A Simple Approach to Fabricate Composite Ceramic Membranes Decorated with Functionalized Carbide-Derived Carbon for Oily Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of CDC
2.3. Membrane Fabrication
2.4. Characterization
2.5. Oil/Water Separation
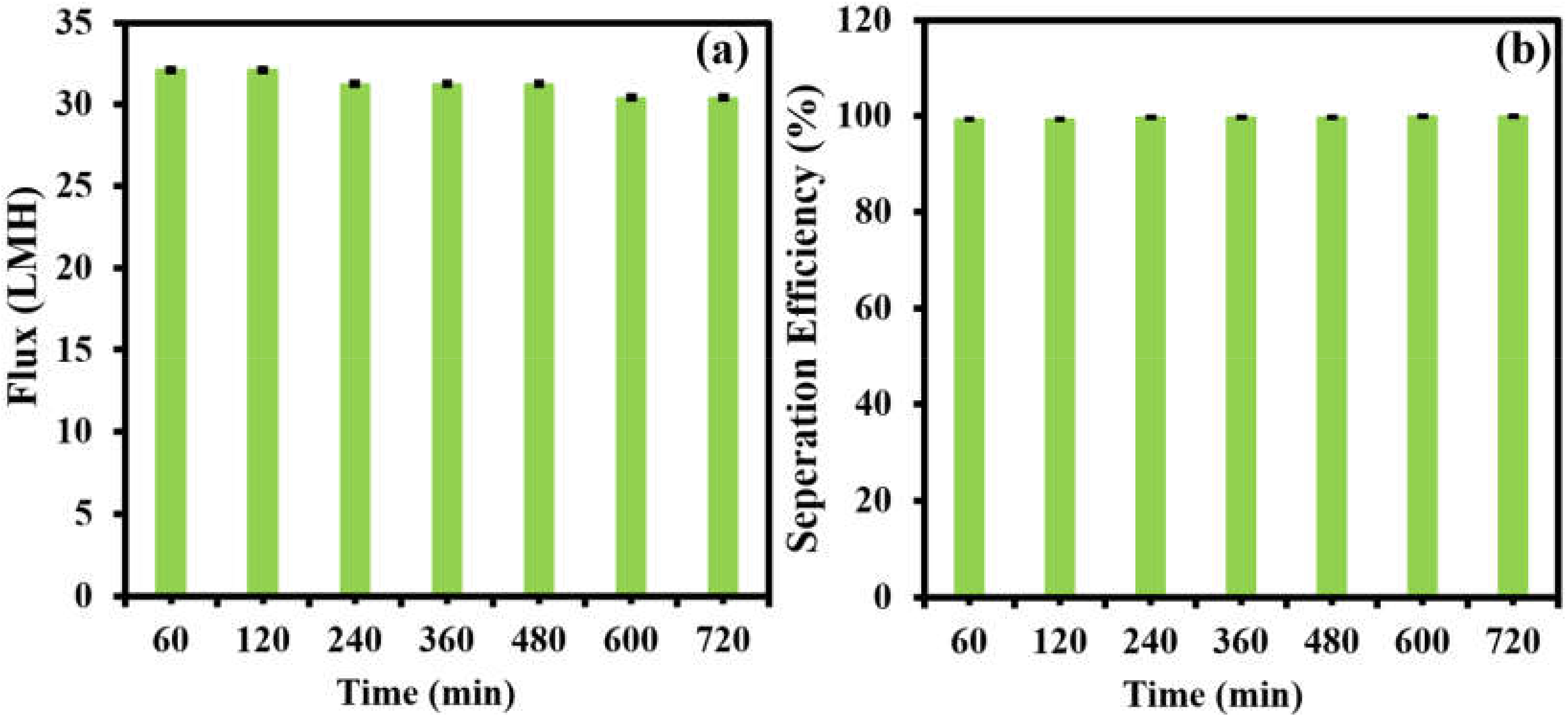
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenny, J.P.; Anneville, O.; Arnaud, F.; Baulaz, Y.; Bouffard, D.; Domaizon, I.; Bocaniov, S.A.; Chèvre, N.; Dittrich, M.; Dorioz, J.M.; et al. Scientists’ Warning to Humanity: Rapid degradation of the world’s large lakes. J. Great Lakes Res. 2020, 46, 686–702. [Google Scholar] [CrossRef]
- Ghanim, A.A. Water Resources Crisis in Saudi Arabia, Challenges and Possible Management Options: An Analytic Review. Int. J. Environ. Ecol. Eng. 2019, 13, 51–56. [Google Scholar] [CrossRef]
- Nehme, D. Saudi Aramco: The Oil Colossus. Available online: https://www.reuters.com/article/us-saudi-aramco-ipo-factbox-idUSKBN1XD03T (accessed on 25 December 2021).
