High Proton-Conductive and Temperature-Tolerant PVC-P4VP Membranes towards Medium-Temperature Water Electrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Water Electrolysis over PVC-P4VP/PA Membrane
2.4. Characterizations
3. Results and Discussion
3.1. Synthesis of PVC-P4VP/PA Membranes through Cross-Linking
3.2. Thermal/Mechanical/Chemical Stability of PVC-P4VP/PA Membranes
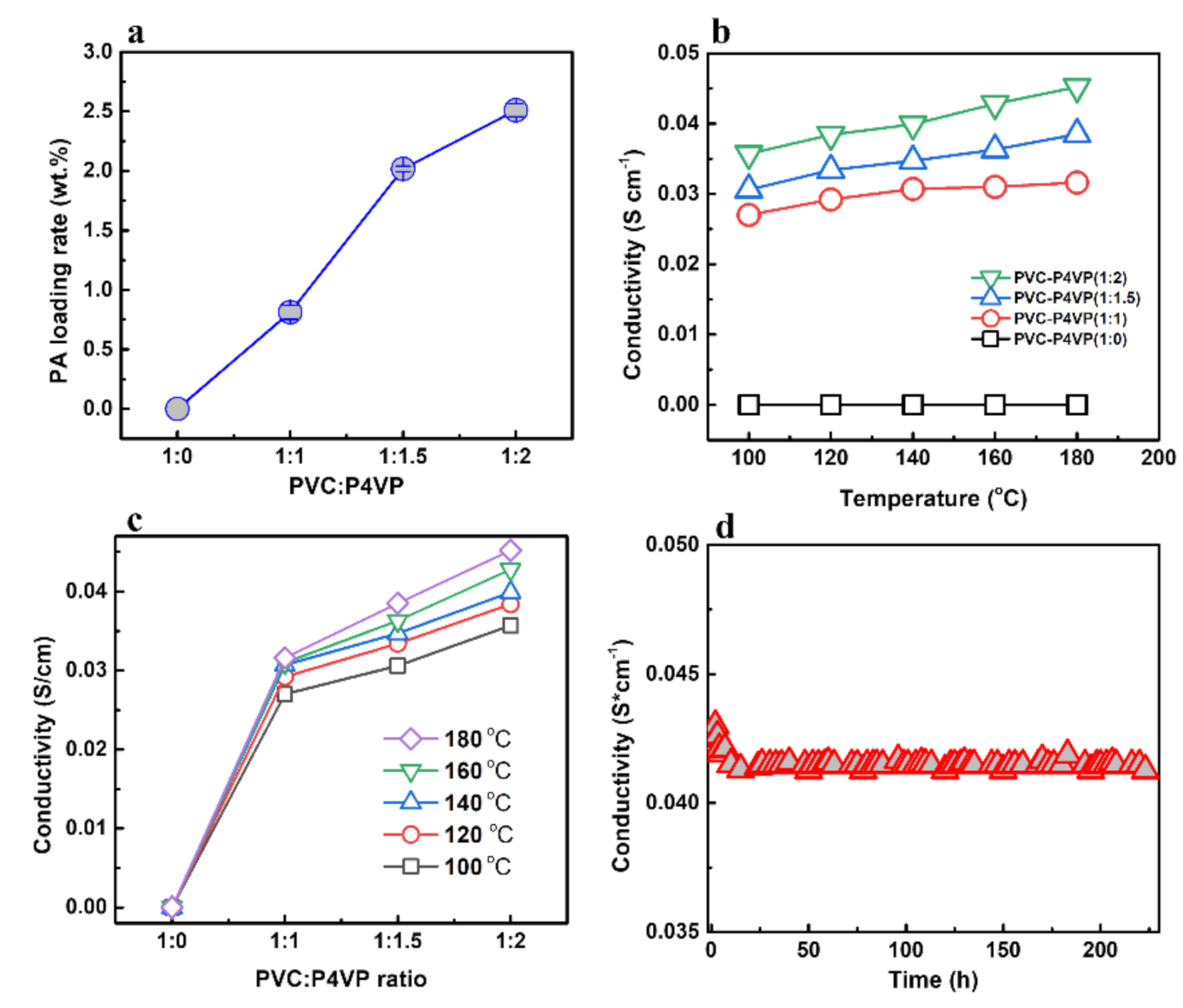
3.3. Electrochemical Properties and Electrolytic Water Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, S.; Xu, Q.; Wu, P.; Jiang, Z.; Zeng, G.F. Hierarchical confinement of PtZn alloy nanoparticles and single-dispersed Zn atoms on COF@MOF-derived carbon towards efficient oxygen reduction reaction. J. Mater. Chem. A 2021, 9, 13625–13630. [Google Scholar] [CrossRef]
- Yang, J.; Gong, D.; Li, G.; Zeng, G.; Wang, Q.; Zhang, Y.; Liu, G.; Wu, P.; Vovk, E.; Peng, Z.; et al. Self-Assembly of Thiourea-Crosslinked Graphene Oxide Framework Membranes toward Separation of Small Molecules. Adv. Mater. 2018, 30, e1705775. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wu, P.; Zeng, G.; Salas-Colerad, E.; Serranod, A.; Castrod, G.; Xu, H.; Sun, C.; Goldbach, A. High-temperature Pd alloy membranes with Cu and Au. J. Membr. Sci. 2017, 544, 151–160. [Google Scholar] [CrossRef]
- Zeng, G.F.; Shi, L.; Liu, Y.Y.; Zhang, Y.F.; Sun, Y.H. A simple approach to uniform PdAg alloy membranes: Comparative study of conventional and silver concentration-controlled co-plating. Int. J. Hydrog. Energy 2014, 39, 4427–4436. [Google Scholar] [CrossRef]
- Zeng, G.F.; Goldbach, A.; Xu, H.Y. Defect sealing in Pd membranes via point plating. J. Membr. Sci. 2009, 328, 6–10. [Google Scholar] [CrossRef]
- Zeng, G.; Goldbach, A.; Xu, H. Impact of support mass flow resistance on low-temperature H2 permeation characteristics of a Pd95Ag5/Al2O3 composite membrane. J. Membr. Sci. 2009, 326, 681–687. [Google Scholar] [CrossRef]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Shiva Kumar, S.; Ramakrishna, S.U.B.; Krishna, S.V.; Srilatha, K.; Devi, B.R.; Himabindu, V. Synthesis of titanium (IV) oxide composite membrane for hydrogen production through alkaline water electrolysis. S. Afr. J. Chem. Eng. 2018, 25, 54–61. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Olateju, B.; Kumar, A. Hydrogen production from wind energy in Western Canada for upgrading bitumen from oil sands. Energy 2011, 36, 6326–6339. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.-t.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Authayanun, S.; Im-orb, K.; Arpornwichanop, A. A review of the development of high temperature proton exchange membrane fuel cells. Chin. J. Catal. 2015, 36, 473–483. [Google Scholar] [CrossRef]
- Guo, Z.; Xiu, R.; Lu, S.; Xu, X.; Yang, S.; Xiang, Y. Submicro-pore containing poly(ether sulfones)/polyvinylpyrrolidone membranes for high-temperature fuel cell applications. J. Mater. Chem. A 2015, 3, 8847–8854. [Google Scholar] [CrossRef]
