Membrane Repairing Capability of Non-Small Cell Lung Cancer Cells Is Regulated by Drug Resistance and Epithelial-Mesenchymal-Transition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Development of Drug Resistant Cell Lines
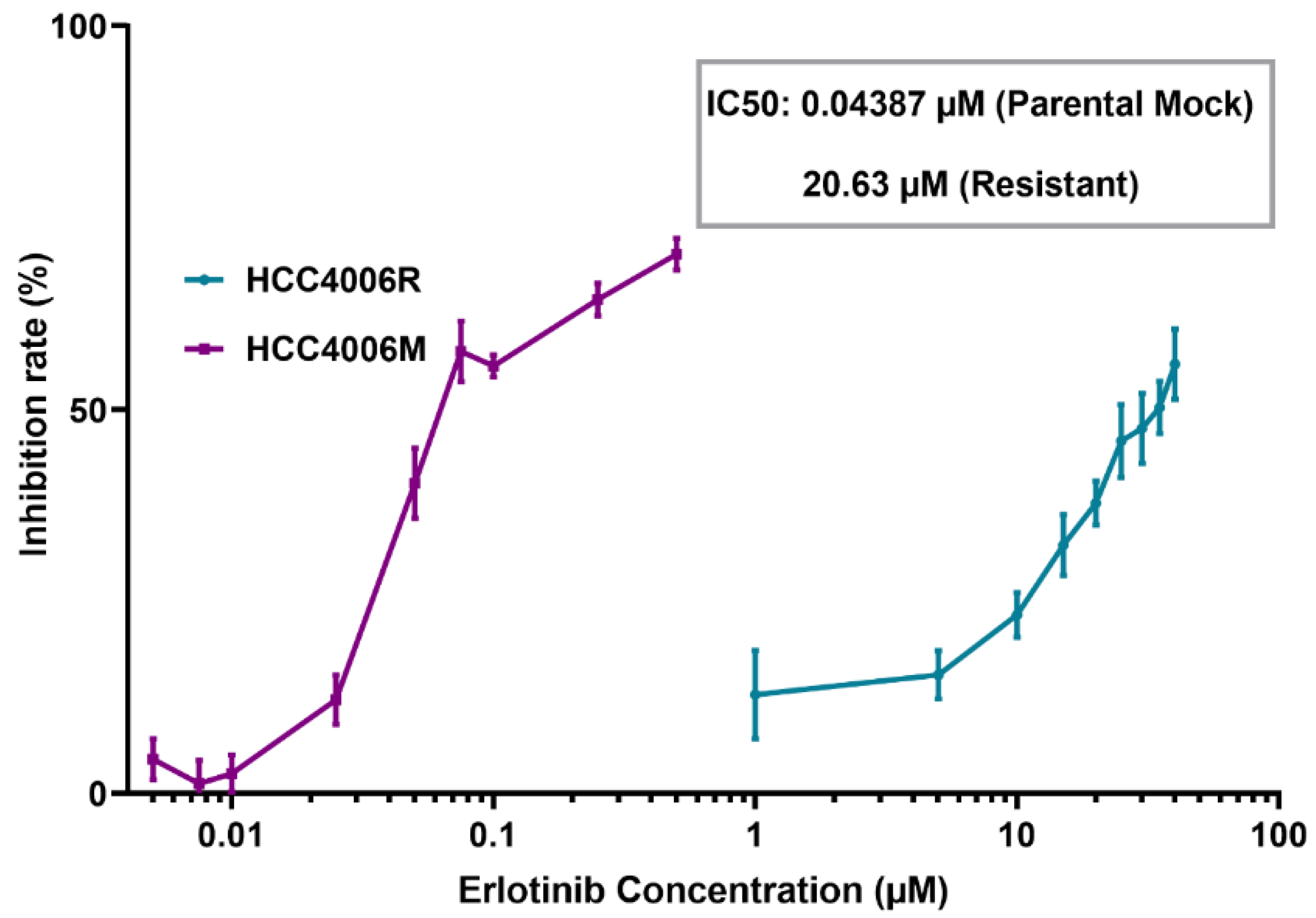
2.3. MTS Assay
2.4. Inducing Cell EMT
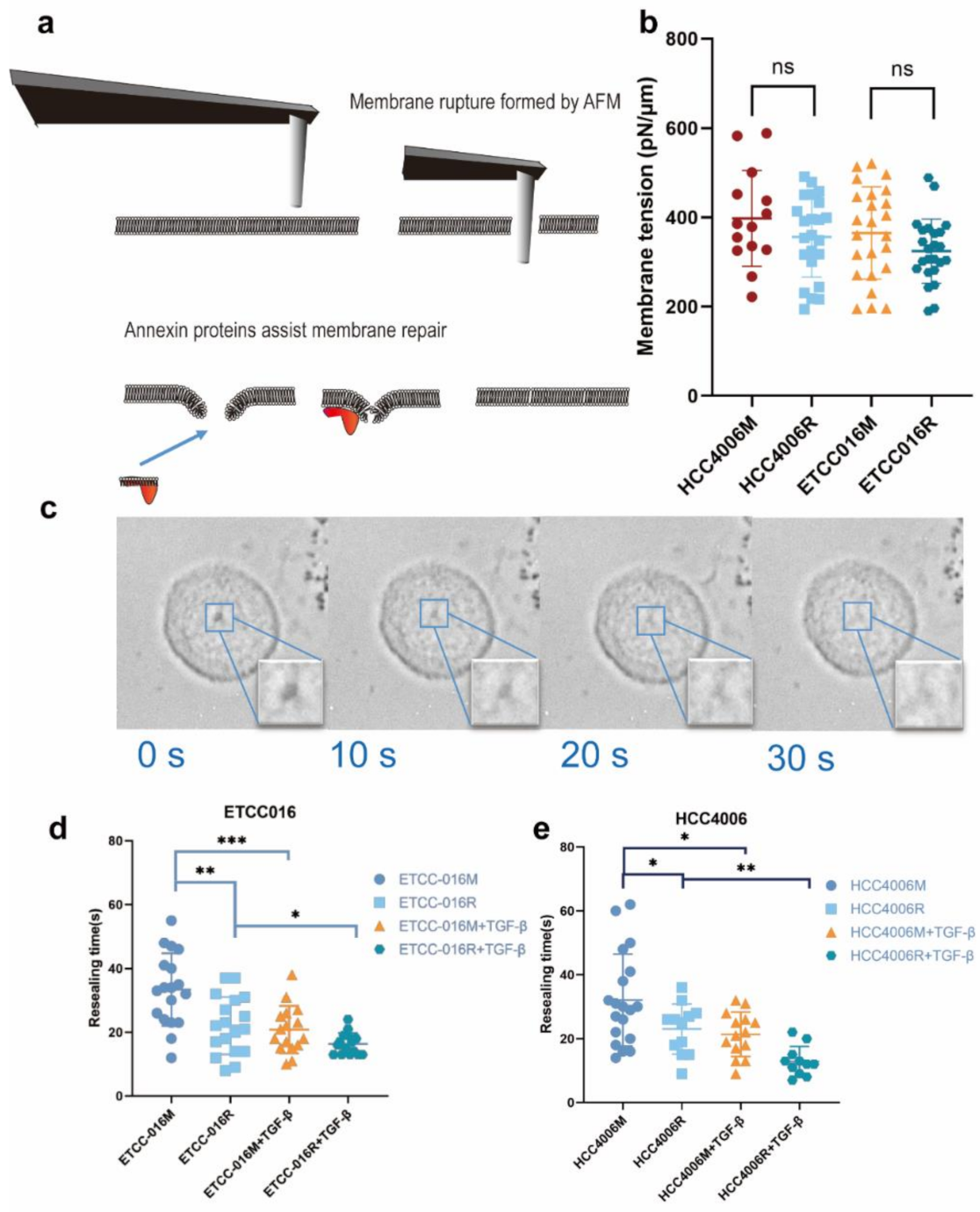
2.5. Membrane Puncturing
2.6. Measuring Membrane Tension
2.7. Immunofluorescent Staining
2.8. Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
3. Results
3.1. Fast Membrane Resealing Response Observed in NSCLC Cancer Cell Lines
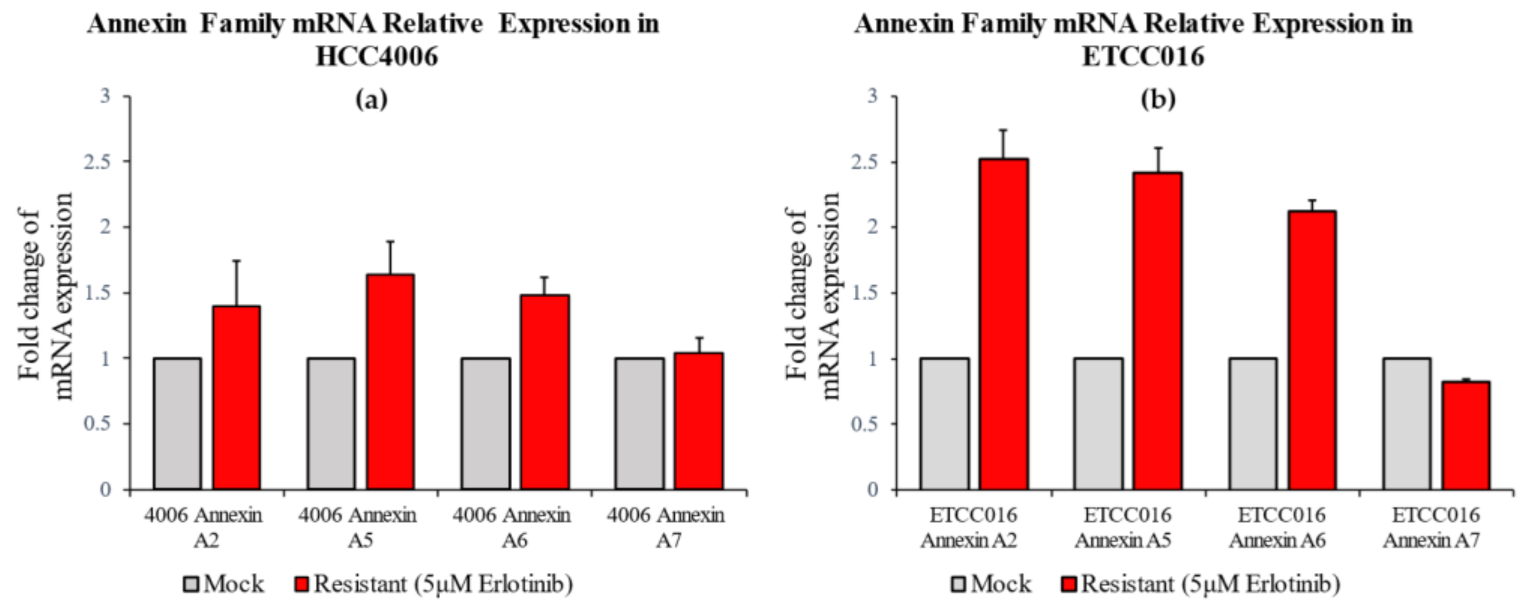
3.2. Membrane Resealing Speed Correlates with Cancer Cells’ Drug Resistivity
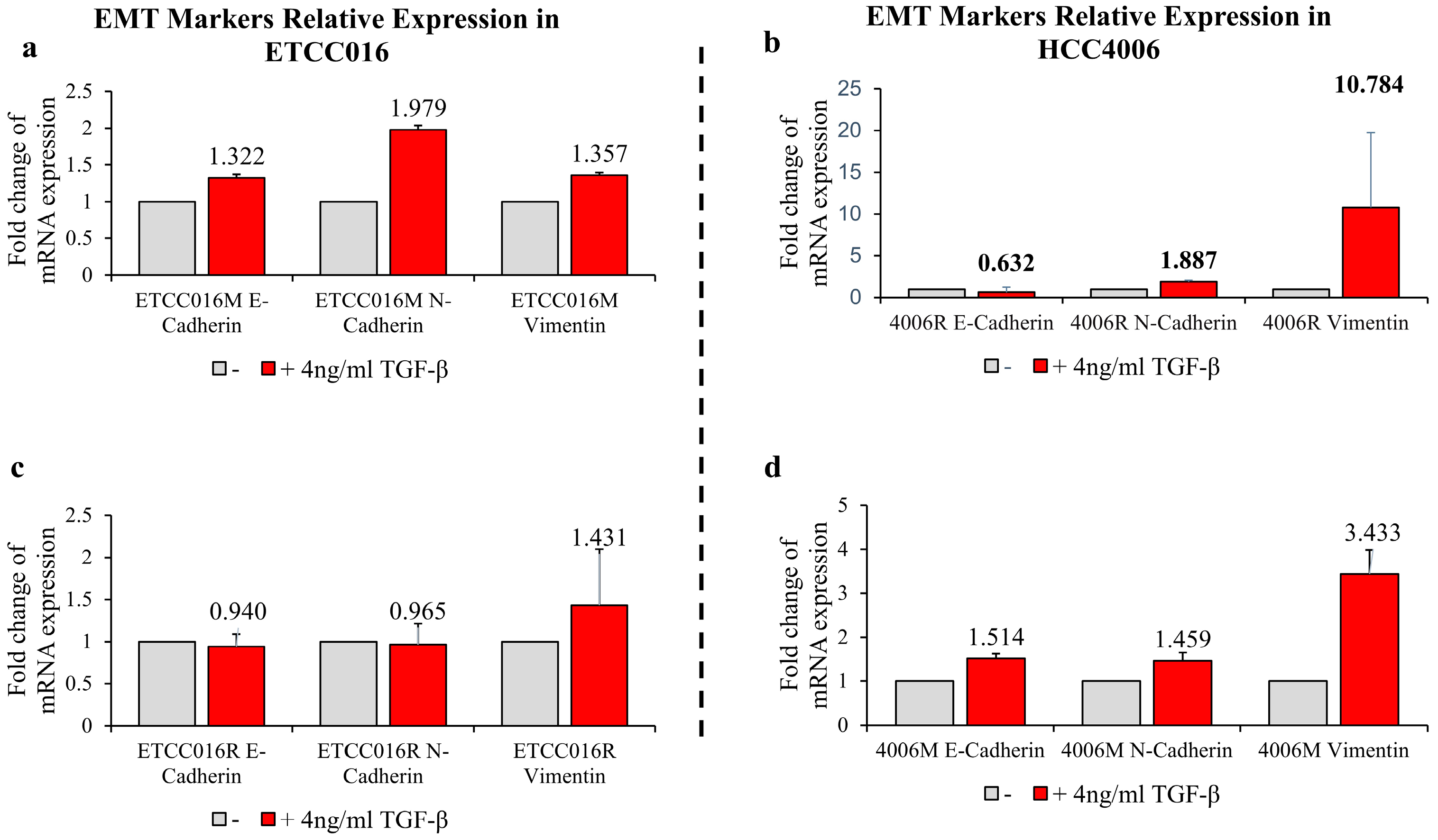
3.3. Epithelial-Mesenchymal-Transition Leads to Faster Membrane Resealing of Cancer Cells
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bagur, R.; Hajnóczky, G. Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, S.T.; McNeil, P.L. Membrane Repair: Mechanisms and Pathophysiology. Physiol. Rev. 2015, 95, 1205–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, C.; Maaser, K.; Daryab, N.; Zänker, K.S.; Bröcker, E.B.; Friedl, P. Release of cell fragments by invading melanoma cells. Eur. J. Cell Biol. 2004, 83, 709–715. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Lauritzen, S.P.; Scheffer, L.; Sakaguchi, M.; Bunkenborg, J.; Simon, S.M.; Kallunki, T.; Jäättelä, M.; Nylandsted, J. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat. Commun. 2014, 5, 3795. [Google Scholar] [CrossRef] [Green Version]
