Construct α-FeOOH-Reduced Graphene Oxide Aerogel as a Carrier for Glucose Oxidase Electrode
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Apparatus
2.2. Preparation of FeOOH-GA
2.3. Preparation of Working Electrodes
2.4. Electrochemical Measurements
Material Characterization
3. Results and Discussion
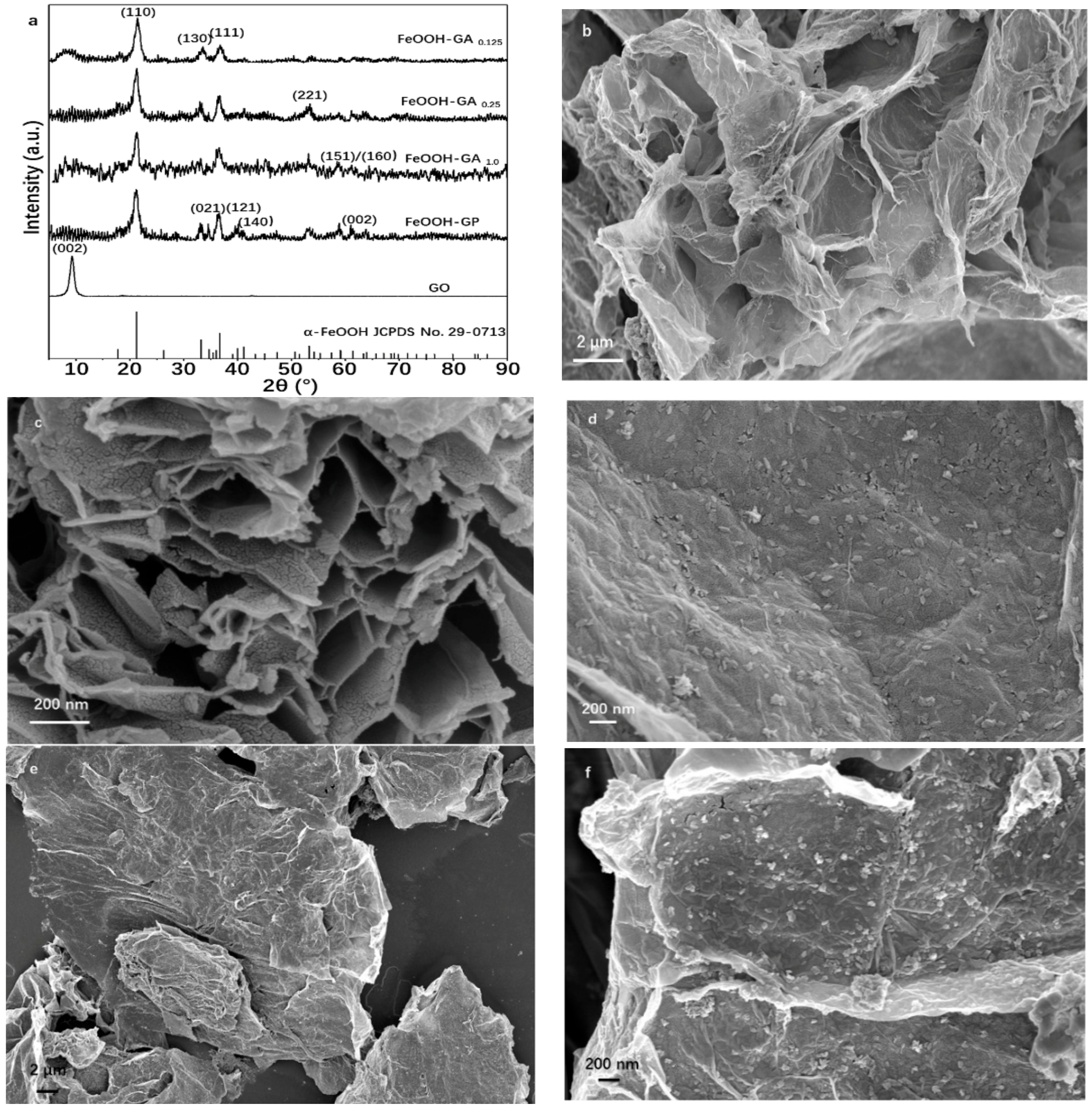
3.1. Structural Characterization
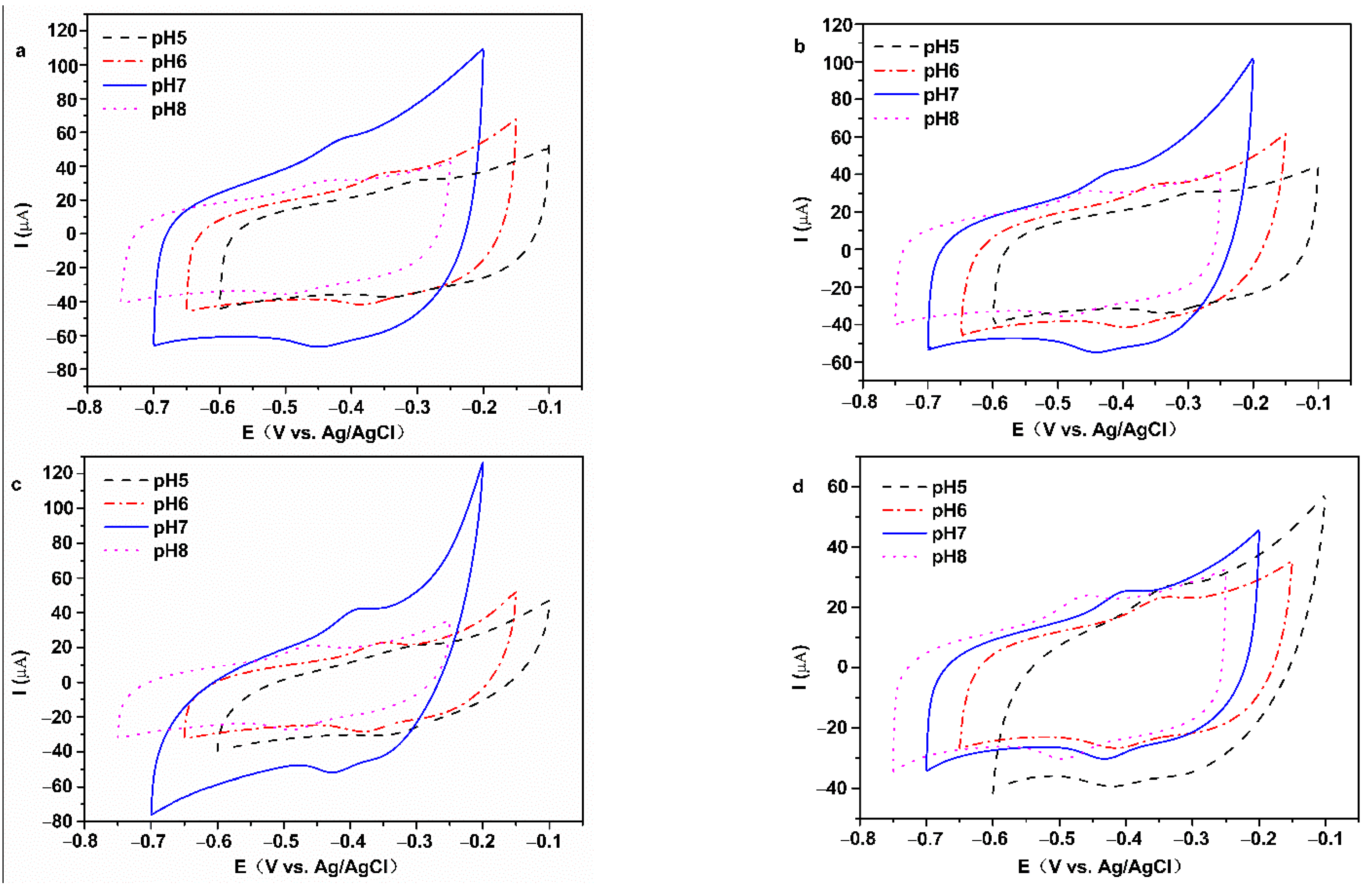
3.2. Electrochemical Properties
3.3. Electrocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, F.; Cao, X.; Pankratov, D.; Zhang, J.; Chi, Q. Graphene Bioelectronics; Tiwari, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; p. 219. [Google Scholar]
- Shen, L.; Ying, J.; Ren, L.; Yao, Y.; Lu, Y.; Dong, Y.; Tian, G.; Yang, X.-Y.; Su, B.-L. 3D Graphene-based macro-mesoporous frameworks as enzymatic electrodes. J. Phys. Chem. Solids 2019, 130, 1–5. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gai, P.; Zhang, J.; Zhu, J.-J. Design of an enzymatic biofuel cell with large power output. J. Mater. Chem. A 2015, 3, 11511. [Google Scholar] [CrossRef]
- Yin, S.; Jin, Z.; Miyake, T. Wearable high-powered biofuel cells using enzyme/carbon nanotube composite fibers on textile cloth. Biosens. Bioelectron. 2019, 141, 111471. [Google Scholar] [CrossRef]
- Campuzano, S.; Yanez-Sedeno, P.; Pingarron, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Cargill, A.A.; Medintz, I.L.; Claussen, J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr. Opin. Biotechnol. 2015, 34, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Zhao, J.; Wang, X.; Shi, J.; Zhang, S.; Jiang, Z. Monolithic biocatalytic systems with enhanced stabilities constructed through biomimetic silicification-induced enzyme immobilization on rGO/FeOOH hydrogel. Biochem. Eng. J. 2017, 117, 52–61. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, R.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y. Well-oriented bioarchitecture for immobilization of chloroperoxidase on graphene oxide nanosheets by site-specific interactions and its catalytic performance. J. Mater. Sci. 2017, 52, 10001–10012. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose Oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ma, Y.; Li, J.; Fan, Q.; Huang, W. Self-Assembly of Reduced Graphene Oxide into Three-Dimensional Architecture by Divalent Ion Linkage. J. Phys. Chem. C 2010, 114, 22462–22465. [Google Scholar] [CrossRef]