- Johnston, C.S.; Morris, R.J. Oily Water Discharge: Regulatory, Technical and Scientific Considerations; Applied Science Publishers Ltd.: London, UK, 1980; p. 322. ISBN 9780853348764. [Google Scholar]
- Lu, D.; Zhang, T.; Ma, J. Ceramic membrane fouling during ultrafiltration of oil/water emulsions: Roles played by stabilization surfactants of oil droplets. Environ. Sci. Technol. 2015, 49, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.S.; Yu, S.L.; Shi, W.X.; Sun, N.; Jin, L.M.; Wang, S.; Zhang, B.; Ma, C.; Sun, L.P. The influence of important factors on ultrafiltration of oil/water emulsion using PVDF membrane modified by nano-sized TiO2/Al2O3. Desalination 2011, 281, 179–184. [Google Scholar] [CrossRef]
- Wang, H. Shale oil production and groundwater: What can we learn from produced water data? PLoS ONE 2021, 16, e0250791. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.; Fane, A.G.; Chew, J.W. Membrane-Based Separation for Oily Wastewater: A Practical Perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Maletskyi, Z. Advances in Membrane Materials and Processes for Water and Wastewater Treatment. ACS Symp. Ser. 2020, 1348, 3–35. [Google Scholar] [CrossRef]
- Mao, H.; Zhou, S.; Shi, S.; Xue, A.; Li, M.; Cai, J.; Zhao, Y.; Xing, W. Anti-fouling and easy-cleaning PVDF membranes blended with hydrophilic thermo-responsive nanofibers for efficient biological wastewater treatment. Sep. Purif. Technol. 2022, 281, 119881. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, S.; Li, M.; Xue, A.; Zhao, Y.; Peng, W.; Xing, W. PVDF mixed matrix ultrafiltration membrane incorporated with deformed rebar-like Fe3O4–palygorskite nanocomposites to enhance strength and antifouling properties. J. Membr. Sci. 2020, 612, 118467. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, S.; Mao, H.; Li, M.; Xue, A.; Zhao, Y.; Xing, W. A novel ceramic microfiltration membrane fabricated by anthurium andraeanum-like attapulgite nanofibers for high-efficiency oil-in-water emulsions separation. J. Membr. Sci. 2021, 630, 119291. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Liu, H.; Chen, M.; Xu, H.; Luo, W.; Zhang, F. Robust functionalization of underwater superoleophobic PVDF-HFP tubular nanofiber membranes and applications for continuous dye degradation and oil/water separation. J. Membr. Sci. 2020, 596, 117583. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Kazemi, F.; Jafarzadeh, Y.; Masoumi, S.; Rostamizadeh, M. Oil-in-water emulsion separation by PVC membranes embedded with GO-ZnO nanoparticles. J. Environ. Chem. Eng. 2021, 9, 104992. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, U.; Ansari, M.A.; Aljundi, I.H. Design and fabrication of fouling resistant cross-linked polyamide thin film composite nanofiltration membrane consisting of an aliphatic triamine and terephthaloyl chloride for water desalting applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 633, 127855. [Google Scholar] [CrossRef]