- Boye, T.L.; Jeppesen, J.C.; Maeda, K.; Pezeshkian, W.; Solovyeva, V.; Nylandsted, J.; Simonsen, A.C. Annexins induce curvature on free-edge membranes displaying distinct morphologies. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Boye, T.L.; Maeda, K.; Pezeshkian, W.; Sønder, S.L.; Haeger, S.C.; Gerke, V.; Simonsen, A.C.; Nylandsted, J. Annexin A4 and A6 induce membrane curvature and constriction during cell membrane repair. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sønder, S.L.; Boye, T.L.; Tölle, R.; Dengjel, J.; Maeda, K.; Jäättelä, M.; Simonsen, A.C.; Jaiswal, J.K.; Nylandsted, J. Annexin A7 is required for ESCRT III-mediated plasma membrane repair. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hakobyan, D.; Gerke, V.; Heuer, A. Modeling of annexin A2—Membrane interactions by molecular dynamics simulations. PLoS ONE 2017, 12, e0185440. [Google Scholar] [CrossRef] [Green Version]
- Bouter, A.; Gounou, C.; Bérat, R.; Tan, S.; Gallois, B.; Granier, T.; d’Estaintot, B.L.; Pöschl, E.; Brachvogel, B.; Brisson, A.R. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nat. Commun. 2011, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef]
- Gozen, I.; Dommersnes, P. Pore dynamics in lipid membranes. Eur. Phys. J. Spec. Top. 2014, 223, 1813–1829. [Google Scholar] [CrossRef]
