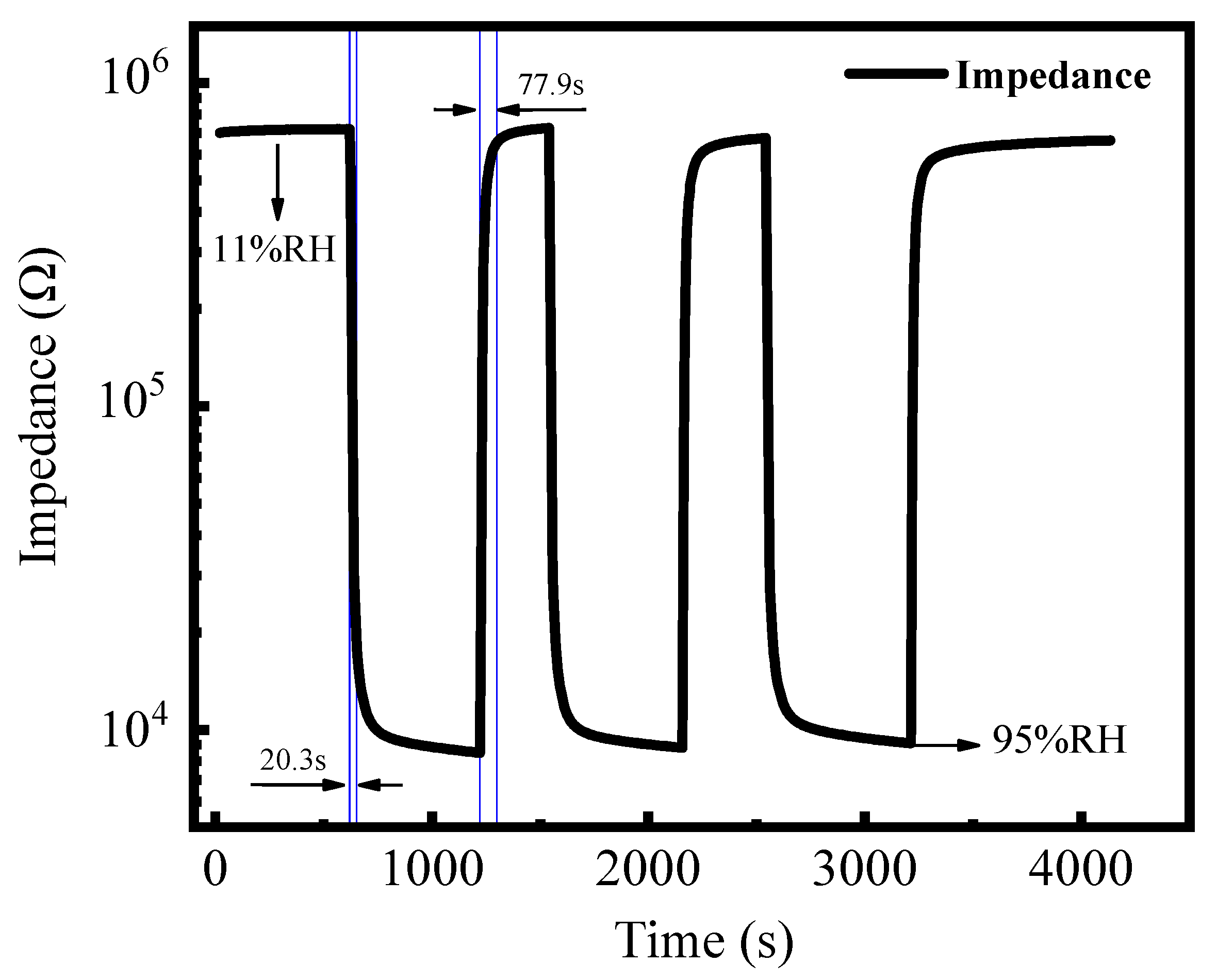
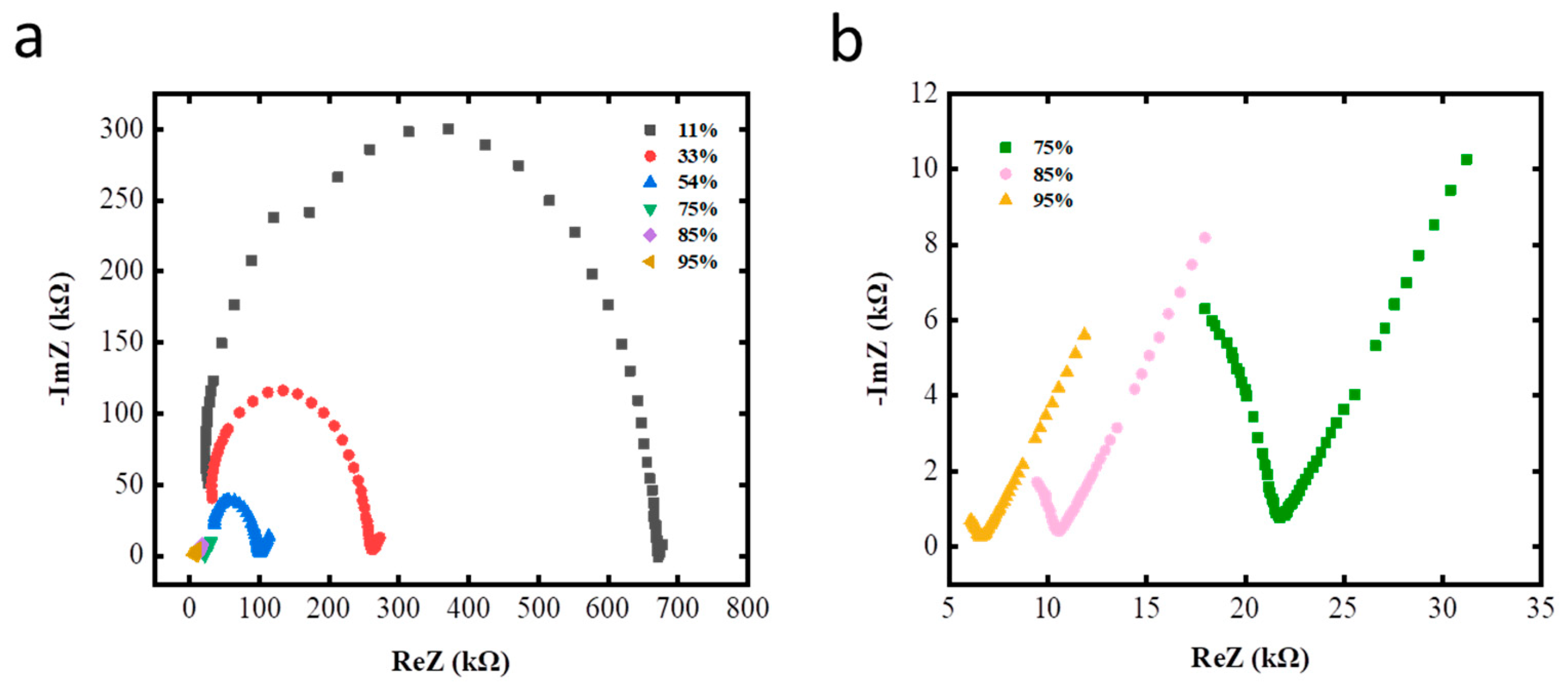
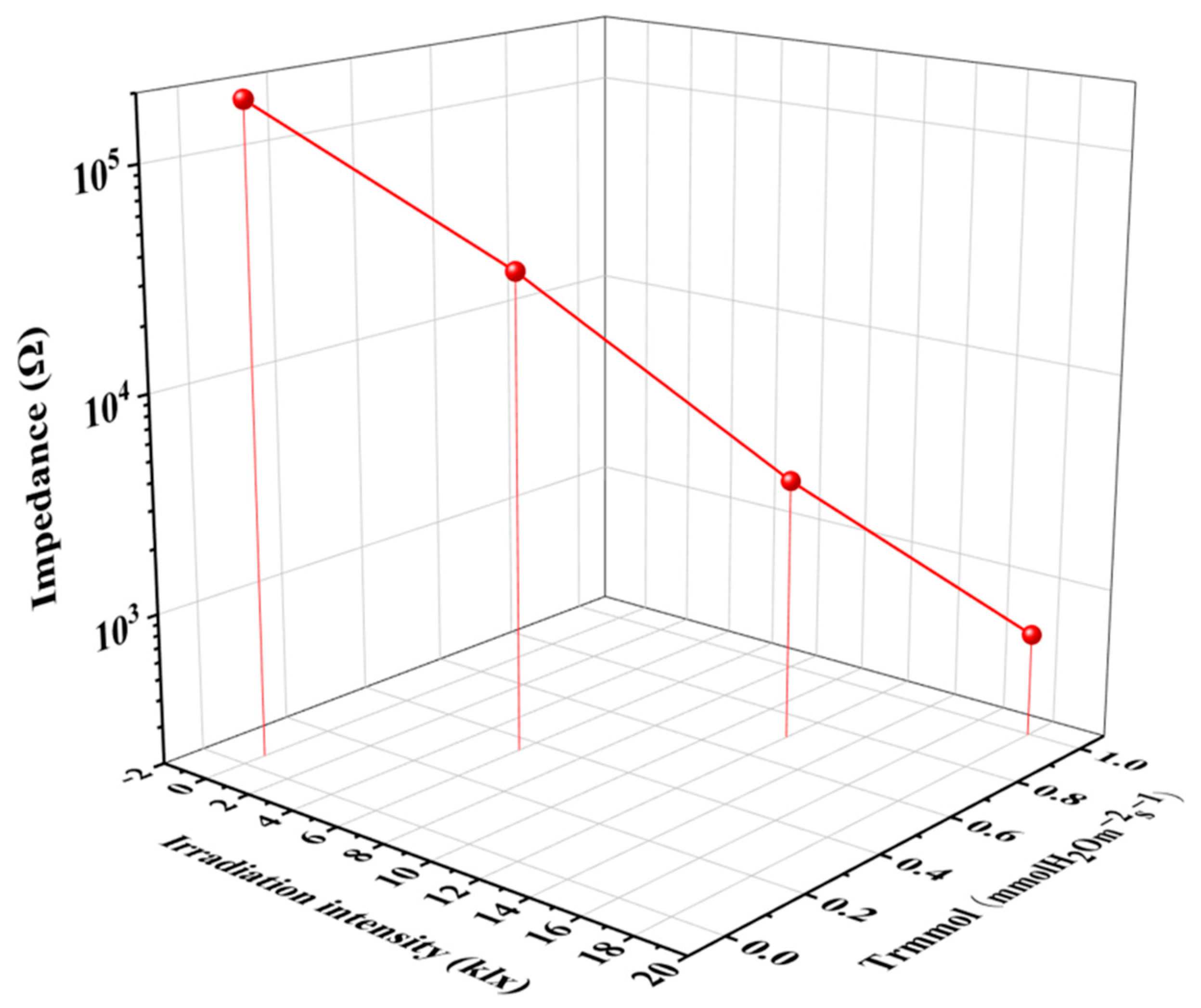
3.2. Sensor Properties and Impedance Analysis
3.3. Sensing Mechanism of the GO Layered Films
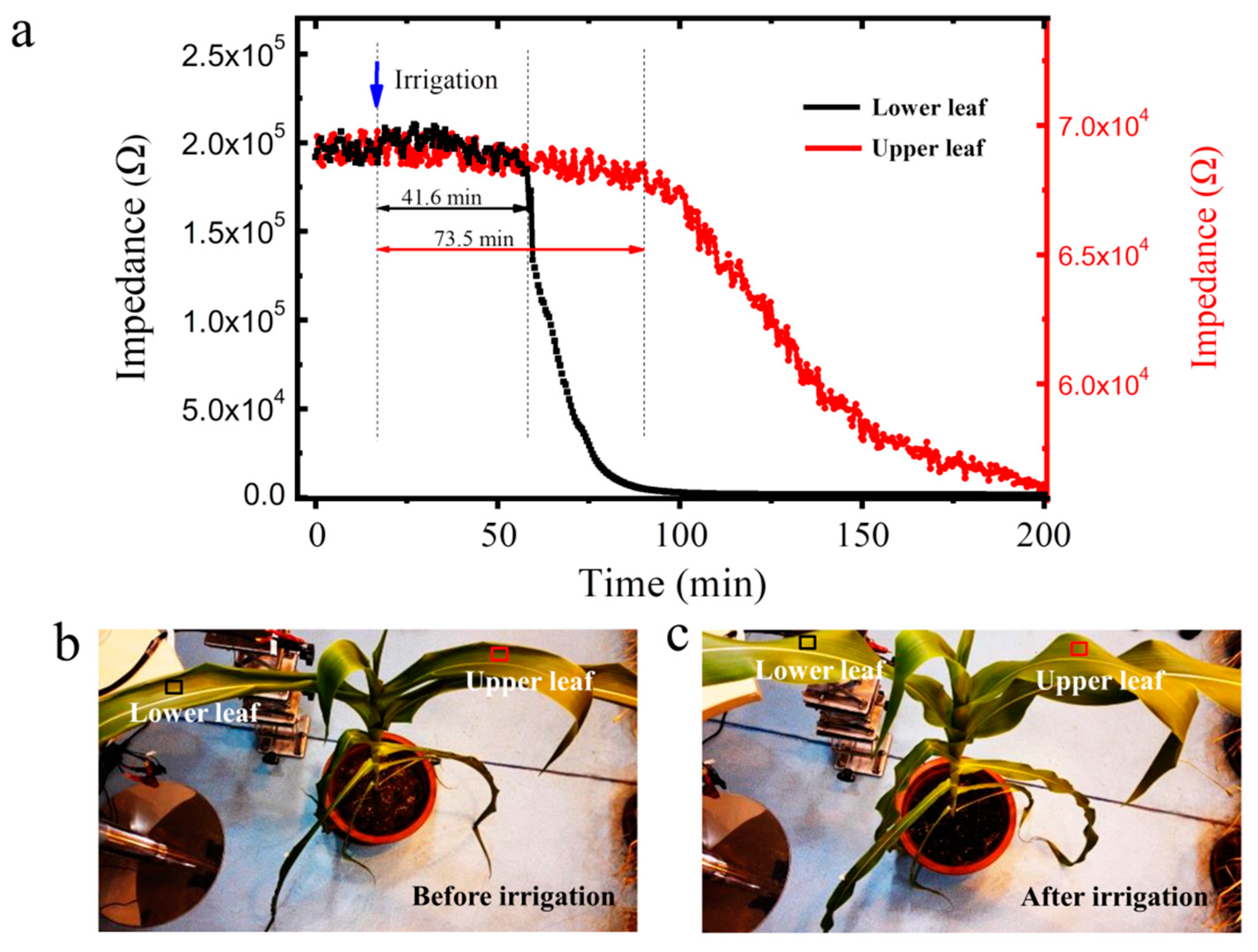
3.4. In Situ Water Movement Monitoring within Plants
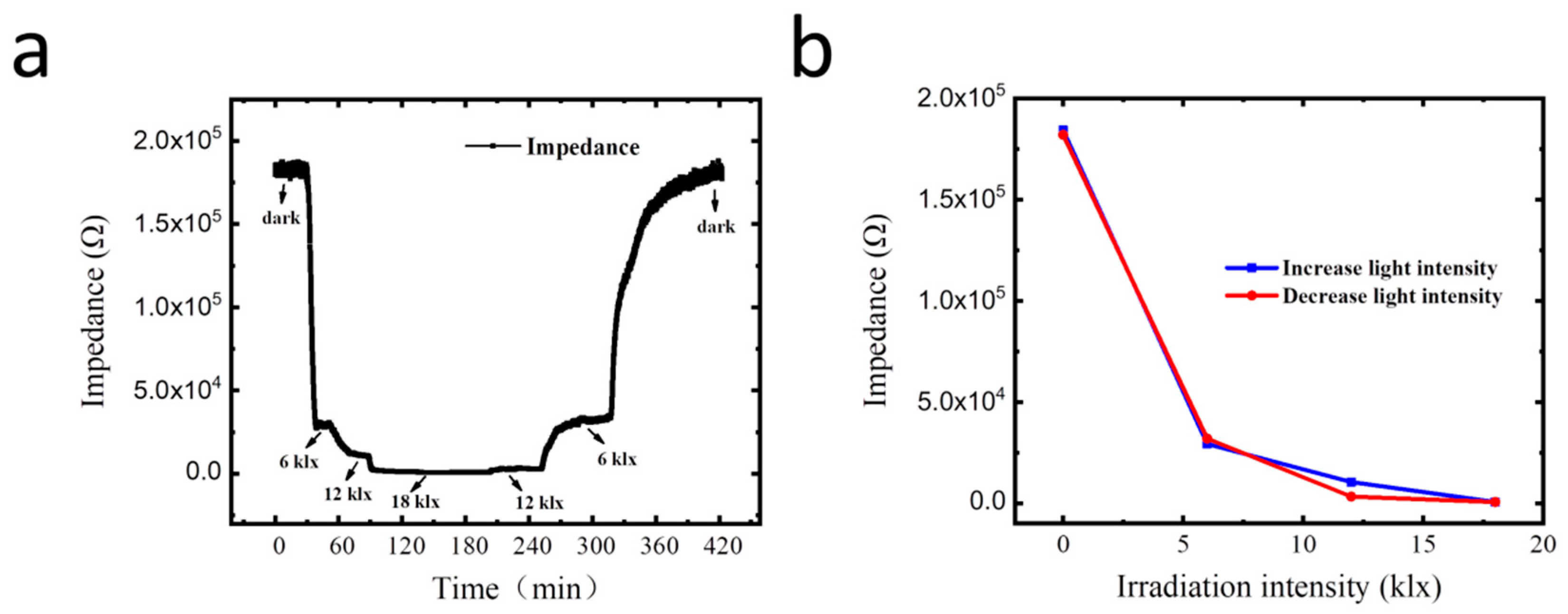
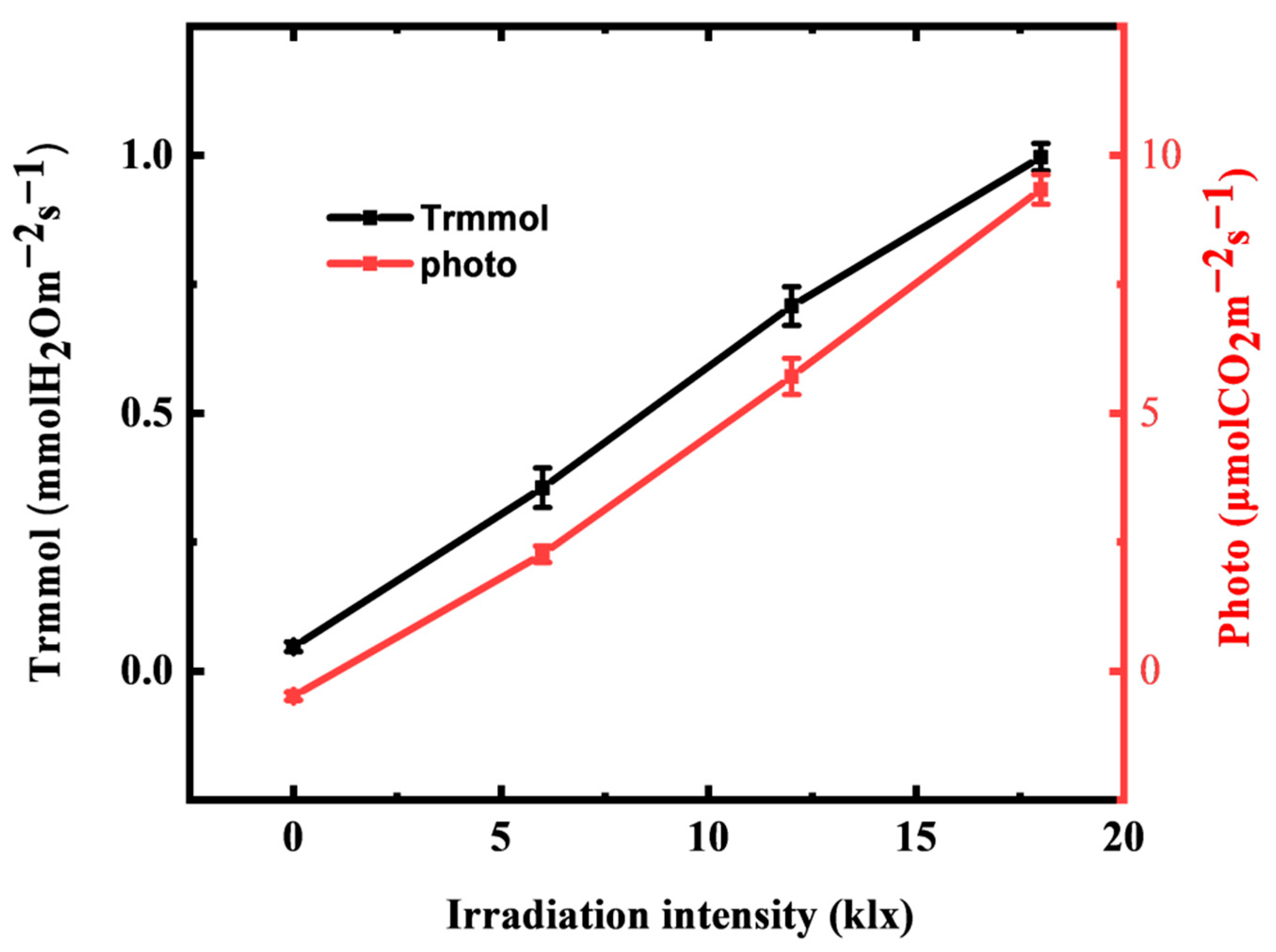
3.5. Correlation Monitoring of Light Regulated Transpiration and Photosynthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scharwies, J.D.; Dinneny, J.R. Water transport, perception, and response in plants. J. Plant Res. 2019, 132, 311–324. [Google Scholar] [PubMed]
- Iglesias, A.; Santillán, D.; Garrote, L. On the barriers to adaption to less water under climate change: Policy choices in mediterranean countries. Water Resour. Manag. 2018, 32, 4819–4832. [Google Scholar]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar]
- Clevers, J.; Kooistra, L.; van den Brande, M. Using Sentinel-2 Data for Retrieving LAI and Leaf and Canopy Chlorophyll Content of a Potato Crop. Remote Sens. 2017, 9, 405. [Google Scholar]
