Clinical Evaluation of Micro-Embolic Activity with Unexpected Predisposing Factors and Performance of Horizon AF PLUS during Cardiopulmonary Bypass
Abstract
:1. Introduction
2. Materials and Methods
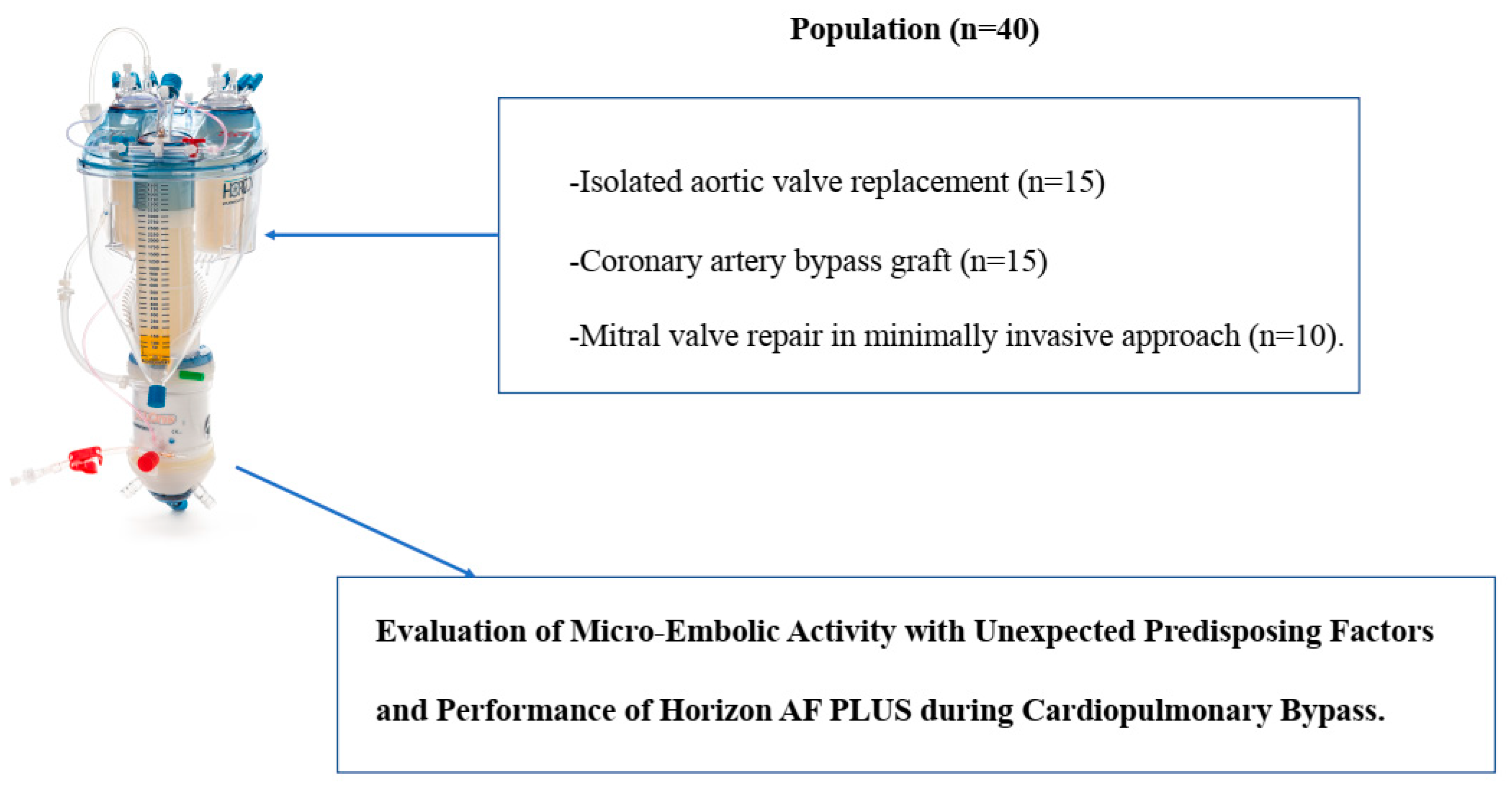
2.1. Population and Study Design
2.2. Anesthetics and Surgical Procedures
2.3. CPB Setting
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Condello, I.; Nasso, G.; Fiore, F.; Azzolina, S.; Bonifazi, R.; Di Bari, N.; Bartolomucci, F.; Massaro, F.; Speziale, G. Fibonacci’s golden ratio—An innovative approach to the design and management of extra-corporeal circulation. Surg. Technol. Int. 2019, 34, 340–350. [Google Scholar] [PubMed]
- Condello, I.; Santarpino, G.; Nasso, G.; Moscarelli, M.; Fiore, F.; Speziale, G. Associations between oxygen delivery and cardiac index with hyperlactatemia during cardiopulmonary bypass. JTCVS Tech. 2020, 2, 92–99. [Google Scholar] [CrossRef] [PubMed]
- De Somer, F.M.; Vetrano, M.R.; Van Beeck, J.P.; Van Nooten, G.J. Extracorporeal bubbles: A word of caution. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 995–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Keshavamurthy, S.; Abraham, J.G.; Toyoda, Y. Massive air embolism caused by a central venous catheter during extracorporeal membrane oxygenation. J. Extra-Corpor. Technol. 2019, 51, 9–11. [Google Scholar] [PubMed]
- De Somer, F. Impact of oxygenator characteristics on its capability to remove gaseous microemboli. J. Extra-Corpor. Technol. 2007, 39, 271–273. [Google Scholar] [PubMed]
- Guan, Y.; Palanzo, D.; Kunselman, A.; Ündar, A. Evaluation of membrane oxygenators and reservoirs in terms of capturing gaseous microemboli and pressure drops. Artif. Organs 2009, 33, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J. Arterial and venous air emboli in health care. J. Extra-Corpor. Technol. 2021, 53, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.L.; Borger, M.A.; Weisel, R.D.; Fedorko, L.; Feindel, C.M. Cerebral microemboli during cardiopulmonary bypass: Increased emboli during perfusionist interventions. Ann. Thorac. Surg. 1999, 68, 89–93. [Google Scholar] [CrossRef]