- Üregen, N.; Pehlivanoğlu, K.; Özdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrog. Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Che, Q.; Zhu, Z.; Chen, N.; Zhai, X. Methylimidazolium group—Modified polyvinyl chloride (PVC) doped with phosphoric acid for high temperature proton exchange membranes. Mater. Des. 2015, 87, 1047–1055. [Google Scholar] [CrossRef]
- Allan, J.T.S.; Prest, L.E.; Easton, E.B. The sulfonation of polyvinyl chloride: Synthesis and characterization for proton conducting membrane applications. J. Membr. Sci. 2015, 489, 175–182. [Google Scholar] [CrossRef]
- Liu, R.; Liu, M.; Wu, S.; Che, X.; Dong, J.; Yang, J. Assessing the influence of various imidazolium groups on the properties of poly(vinyl chloride) based high temperature proton exchange membranes. Eur. Polym. J. 2020, 137, 109948. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Xu, Y.; Yang, J.; He, R. Quaternized poly(aromatic ether sulfone) with siloxane crosslinking networks as high temperature proton exchange membranes. Appl. Surf. Sci. 2018, 452, 473–480. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Bai, H.; Tan, Q.; Wang, H.; He, B.; Xiang, Y.; Lu, S. A new high temperature polymer electrolyte membrane based on tri-functional group grafted polysulfone for fuel cell application. J. Membr. Sci. 2019, 572, 496–503. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Jensen, J.O.; Pan, C.; Cleemann, L.N.; Bjerrum, N.J.; He, R. Phosphoric acid doped imidazolium polysulfone membranes for high temperature proton exchange membrane fuel cells. J. Power Sources 2012, 205, 114–121. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, C.; Gao, L.; Xu, Y.; Che, Q.; He, R. Influences of the structure of imidazolium pendants on the properties of polysulfone-based high temperature proton conducting membranes. J. Membr. Sci. 2015, 493, 80–87. [Google Scholar] [CrossRef]
- Nasef, M.M.; Shamsaei, E.; Saidi, H.; Ahmad, A.; Dahlan, K.Z.M. Preparation and characterization of phosphoric acid composite membrane by radiation induced grafting of 4-vinylpyridine onto poly(ethylene-co-tetrafluoroethylene) followed by phosphoric acid doping. J. Appl. Polym. Sci. 2013, 128, 549–557. [Google Scholar] [CrossRef]
- Takrori, F. Grafting of nitrogen containing monomers onto poly(ethylene-alt-tetrafluoroethylene) films by bulk polymerization for proton exchange membranes. J. Radioanal. Nucl. Chem. 2015, 308, 1089–1094. [Google Scholar] [CrossRef]
- Işıkel Şanlı, L.; Alkan Gürsel, S. Synthesis and characterization of novel graft copolymers by radiation-induced grafting. J. Appl. Polym. Sci. 2011, 120, 2313–2323. [Google Scholar] [CrossRef] [Green Version]
- Arico, A.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer electrolyte membrane water electrolysis: Status of technologies and potential applications in combination with renewable power sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Jiang, F.; Kaltbeitzel, A.; Zhang, J.; Meyer, W.H. Nano-spheres stabilized poly(vinyl phosphonic acid) as proton conducting membranes for PEMFCs. Int. J. Hydrog. Energy 2014, 39, 11157–11164. [Google Scholar] [CrossRef]
- Woong, J.C.; Venkataramani, S.; Kim, S.C. Modification of Nafion membrane using poly(4-vinyl pyridine) for direct methanol fuel cell. Polym. Int. 2006, 55, 491–499. [Google Scholar] [CrossRef]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. TG/FTIR analysis on co-pyrolysis behavior of PE, PVC and PS. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef]