- Gao, H.; Duan, H. 2D and 3D graphene materials: Preparation and bioelectrochemical applications. Biosens. Bioelectron. 2015, 65, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, X.; Shi, J.; Wang, X.; Zhang, S.; Han, P.; Jiang, Z. In situ synthesized rGO-Fe3O4 nanocomposites as enzyme immobilization support for achieving high activity recovery and easy recycling. Biochem. Eng. J. 2016, 105, 273–280. [Google Scholar] [CrossRef]
- Lin, J.; Wen, Q.; Chen, S.; Le, X.; Zhou, X.; Huang, L. Synthesis of amine-functionalized Fe3O4@C nanoparticles for laccase immobilization. Int. J. Biol. Macromol. 2017, 96, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Huang, W.; Pan, S.; Li, Y.; Yu, L.; He, D. Covalent immobilization and characterization of penicillin G acylase on magnetic Fe2O3/Fe3O4 heterostructure nanoparticles prepared via a novel solution combustion and gel calcination process. Int. J. Biol. Macromol. 2020, 162, 1587–1596. [Google Scholar] [CrossRef]
- Tavares, T.S.; Rocha, E.P.; Nogueira, F.G.E.; Torres, J.A.; Silva, M.C.; Kuca, K.; Ramalho, T.C. Delta-FeOOH as Support for Immobilization Peroxidase: Optimization via a Chemometric Approach. Molecules 2020, 25, 259. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S., Jr.; Offenman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Graphene Nanosheets and Graphite Oxide as Promising Adsorbents for Removal of Organic Contaminants from Aqueous Solution. J. Environ. Qual. 2013, 42, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced Self-Assembly Process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef]
- Aunkora, M.T.H.; Mahbubulb, I.M.; Saidurb, R.; Metselaar, H.S.C. The green reduction of graphene oxide. RSC Adv. 2016, 6, 27807–27828. [Google Scholar] [CrossRef]
- Huang, N.M.; Lim, H.N.; Chia, C.H.; Yarmo, M.A.; Muhamad, M.R. Simple room-temperature preparation of high-yield large-area graphene oxide. Int. J. Nanomed. 2011, 6, 3443–3448. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Ding, R.; Zhang, C.; Lv, B.; Wang, Y.; Chen, C.; Wang, X.; Xu, J.; Yang, Y.; Li, Y. Facile synthesis of self-assembled ultrathin α-FeOOH nanorod/graphene oxide composites for supercapacitors. J. Colloid Interf. Sci. 2017, 504, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, R.; Yan, Z.; Liu, X.; Chen, R.; Ma, Y.; Li, X.; Fan, Q.; Huang, W. Preparation of Graphene/Polypyrrole Composite Film via Electrodeposition for Supercapacitors. IEEE T. Nanotechnology 2012, 11, 1080–1086. [Google Scholar]