- Borger, M.A.; Peniston, C.M.; Weisel, R.D.; Vasiliou, M.; Green, R.; Feindel, C.M. Neuropsychologic impairment after coronary bypass surgery: Effect of gaseous microemboli during perfusionist interventions. J. Thorac. Cardiovasc. Surg. 2001, 121, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, J.B. Arterial line filters ranked for gaseous micro-emboli separation performance: An in vitro study. J. Extra-Corpor. Technol. 2008, 40, 21–26. [Google Scholar] [PubMed]
- Sutton, R.G.; Riley, J.B.; Merrill, J.H. Comparison of gaseous microemboli counts in arterial, simultaneous and venous heat exchange with a hollow fiber membrane oxygenator. J. Extra-Corpor. Technol. 1994, 26, 56. [Google Scholar] [PubMed]
- Svenarud, P.; Persson, M.; van der Linden, J. Effect of CO2 insufflation on the number and behavior of air microemboli in open-heart surgery: A randomized clinical trial. Circulation 2004, 109, 1127–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uplaonkar, D.S.; Appa, V.; Patil, N. An efficient discrete wavelet transform based partial hadamard feature extraction and hybrid neural network based monarch butterfly optimization for liver tumor classification. Eng. Sci. 2021, 16, 354–365. [Google Scholar] [CrossRef]
- Musunuri, B.; Shetty, S.; Shetty, D.K.; Vanahalli, M.K.; Pradhan, A.; Naik, N.; Paul, R. Acute-on-chronic liver failure mortality prediction using an artificial neural network. Eng. Sci. 2021, 15, 187–196. [Google Scholar] [CrossRef]
- Cenitta, D.; Arjunan, R.V.; Prema, K.V. Ischemic heart disease multiple imputation technique using machine learning algorithm. Eng. Sci. 2022. [Google Scholar] [CrossRef]





| Characteristic | Conventional Cardiac Surgery (n = 30) | Minimally Invasive Mitral Valve Repair (n = 10) |
|---|---|---|
| Mean age (y) | 69.9 | 72.5 |
| Male sex | 15 (50) | 6 (60) |
| Mean body surface area (m2) | 1.73 | 1.78 |
| Mean left ventricular ejection fraction (%) | 45 | 50 |
| Median NYHA functional class | 2 | 2 |
| EuroSCORE II (mean) | 2.1 | 2.4 |
| Pre-CPB hematocrit (%) | 34.4 ± 1.2 | 34.4 ± 1.7 |
| Pre-CPB Hb (g/dL) | 10.4 ± 1.1 | 10.8 ± 1.2 |
| No. of chronic obstructive pulmonary disease cases (mean) | 27 | 28 |
| Creatinine (mg/dL) | 1.11 ± 0.4 | 1.09 ± 0.5 |
| Obstructive coronary artery disease (%) | 15 | 0 |
| Parameter | Conventional Cardiac Surgery (n = 30) | Minimally Invasive Mitral Valve Repair (n = 10) | p-Value |