- Vijayalakshmi Rao, R.; Mohan Rao, P.; Shridhar, M.H. Effect of electron irradiation on P4VP/PTSA complex and P4VP/Phthalocyanine composites. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2002, 187, 331–339. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Shi, L.; Zeng, G.; Zhang, H.; Li, S.; Wu, P.; Zhang, Y.; Fan, Y.; Liu, G.; et al. Ultralow Pt Catalyst for Formaldehyde Removal: The Determinant Role of Support. iScience 2018, 9, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Rama Mohan, K.; Achari, V.B.S.; Rao, V.V.R.N.; Sharma, A.K. Electrical and optical properties of (PEMA/PVC) polymer blend electrolyte doped with NaClO4. Polym. Test. 2011, 30, 881–886. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, C.; Qiu, Y.; Chang, X.; Tan, G.; Ren, B. Effect of topology structure on the electrochemical behavior of hydrogen-bonded self-assembled Poly(4-vinylpyridine)-ferrocenyl dendron complexes. J. Organomet. Chem. 2017, 846, 223–229. [Google Scholar] [CrossRef]
- Maaz, M.; Elzein, T.; Bejjani, A.; Barroca-Aubry, N.; Lepoittevin, B.; Dragoe, D.; Mazerat, S.; Nsouli, B.; Roger, P. Surface initiated supplemental activator and reducing agent atom transfer radical polymerization (SI-SARA-ATRP) of 4-vinylpyridine on poly(ethylene terephthalate). J. Colloid. Interface Sci. 2017, 500, 69–78. [Google Scholar] [CrossRef]
- Li, G.; Zhu, Z.; Qi, B.; Liu, G.; Wu, P.; Zeng, G.; Zhang, Y.; Wang, W.; Sun, Y. Rapid capture of Ponceau S via a hierarchical organic–inorganic hybrid nanofibrous membrane. J. Mater. Chem. A 2016, 4, 5423–5427. [Google Scholar] [CrossRef]
- Feliu, S.; Fierro, J.L.G.; Maffiotie, C.; Chico, B.; Morcillo, M. Study of the interfacial chemistry of poly(vinyl chloride) paint on steel exposed to the ultraviolet-water condensation test. J. Adhes. Sci. Technol. 1997, 11, 591–611. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Tian, X.; Li, J.; Liu, F.; Wang, X.; Chen, H.; Mao, T.; Liu, G. Ethyl phosphoric acid grafted amino-modified polybenzimidazole with improved long-term stability for high-temperature proton exchange membrane applications. Int. J. Hydrog. Energy 2020, 45, 3176–3185. [Google Scholar] [CrossRef]
- Mansri, A.; Benabadji, K.I.; Desbrières, J.; François, J. Chromium removal using modified poly(4-vinylpyridinium) bentonite salts. Desalination 2009, 245, 95–107. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, Y.; Yuan, J.; Niu, L.; Xia, F. In situ preparation of caterpillar-like polyaniline/carbon nanotube hybrids with core shell structure for high performance supercapacitors. Carbon 2014, 78, 279–287. [Google Scholar] [CrossRef]
- Güler, E.; Sadeghi, S.; Alkan Gürsel, S. Characterization and fuel cell performance of divinylbenzene crosslinked phosphoric acid doped membranes based on 4-vinylpyridine grafting onto poly(ethylene-co-tetrafluoroethylene) films. Int. J. Hydrog. Energy 2018, 43, 8088–8099. [Google Scholar] [CrossRef]