- Feng, L.; Chen, S.; Zhang, C.; Zhang, Y.; He, Y. A comprehensive review on recent applications of unmanned aerial vehicle remote sensing with various sensors for high-throughput plant phenotyping. Comput. Electron. Agric. 2021, 182, 106033. [Google Scholar]
- Altangerel, N.; Ariunbold, G.O.; Gorman, C.; Alkahtani, M.H.; Borrego, E.J.; Bohlmeyer, D.; Hemmer, P.; Kolomiets, M.V.; Yuan, J.S.; Scully, M.O. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 3393–3396. [Google Scholar] [PubMed] [Green Version]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [PubMed]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [PubMed] [Green Version]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [PubMed] [Green Version]
- Lee, G.; Wei, Q.; Zhu, Y. Emerging wearable sensors for plant health monitoring. Adv. Funct. Mater. 2021, 31, 2106475. [Google Scholar]
- Wong, M.H.; Giraldo, J.P.; Kwak, S.Y.; Koman, V.B.; Sinclair, R.; Lew, T.; Bisker, G.; Liu, P.; Strano, M.S. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 2017, 16, 264–272. [Google Scholar] [PubMed]
- Lee, K.; Park, J.; Lee, M.; Kim, J.; Hyun, B.G.; Kang, D.J.; Na, K.; Lee, C.Y.; Bien, F.; Park, J. In-situ synthesis of carbon nanotube-graphite electronic devices and their integrations onto surfaces of live plants and insects. Nano Lett. 2014, 14, 2647–2654. [Google Scholar] [PubMed]
