Recovery of Nutrients from Residual Streams Using Ion-Exchange Membranes: Current State, Bottlenecks, Fundamentals and Innovations
Abstract
:1. Introduction: Nutrient Sources, Environmental Impact
2. Conventional Methods of the Residual Streams Processing
2.1. Classification of Nutrient-Containing Wastes
2.2. Stabilization of Wastewater and Transformation of Nutrients
2.3. Phases Separation
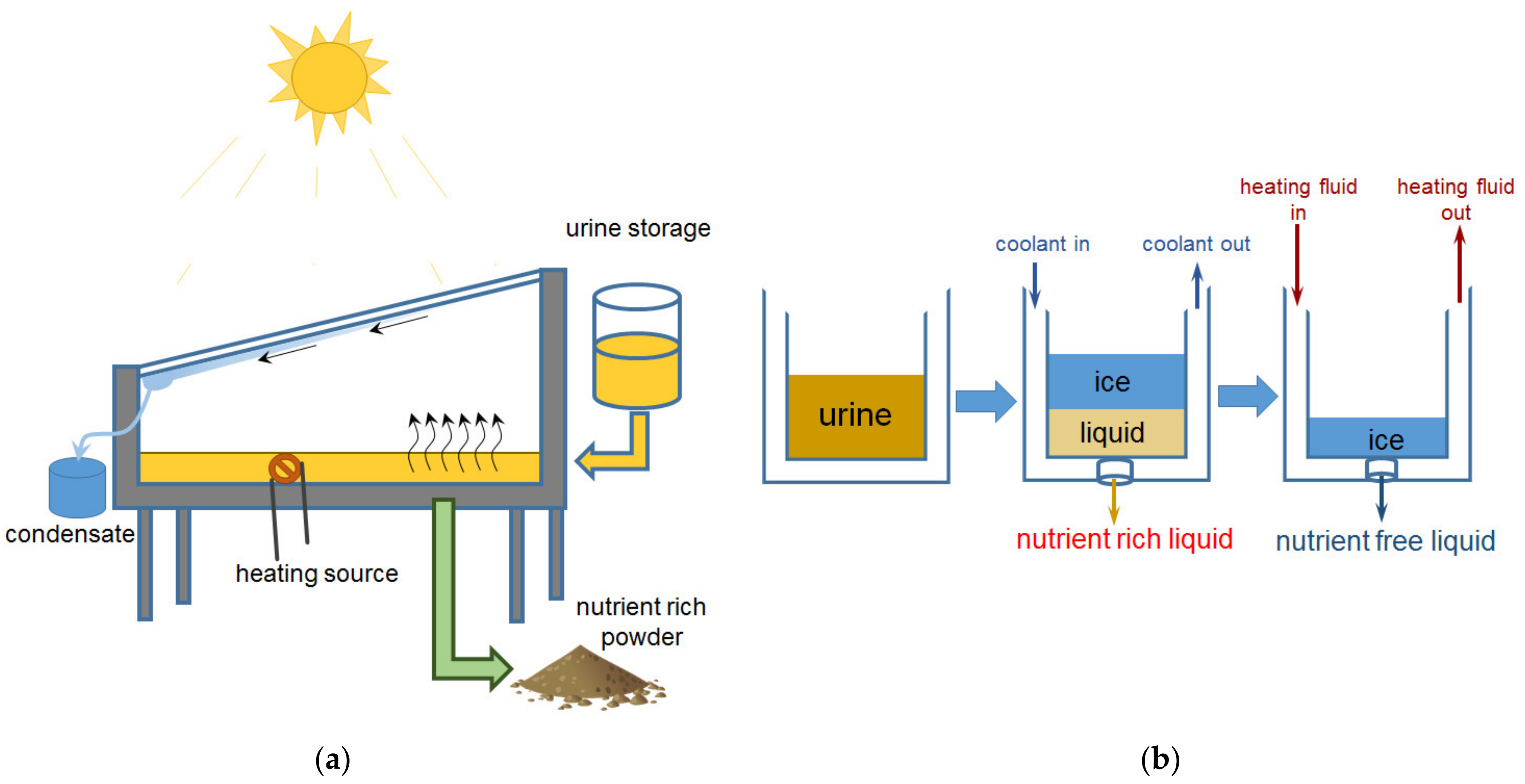
2.4. Nutrient Concentration
2.5. Fractionation and Selective Recovery of Nutrients
3. Modern Trends in Nutrients Recovery
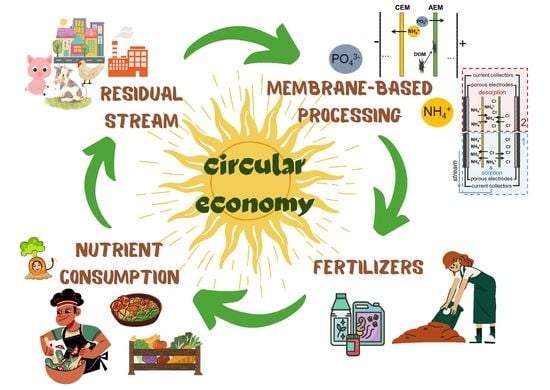
3.1. The Place of Membrane Processes in the Circular Economy of Nutrients
3.2. Main Types of Membranes
3.3. Membrane Bioreactors and Membrane Microbiological Fuel Cells
3.4. Recovery of Volatile Fractions (NH3) Using Gas Separation Membranes
3.5. Forward Osmosis and Baromembrane Processes
3.6. Electrochemically Induced Precipitation (NH4+, PO43−) and NH4+ Transformation to N2 or Nitrates
3.7. Capacitive Deionization and Electrodialysis
3.7.1. Nutrients Recovery and Concentration
| Method | Experiment Details | Feed Solution | Results Achieved | Bottlenecks | The Objective | Ref. |
|---|---|---|---|---|---|---|
| MFCDI | Three-chamber reactor consisting of cathode, anode, two AEM (TWEDA-I), and CEM (TWEDC-I) membranes (TIANWEI, China) separated by a nylon sheet. The flow-electrode: graphite carbon 5 wt%. Membrane surface, S = 48.6 cm2, Current density, I = 10 A m−2 (charging stage), t = 120 min (charging stage