- Rodrigues, R.; Mierzwa, J.C.; Vecitis, C.D. Mixed matrix polysulfone/clay nanoparticles ultrafiltration membranes for water treatment. J. Water Process Eng. 2019, 31, 100788. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Ibrahim, Y.; Almarzooqi, F.; Naddeo, V.; Karanikolos, G.N.; Alhseinat, E.; Banat, F.; Hasan, S.W. Synthesis of polydopamine coated tungsten oxide@ poly(vinylidene fluoride-co-hexafluoropropylene) electrospun nanofibers as multifunctional membranes for water applications. Chem. Eng. J. 2022, 427, 131021. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An Intelligent Superwetting PVDF Membrane Showing Switchable Transport Performance for Oil/Water Separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, H.; Liu, F.; Xiao, P.; Wu, Z.; Li, T.; Li, D. Flexible PVDF membranes with exceptional robust superwetting surface for continuous separation of oil/water emulsions. Sci. Rep. 2017, 7, 14099. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Guria, C.; Mandal, A. Enhanced performance of salt-induced Pluronic F127 and bentonite blended polyvinyl chloride ultrafiltration membrane for the processing of oilfield produced water. J. Water Process Eng. 2020, 34, 101144. [Google Scholar] [CrossRef]
- Raji, Y.O.; Othman, M.H.D.; Nordin, N.A.H.S.M.; Kurniawan, T.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Adam, M.R.B.; Alftessi, S.A.; El-badawy, T.; et al. Wettability improvement of ceramic membrane by intercalating nano-Al2O3 for oil and water separation. Surf. Interfaces 2021, 25, 101178. [Google Scholar] [CrossRef]
- Alftessi, S.A.; Othman, M.H.D.; Adam, M.R.; Farag, T.M.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Habib, M.A.; Raji, Y.O.; Hubadillah, S.K. Novel silica sand hollow fibre ceramic membrane for oily wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104975. [Google Scholar] [CrossRef]
- Liu, W.; Yang, G.; Huang, M.; Liang, J.; Zeng, B.; Fu, C.; Wu, H. Ultrarobust and Biomimetic Hierarchically Macroporous Ceramic Membrane for Oil-Water Separation Templated by Emulsion-Assisted Self-Assembly Method. ACS Appl. Mater. Interfaces 2020, 12, 35555–35562. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, T.; Gutierrez, L.; Ma, J.; Croué, J.P. Influence of Surface Properties of Filtration-Layer Metal Oxide on Ceramic Membrane Fouling during Ultrafiltration of Oil/Water Emulsion. Environ. Sci. Technol. 2016, 50, 4668–4674. [Google Scholar] [CrossRef] [Green Version]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-Derived Carbons—From Porous Networks to Nanotubes and Graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Awad, A.; Aljundi, I.H. Layer-by-layer assembly of carbide derived carbon-polyamide membrane for CO2 separation from natural gas. Energy 2018, 157, 188–199. [Google Scholar] [CrossRef]
- Najimu, M.O.; Aljundi, I.H. Separation performance of CO2 by hybrid membrane comprising nanoporous carbide derived carbon. J. Nat. Gas Sci. Eng. 2018, 59, 9–20. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Dash, R.K.; Yushin, G.; Yildirim, T.; Laudisio, G.; Fischer, J.E. Tailoring of nanoscale porosity in carbide-derived carbons for hydrogen storage. J. Am. Chem. Soc. 2005, 127, 16006–16007. [Google Scholar] [CrossRef]
- Schlange, A.; Dos Santos, A.R.; Hasse, B.; Etzold, B.J.M.; Kunz, U.; Turek, T. Titanium carbide-derived carbon as a novel support for platinum catalysts in direct methanol fuel cell application. J. Power Sources 2012, 199, 22–28. [Google Scholar] [CrossRef]
- Khan, A.U.H.; Khan, Z.; Aljundi, I.H. Improved hydrophilicity and anti-fouling properties of polyamide TFN membrane comprising carbide derived carbon. Desalination 2017, 420, 125–135. [Google Scholar] [CrossRef]