- Travan, C.; Bergmann, A. NO2 and NH3 sensing characteristics of inkjet printing graphene gas sensors. Sensors 2019, 19, 3379. [Google Scholar]
- Yu, C.; Guo, Y.; Liu, H.; Yan, N.; Xu, Z.; Yu, G.; Fang, Y.; Liu, Y. Ultrasensitive and selective sensing of heavy metal ions with modified graphene. Chem. Commun. 2013, 49, 6492–6494. [Google Scholar]
- Stebunov, Y.V.; Aftenieva, O.A.; Arsenin, A.V.; Volkov, V.S. Highly sensitive and selective sensor chips with graphene-oxide linking layer. ACS Appl. Mater. Inter. 2015, 7, 21727–21734. [Google Scholar]
- Duan, K.; Li, L.; Hu, Y.; Wang, X. Pillared graphene as an ultra-high sensitivity mass sensor. Sci. Rep. 2017, 7, 14012. [Google Scholar] [PubMed] [Green Version]
- Pang, K.; Song, X.; Xu, Z.; Liu, X.; Liu, Y.; Zhong, L.; Peng, Y.; Wang, J.; Zhou, J.; Meng, F.; et al. Hydroplastic foaming of graphene aerogels and artificially intelligent tactile sensors. Sci. Adv. 2020, 6, 1–8. [Google Scholar]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen bond networks in graphene oxide composite paper: Structure and mechanical properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [PubMed]