|---|---|---|---|
| CPB time (min) | 104 ± 11.1 | 102 ± 9.34 | 0.92 |
| Aortic cross-clamp time (min) | 78 ± 5 | 44 ± 6 | 0.75 |
| Nadir temperature (°C) during CPB | 34.9 ± 1.1 | 34.7 ± 2.1 | 0.75 |
| Nadir hemoglobin value (mg/dL) during CPB | 8.73 ± 1.53 | 8.6 ± 1.25 | 0.88 |
| Nadir hematocrit (%) during CPB | 26.6 ± 3.4 | 26.3 ± 3.9 | 0.89 |
| Nadir DO2i (mL/min/m2) during CPB | 294 ± 29 | 289 ± 14 | 0.99 |
| O2ERi (%) during CPB | 23 ± 1 | 23 ± 5 | 0.89 |
| Nadir CI (L/min/m2) during CPB | 2.5 ± 0.2 | 2.5 ± 0.1 | 0.91 |
| Nadir SvO2 (%) | 81 ± 2 | 80 ± 5 | 0.93 |
| Unexpected Predisposing Factors for Micro-Embolic Activity n = 18 (45%) | Conventional Cardiac Surgery (n = 30) | Minimally Invasive Mitral Valve Repair (n = 10) | Phenomena Duration (min) Mean Values |
|---|---|---|---|
| Accidental air embolism from venous line (n = 4) | 1 | 3 | 3 ± 1 |
| Low levels in venous reservoir (<250 mL) in association with vacuum-assisted venous drainage (≥40 mmHg) (n = 10) | 8 | 2 | 8 ± 5 |
| Combination of two excessive suctions of aspirators (>2 L/min, >77 RPM) with low levels in venous reservoir (<250 mL) in association with vacuum-assisted venous drainage (≥40 mmHg) (n = 4) | 3 | 1 | 10 ± 2 |
| Absence of predisposing factors for micro-embolic activity n = 22 (55%) | 18 | 4 | 0 |
| Unexpected Predisposing Factors for Micro-Embolic Activity on Horizon AF PLUS (n = 18) | Venous Inlet Line of Venous Reservoir | Outlet Line of Venous Reservoir | Arterial Line |
|---|---|---|---|
| Accidental Air Embolism from venous line (n = 4) | |||
| Gaseous micro-emboli numbers | 349.299 ± 28 | 129.321 ± 60 | 1.235 ± 73 |
| Diameter >500 μm (%) | 45 | 3.5 | 0 |
| Volume (μL) | 79.9 ± 2 | 1.49 ± 5 | 0.89 ± 1 |
| Low levels in venous reservoir (<250 mL) in association with vacuum-assisted venous drainage (≥40 mmHg) (n = 10) | |||
| Gaseous micro-emboli numbers | 199.111 ± 76 | 35.720 ± 40 | 899 ± 47 |
| Diameter > 500 μm (%) | 26 | 1.6 | 0 |
| Volume (μL) | 19.2 ± 2 | 1.19 ± 6 | 0.57 ± 1 |
| Combination of two excessive suctions of aspirators (>2 L/min, >77 RPM) with low levels in venous reservoir (<250 mL) in association with vacuum-assisted venous drainage (≥40 mmHg) (n = 4) | |||
| Gaseous micro-emboli numbers | 230.889 ± 100 | 39.119 ± 25 | 1.001 ± 37 |