- Porada, S.; Weinstein, L.; Dash, R.; Van Der Wal, A.; Bryjak, M.; Gogotsi, Y.; Biesheuvel, P.M. Water desalination using capacitive deionization with microporous carbon electrodes. ACS Appl. Mater. Interfaces 2012, 4, 1194–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, R.K.; Nikitin, A.; Gogotsi, Y. Microporous carbon derived from boron carbide. Microporous Mesoporous Mater. 2004, 72, 203–208. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, U.; Abussaud, B.; Aljundi, I.H. Fabrication of supported carbide-derived-carbon membrane by two phases of interfacial polymerization for oil/water separation. Ceram. Int. 2021, 48, 8125–8135. [Google Scholar] [CrossRef]
- Karade, V.C.; Sharma, A.; Dhavale, R.P.; Dhavale, R.P.; Shingte, S.R.; Patil, P.S.; Kim, J.H.; Zahn, D.R.T.; Chougale, A.D.; Salvan, G.; et al. APTES monolayer coverage on self-assembled magnetic nanospheres for controlled release of anticancer drug Nintedanib. Sci. Rep. 2021, 11, 5674. [Google Scholar] [CrossRef]
- Bai, H.; Wang, X.; Zhou, Y.; Zhang, L. Preparation and characterization of poly(vinylidene fluoride) composite membranes blended with nano-crystalline cellulose. Prog. Nat. Sci. 2012, 22, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Xianmiao, C.; Yubao, L.; Yi, Z.; Li, Z.; Jidong, L.; Huanana, W. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater. Sci. Eng. C 2009, 29, 29–35. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Rice- and butterfly-wing effect inspired self-cleaning and low drag micro/nanopatterned surfaces in water, oil, and air flow. Nanoscale 2014, 6, 76–96. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Quintanar-Guerrero, D.; Del Real-López, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, M.L. Effect of sucrose concentration and pH onto the physical stability of β-carotene nanocapsules. LWT 2018, 90, 354–361. [Google Scholar] [CrossRef]
- Alade, O.S.; Mahmoud, M.; Al Shehri, D.A.; Sultan, A.S. Rapid determination of emulsion stability using turbidity measurement incorporating artificial neural network (ANN): Experimental validation using video/optical microscopy and kinetic modeling. ACS Omega 2021, 6, 5910–5920. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Baig, U.; Khan, A.A. Polyurethane-based cation exchange composite membranes: Preparation, characterization and its application in development of ion-selective electrode for detection of copper(II). J. Ind. Eng. Chem. 2015, 29, 392–399. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, U.; Waheed, A.; Abussaud, B.; Aljundi, I.H. A Simple Approach to Fabricate Composite Ceramic Membranes Decorated with Functionalized Carbide-Derived Carbon for Oily Wastewater Treatment. Membranes 2022, 12, 394. https://doi.org/10.3390/membranes12040394
Baig U, Waheed A, Abussaud B, Aljundi IH. A Simple Approach to Fabricate Composite Ceramic Membranes Decorated with Functionalized Carbide-Derived Carbon for Oily Wastewater Treatment. Membranes. 2022; 12(4):394. https://doi.org/10.3390/membranes12040394
Chicago/Turabian StyleBaig, Umair, Abdul Waheed, Basim Abussaud, and Isam H. Aljundi. 2022. "A Simple Approach to Fabricate Composite Ceramic Membranes Decorated with Functionalized Carbide-Derived Carbon for Oily Wastewater Treatment" Membranes 12, no. 4: 394. https://doi.org/10.3390/membranes12040394