- Ghosh, S.; Ghosh, R.; Guha, P.K.; Bhattacharyya, T.K. Humidity sensor based on high proton conductivity of graphene oxide. IEEE Trans. Nanotechnol. 2015, 14, 931–937. [Google Scholar]
- Kwak, S.Y.; Wong, M.H.; Lew, T.T.S.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R.; et al. Nanosensor technology applied to living plant systems. Annu. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar]
- Giraldo, J.P.; Wu, H.; Newkirk, G.M.; Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 2019, 14, 541–553. [Google Scholar] [PubMed]
- Lu, Y.; Xu, K.; Zhang, L.; Deguchi, M.; Shishido, H.; Arie, T.; Pan, R.; Hayashi, A.; Shen, L.; Akita, S.; et al. Multimodal plant healthcare flexible sensor system. ACS Nano 2020, 14, 10966–10975. [Google Scholar] [PubMed]
- Joonkim, J.; Allison, L.; Andrew, T. Vapor-printed polymer electrodes for long-term, on-demand health monitoring. Scie. Adv. 2019, 5, eaaw0463. [Google Scholar]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-film particles of graphite oxide 1:: High-yield synthesis and flexibility of the particles. Carbon 2004, 42, 2929–2937. [Google Scholar]
- Zhou, X.; Huang, X.; Qi, X.; Wu, S.; Xue, C.; Boey, F.Y.C.; Yan, Q.; Chen, P.; Zhang, H. In situ synthesis of metal nanoparticles on single-layer graphene oxide and reduced graphene oxide surfaces. J. Phys. Chem. C 2009, 113, 10842–10846. [Google Scholar]