| Diameter >500 μm (%) | 29 | 1.9 | 0 |
| Volume (μL) | 23.8 ± 2 | 1.25 ± 3 | 0.69 ± 2 |
| Without predisposing factors for micro-embolic activity on Horizon AF PLUS (n = 22) | |||
| Gaseous micro-emboli numbers | 99.000 ± 35 | 18.000 ± 65 | 559 ± 56 |
| Diameter >500 μm (%) | 15 | 1.2 | 0 |
| Volume (μL) | 12.2 ± 3 | 0.97 ± 5 | 0.59 |
| Unexpected Predisposing Factors for Micro-Embolic Activity on Horizon AF (n = 18) | Without Predisposing Factors for Micro-Embolic Activity on Horizon AF (n = 22) | p-Value | |
|---|---|---|---|
| Gaseous micro-emboli volume (μL) in arterial line at the end of CPB | 0.71 ± 0.2 | 0.59 ± 0.1 | 0.67 |
| PaO2 mmHg | PaCO2 mmHg | |
|---|---|---|
| Unexpected predisposing factors for micro-embolic activity on Horizon AF PLUS (n = 18) | 260 ± 25 | 38 ± 4 |
| Without predisposing factors for micro-embolic activity on Horizon AF PLUS (n = 22) | 260 ± 23 | 38 ± 6 |
| Nasopharyngeal Temperature | From 36 °C to 34 °C HCD Setting 34 °C (min) | From 34 °C to 36 °C HCD Setting 36.5 °C (min) |
|---|---|---|
| Unexpected predisposing factors for micro-embolic activity on Horizon AF PLUS (n = 18) | 5.5 ± 2 | 6.5 ± 1 |
| Without predisposing factors for micro-embolic activity on Horizon AF PLUS (n = 22) | 5.5 ± 1 | 6.5 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condello, I.; Lorusso, R.; Santarpino, G.; Fiore, F.; Nasso, G.; Speziale, G. Clinical Evaluation of Micro-Embolic Activity with Unexpected Predisposing Factors and Performance of Horizon AF PLUS during Cardiopulmonary Bypass. Membranes 2022, 12, 465. https://doi.org/10.3390/membranes12050465
Condello I, Lorusso R, Santarpino G, Fiore F, Nasso G, Speziale G. Clinical Evaluation of Micro-Embolic Activity with Unexpected Predisposing Factors and Performance of Horizon AF PLUS during Cardiopulmonary Bypass. Membranes. 2022; 12(5):465. https://doi.org/10.3390/membranes12050465
Chicago/Turabian StyleCondello, Ignazio, Roberto Lorusso, Giuseppe Santarpino, Flavio Fiore, Giuseppe Nasso, and Giuseppe Speziale. 2022. "Clinical Evaluation of Micro-Embolic Activity with Unexpected Predisposing Factors and Performance of Horizon AF PLUS during Cardiopulmonary Bypass" Membranes 12, no. 5: 465. https://doi.org/10.3390/membranes12050465