- Liang, C.; Zhao, W.; Song, Z.; Xing, S. Influence of precursor pH on the structure and photo-Fenton performance of Fe/hydrochar. RSC Adv. 2017, 7, 35257–35264. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Liang, H.; Jia, H.; Chen, S.; Guo, J.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. In-situ encapsulate Fe3O4 nanosheet arrays with graphene layers as anode for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 24594–24601. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Q.; Kong, Y.; Shen, C.; Hao, H.; Dionysioud, D.D.; Shi, B. Enhanced antibiotic removal through a dualreaction-center Fenton-like process in 3D graphene based hydrogels. Environ. Sci. Nano 2019, 6, 388–398. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhao, Y.; Dionysiou, D.D. Aligned α-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst. Appl. Catal. B 2017, 213, 74–86. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, L.; Cai, M.; Li, Y.; Jiang, K.; Zhang, X.; Shen, P.K. Preparation and charaterization of Pt/functionalized graphene and its electrocatalysis for methanol oxidation. Electrochim. Acta 2013, 111, 275–283. [Google Scholar] [CrossRef]
- Liu, S.; Ju, H. Reagentless glucose biosensor based on direct electron transfer of glucose oxidase immobilized on colloidal gold modified carbon paste electrode. Biosens. Bioelectron. 2003, 19, 177–183. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, J.; Chen, P.; Liu, Y.; Hou, H.; You, T. Simultaneous determination of catechol and hydroquinone using electrospun carbon nanofibers modified electrode. Sensor Actuat. B Chem. 2012, 163, 179–185. [Google Scholar] [CrossRef]
- Kang, Z.; Jiao, K.; Yu, C.; Dong, J.; Peng, R.; Hu, Z.; Jiao, S. Direct electrochemistry and bioelectrocatalysis of glucose oxidase in CS/CNC film and its application in glucose biosensing and biofuel cells. RSC Adv. 2017, 7, 4572–4579. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yan, S.; Lin, Z.; Shi, Y.; Xu, X.; Fu, L.; Jiang, J. Fabrication of Graphene Aerogel/Platinum Nanoparticle Hybrids for the Direct Electrochemical Analysis of Glucose. J. Nanosci. Nanotechnol. 2016, 16, 6895–6902. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamental and Applications, 2nd ed.; John Wiley& Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the case of diffusionless electrochemical ayatems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Campbell, A.S.; Jeong, Y.J.; Geier, S.M.; Koepsel, R.R.; Russell, A.J.; Islam, M.F. Membrane/Mediator-Free Rechargeable Enzymatic Biofuel Cell Utilizing Graphene/Single-Wall Carbon Nanotube Cogel Electrodes. ACS Appl. Mater. Inter. 2015, 7, 4056–4065. [Google Scholar] [CrossRef]
- Laviron, E. Surface linear potential sweep voltammetry: Equation of the peaks for a reversible reaction when interactions between the adsorbed molecules are taken into account. J. Electroanal. Chem. Inter. Electrochem. 1974, 52, 395–402. [Google Scholar] [CrossRef]
- Zaib, M.; Athar, M.M. Electrochemical Evaluation of Phanerocheaete Chrysosporium Based Carbon Paste Electrode with Potassium Ferricyanide Redox System. Int. J. Electrochem. Sci. 2015, 10, 6690–6702. [Google Scholar]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Glassy carbon electrode modified with horse radish peroxidase/organic nucleophilic-functionalized carbon nanotube composite for enhanced electrocatalytic oxidation and efficient voltammetric sensing of levodopa. Mat. Sci. Eng. C 2016, 58, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Navaee, A.; Salimi, A. Graphene-supported pyrene-functionalizedamino-carbonnanotube: A novel hybrid architecture of laccase immobilization as effective bioelectrocatalyst for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 7623–7630. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Hou, C.; Zhang, X. Construct α-FeOOH-Reduced Graphene Oxide Aerogel as a Carrier for Glucose Oxidase Electrode. Membranes 2022, 12, 447. https://doi.org/10.3390/membranes12050447
Yao Y, Hou C, Zhang X. Construct α-FeOOH-Reduced Graphene Oxide Aerogel as a Carrier for Glucose Oxidase Electrode. Membranes. 2022; 12(5):447. https://doi.org/10.3390/membranes12050447
Chicago/Turabian StyleYao, Yue, Changyu Hou, and Xin Zhang. 2022. "Construct α-FeOOH-Reduced Graphene Oxide Aerogel as a Carrier for Glucose Oxidase Electrode" Membranes 12, no. 5: 447. https://doi.org/10.3390/membranes12050447
APA StyleYao, Y., Hou, C., & Zhang, X. (2022). Construct α-FeOOH-Reduced Graphene Oxide Aerogel as a Carrier for Glucose Oxidase Electrode. Membranes, 12(5), 447. https://doi.org/10.3390/membranes12050447