- Geng, L.; Wu, S.; Zou, Y. Correlation between the microstructures of graphite oxides and their catalytic behaviors in air oxidation of benzyl alcohol. J. Colloid Inter. Sci. 2014, 421, 71–77. [Google Scholar]
- Li, Y.; Deng, C.; Yang, M. A composite of quaternized and crosslinked poly(4-vinylpyridine) with processable polypyrrole for the construction of humidity sensors with improved sensing properties. Synth. Met. 2012, 162, 205–211. [Google Scholar]
- Li, N.; Chen, X.; Chen, X.; Ding, X.; Zhao, X. Ultrahigh humidity sensitivity of graphene oxide combined with Ag nanoparticles. RSC Adv. 2017, 7, 45988–45996. [Google Scholar]
- Yao, Y.; Chen, X.; Zhu, J.; Zeng, B.; Wu, Z.; Li, X. The effect of ambient humidity on the electrical properties of graphene oxide films. Nanoscale Res. Lett. 2012, 7, 363. [Google Scholar] [PubMed] [Green Version]
- Smith, A.D.; Elgammal, K.; Niklaus, F.; Delin, A.; Fischer, A.C.; Vaziri, S.; Forsberg, F.; Råsander, M.; Hugosson, H.; Bergqvist, L.; et al. Resistive graphene humidity sensors with rapid and direct electrical read-out. Nanoscale 2015, 7, 19099–19109. [Google Scholar]