- Hui, T.H.; Zhou, Z.L.; Fong, H.W.; Ngan, R.K.C.; Lee, T.Y.; Au, J.S.K.; Ngan, A.H.W.; Yip, T.T.C.; Lin, Y. Characterizing the malignancy and drug resistance of cancer cells from their membrane resealing response. Sci. Rep. 2016, 6, 26692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaltriti, M.; Baselga, J. The Epidermal Growth Factor Receptor Pathway: A Model for Targeted Therapy. Clin. Cancer Res. 2006, 12, 5268–5272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.T.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Yu, C.J.; Kim, S.W.; Lin, M.C.; Sriuranpong, V.; Tsai, C.M.; Lee, J.S.; Kang, J.H.; Chan, K.C.; Perez-Moreno, P.; et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol. 2016, 2, 305–312. [Google Scholar] [CrossRef]
- Kobayashi, S.; Boggon, T.J.; Dayaram, T.; Jänne, P.A.; Kocher, O.; Meyerson, M.; Johnson, B.E.; Eck, M.J.; Tenen, D.G.; Halmos, B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005, 352, 786–792. [Google Scholar] [CrossRef]
- Wu, S.G.; Liu, Y.N.; Tsai, M.F.; Chang, Y.L.; Yu, C.J.; Yang, P.C.; Yang, J.C.; Wen, Y.F.; Shih, J.Y. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget 2016, 7, 12404–12413. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Han, Y.; Chen, Y.; Xie, J.T. Field-induced electroconformational damages in cell membrane proteins: A new mechanism involved in electrical injury. Bioelectrochemistry Bioenerg. 1998, 47, 237–245. [Google Scholar] [CrossRef]