- Sanlı, L.I.; Tas, S.; Yürüm, Y.; Gürsel, S.A. Water Free Operated Phosphoric Acid Doped Radiation-Grafted Proton Conducting Membranes for High Temperature Polymer Electrolyte Membrane Fuel Cells. Fuel Cells 2014, 14, 914–925. [Google Scholar] [CrossRef]
- Brown, E.H.; Whitt, C.D. Vapor Pressure of Phosphoric Acids. Ind. Eng. Chem. 1952, 44, 615–618. [Google Scholar] [CrossRef]
- Munoz, M.; Domínguez, C.M.; de Pedro, Z.M.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Ionic liquids breakdown by Fenton oxidation. Catal. Today 2015, 240, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Che, Q.; Zhou, L.; Wang, J. Fabrication and characterization of phosphoric acid doped imidazolium ionic liquid polymer composite membranes. J. Mol. Liq. 2015, 206, 10–18. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Ticianelli, E.A. A performance and degradation study of Nafion 212 membrane for proton exchange membrane fuel cells. J. Power Sources 2009, 193, 547–554. [Google Scholar] [CrossRef]
- Pu, H.T. Studies on polybenzimidazole/poly (4-vinylpyridine) blends and their proton conductivity after doping with acid. Polym. Int. 2003, 52, 1540–1545. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, H.J.; Ren, Q.; Luo, H.B.; Ren, X.M.; Tian, Z.F.; Lu, S. Extra Water- and Acid-Stable MOF-801 with High Proton Conductivity and Its Composite Membrane for Proton-Exchange Membrane. ACS Appl. Mater. Interfaces 2018, 10, 28656–28663. [Google Scholar] [CrossRef]
- Ma, Y.L.; Wainright, J.S.; Litt, M.H.; Savinell, R.F. Conductivity of PBI Membranes for High-Temperature Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2004, 151, A8. [Google Scholar] [CrossRef]





| Membrane Material | Conductivity (10−2 S cm−1) | Temperature (°C) | References |
|---|---|---|---|
| PBI/P4VP (50/50) (W/W) | 1.8 | 140 | [47] |
| PVPA/P4VP-NS blends | 0.02 | 140 | [28] |
| PVC-P4VP(1:2)/PA | 4.0 | 140 | This work |
| ETFE-g-4VP (0% DVB 30%GL) | 2.8 | 120 | [42] |
| ETFE-g-P4VP | 4.0 | 120 | [43] |
| PVC-P4VP(1:2)/PA | 3.8 | 120 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Ying, Y.; Liu, G.; Chen, H.; Fan, J.; Li, Z.; Wang, C.; Guo, Z.; Zeng, G. High Proton-Conductive and Temperature-Tolerant PVC-P4VP Membranes towards Medium-Temperature Water Electrolysis. Membranes 2022, 12, 363. https://doi.org/10.3390/membranes12040363
Yin Y, Ying Y, Liu G, Chen H, Fan J, Li Z, Wang C, Guo Z, Zeng G. High Proton-Conductive and Temperature-Tolerant PVC-P4VP Membranes towards Medium-Temperature Water Electrolysis. Membranes. 2022; 12(4):363. https://doi.org/10.3390/membranes12040363
Chicago/Turabian StyleYin, Yichen, Yiming Ying, Guojuan Liu, Huiling Chen, Jingrui Fan, Zhi Li, Chuhao Wang, Zhuangyan Guo, and Gaofeng Zeng. 2022. "High Proton-Conductive and Temperature-Tolerant PVC-P4VP Membranes towards Medium-Temperature Water Electrolysis" Membranes 12, no. 4: 363. https://doi.org/10.3390/membranes12040363
APA StyleYin, Y., Ying, Y., Liu, G., Chen, H., Fan, J., Li, Z., Wang, C., Guo, Z., & Zeng, G. (2022). High Proton-Conductive and Temperature-Tolerant PVC-P4VP Membranes towards Medium-Temperature Water Electrolysis. Membranes, 12(4), 363. https://doi.org/10.3390/membranes12040363