- Wang, J.; Wan, H.; Lin, Q. Properties of a nanocrystalline barium titanate on silicon humidity sensor. Meas. Sci. Technol. 2003, 14, 172–175. [Google Scholar]
- Geng, W.; Yuan, Q.; Jiang, X.; Tu, J.; Duan, L.; Gu, J.; Zhang, Q. Humidity sensing mechanism of mesoporous MgO/KCl–SiO2 composites analyzed by complex impedance spectra and bode diagrams. Sens. Actuators B-Chem. 2012, 174, 513–520. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [PubMed]
- Zhang, T.; Wang, R.; Geng, W.; Li, X.; Qi, Q.; He, Y.; Wang, S. Study on humidity sensing properties based on composite materials of Li-doped mesoporous silica A-SBA-15. Sens. Actuators B-Chem. 2008, 128, 482–487. [Google Scholar]
- Casalbore-Miceli, G.; Yang, M.J.; Li, Y.; Zanelli, A.; Martelli, A.; Chen, S.; She, Y.; Camaioni, N. A polyelectrolyte as humidity sensing material: Influence of the preparation parameters on its sensing property. Sens. Actuators B 2006, 114, 584–590. [Google Scholar]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhänen, T. Ultrafast graphene oxide humidity sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar]
- Leng, X.; Luo, D.; Xu, Z.; Wang, F. Modified graphene oxide/Nafion composite humidity sensor and its linear response to the relative humidity. Sens. Actuators B 2018, 257, 372–381. [Google Scholar]