- Rosenbluth, M.J.; Lam, W.A.; Fletcher, D.A. Force microscopy of nonadherent cells: A comparison of leukemia cell deformability. Biophys. J. 2006, 90, 2994–3003. [Google Scholar] [CrossRef] [Green Version]
- Hui, T.H.; Zhou, Z.L.; Qian, J.; Lin, Y.; Ngan, A.H.; Gao, H. Volumetric deformation of live cells induced by pressure-activated cross-membrane ion transport. Phys. Rev. Lett. 2014, 113, 118101. [Google Scholar] [CrossRef] [Green Version]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Pigault, C.; Follenius-Wund, A.; Schmutz, M.; Freyssinet, J.-M.; Brisson, A. Formation of two-dimensional arrays of annexin V on phosphatidylserine-containing liposomes. J. Mol. Biol. 1994, 236, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Creutz, C.; Pazoles, C.; Pollard, H. Identification and purification of an adrenal medulla (synexin) that causes calcium-dependent aggregation of isolated chromamn granules. J. Biol. Chem. 1978, 253, 2858–2866. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, M.; Stephens, M.A.; Pathak, H.; Rangarajan, A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011, 2, 179. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ma, D.; Jing, X.; Wang, B.; Yang, W.; Qiu, W. Overexpression of ANXA2 predicts adverse outcomes of patients with malignant tumors: A systematic review and meta-analysis. Med. Oncol. 2014, 32, 392. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; Zhang, Z.; Gu, Y.; Qiu, H.; He, Z. Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 123. [Google Scholar] [CrossRef] [Green Version]