- Jiang, K.; Zhao, H.; Dai, J.; Kuang, D.; Fei, T.; Zhang, T. Excellent humidity sensor based on LiCl loaded hierarchically porous polymeric microspheres. ACS Appl. Mater. Inter. 2016, 8, 25529–25534. [Google Scholar]
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B-Chem. 2009, 138, 368–373. [Google Scholar]
- Su, M.; Wang, J. Preparation and humidity sensitivity of multi-layered zirconia thin films by sol-gel method. Sens. Lett. 2011, 9, 670–674. [Google Scholar]
- Wang, S.; Chen, Z.; Umar, A.; Wang, Y.; Tian, T.; Shang, Y.; Fan, Y.; Qi, Q.; Xu, D. Supramolecularly Modified Graphene for Ultrafast Responsive and Highly Stable Humidity Sensor. J. Phys. Chem. C 2015, 119, 28640–28647. [Google Scholar]
- Jezek, M.; Blatt, M.R. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol. 2017, 174, 487–519. [Google Scholar] [PubMed] [Green Version]
- Plett, D.C.; Ranathunge, K.; Melino, V.J.; Kuya, N.; Uga, Y.; Kronzucker, H.J. The intersection of nitrogen nutrition and water use in plants: New paths toward improved crop productivity. J. Exp. Bot. 2020, 71, 4452–4468. [Google Scholar] [PubMed]
- China Meteorological Administration. Specifications for Agrometeorological Observation; China Meteorological Press: Beijing, China, 1993.
- Głowacka, K.; Kromdijk, J.; Kucera, K.; Xie, J.; Cavanagh, A.P.; Leonelli, L.; Leakey, A.; Ort, D.R.; Niyogi, K.K.; Long, S.P. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 2018, 9, 868. [Google Scholar] [PubMed] [Green Version]
- Lawson, T.; Simkin, A.J.; Kelly, G.; Granot, D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 2014, 203, 1064–1081. [Google Scholar] [PubMed] [Green Version]
- Mott, E.A. Guard cell photosynthesis and stomatal function. Plant Cell Environ. 2009, 32, 1479–1486. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, G.; Li, J.; Xu, S. Wearable Crop Sensor Based on Nano-Graphene Oxide for Noninvasive Real-Time Monitoring of Plant Water. Membranes 2022, 12, 358. https://doi.org/10.3390/membranes12040358
Li D, Li G, Li J, Xu S. Wearable Crop Sensor Based on Nano-Graphene Oxide for Noninvasive Real-Time Monitoring of Plant Water. Membranes. 2022; 12(4):358. https://doi.org/10.3390/membranes12040358
Chicago/Turabian StyleLi, Denghua, Ganqiong Li, Jianzheng Li, and Shiwei Xu. 2022. "Wearable Crop Sensor Based on Nano-Graphene Oxide for Noninvasive Real-Time Monitoring of Plant Water" Membranes 12, no. 4: 358. https://doi.org/10.3390/membranes12040358