- Namee, N.M.; O’Driscoll, L. Extracellular vesicles and anti-cancer drug resistance. Biochim. Et Biophys. Acta—Rev. Cancer 2018, 1870, 123–136. [Google Scholar] [CrossRef]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Wang, H.; Fang, C.; Yang, Y.; Xia, X.; Yang, B.; Lin, Y.; Li, G.; Bian, L. Microscopic local stiffening in supramolecular hydrogel network expedites stem cell mechanosensing in 3D and bone regeneration. Mater. Horiz. 2021, 8, 1722–1734. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Shenoy, V.; Hu, B.; Bai, L. A microscopic formulation for the actin-driven motion of Listeria in curved paths. Biophys. J. 2010, 99, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef] [Green Version]
- Hui, T.H.; Kwan, K.W.; Chun Yip, T.T.; Fong, H.W.; Ngan, K.C.; Yu, M.; Yao, S.; Wan Ngan, A.H.; Lin, Y. Regulating the Membrane Transport Activity and Death of Cells via Electroosmotic Manipulation. Biophys. J. 2016, 110, 2769–2778. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Sun, S.X. Cellular pressure and volume regulation and implications for cell mechanics. Biophys. J. 2013, 105, 609–619. [Google Scholar] [CrossRef] [Green Version]

| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| E-Cadherin | TGCTGATGCCCCCAATACCCCA | GTGATTTCCTGGCCCACGCCAA |
| N-Cadherin | TGACTCCAACGGGGACTGCACA | AGCTCAAGGACCCAGCAGTGGA |
| Vimentin | AACCAACGACAAAGCCCGCGTC | TTCCGGTTGGCAGCCTCAGAGA |
| Annexin A2 | TCGGACACATCTGGTGACTTCC | CCTCTTCACTCCAGCGTCATAG |
| Annexin A5 | GTGGCTCTGATGAAACCCTCTC | GGCTCTCAGTTCTTCAGGTGTC |
| Annexin A6 | GACTGACGAAGACACAATCATCG | CAGAATCAGCCTTGCCAGGTCT |
| Annexin A7 | CGGATTGTGGTCACTCGAAGTG | CGGTAATCTCCACTCGTGTCAC |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Yang, H.; Au, D.W.-Y.; Lai, S.P.-H.; Lin, Y.; Cho, W.C.-S. Membrane Repairing Capability of Non-Small Cell Lung Cancer Cells Is Regulated by Drug Resistance and Epithelial-Mesenchymal-Transition. Membranes 2022, 12, 428. https://doi.org/10.3390/membranes12040428
Xia X, Yang H, Au DW-Y, Lai SP-H, Lin Y, Cho WC-S. Membrane Repairing Capability of Non-Small Cell Lung Cancer Cells Is Regulated by Drug Resistance and Epithelial-Mesenchymal-Transition. Membranes. 2022; 12(4):428. https://doi.org/10.3390/membranes12040428
Chicago/Turabian StyleXia, Xingyu, Hanbo Yang, Dennis Wai-Yin Au, Syrus Pak-Hei Lai, Yuan Lin, and William Chi-Shing Cho. 2022. "Membrane Repairing Capability of Non-Small Cell Lung Cancer Cells Is Regulated by Drug Resistance and Epithelial-Mesenchymal-Transition" Membranes 12, no. 4: 428. https://doi.org/10.3390/membranes12040428
APA StyleXia, X., Yang, H., Au, D. W.-Y., Lai, S. P.-H., Lin, Y., & Cho, W. C.-S. (2022). Membrane Repairing Capability of Non-Small Cell Lung Cancer Cells Is Regulated by Drug Resistance and Epithelial-Mesenchymal-Transition. Membranes, 12(4), 428. https://doi.org/10.3390/membranes12040428