t = 30 min (discharging stage), ttot = 7.5 h | Synthetic urine: prepared with ∼1200 mg L−1 NaCl and ∼720 mg L−1 Na2HPO4·12H2O | Recovery efficiency per cycle: 164 mg L−1 PV. Selective recovery factor for PV versus Cl−: 2. Energy consumption: 27.8 kWh kgPV | Migration uncharged H3PO4 from anode chamber | Selective recovery of PV | [156] |
| MFCDI | Three-chamber reactor consisting of cathode, anode, CEM (CMI), and AEM (AMI) membranes (Membrane International INC, Ringwood, USA) with nylon spacer between them. The flow-electrode: activated carbon powder (particle size ∼10 μm, Yihuan Carbon Inc.) mixed in 3.55 g L−1 Na2SO4 solution. S = 11.7 cm2, Voltage, U = 1.2 V (charging stage), ttot = 7 h | Synthetic wastewater: 40 mg L−1 (NH4Cl), 30 mg L−1 (NaH2PO4·H2O), 30 mg L−1 (Na2HPO4·7H2O), 120 mg L−1 (NaNO3), 200 mg L−1 (Na2SO4) | Removal efficiency: 70−98.5% (salinity), 49−91% (PO43−), 89−99% (NH4+), 83−99% (NO3−) under the 5−15 wt% electrode loadings | Low phosphate recovery rate. Negatively charged organics may contribute to fouling and microbial growth | Selective recovery of NH4+ and NO3−, PO43− | [157] |
| MCDI | Three-chamber reactor that consists of cathode, anode, and CEM, AEM. Run 1: standard monopolar CEM-DF-120 and AEM-DF-120 (Tianwei Membrane Technology Co., Ltd., Shandong, China) membranes. Run 2: selective to monovalent cations M-CEM (Astom, Japan) and standard monopolar AEM-DF-120 (Tianwei Membrane Technology Co., Ltd., Shandong, China) S = 35.23 cm2, Flow rate, W = 5.00 mL min−1, U = 1.2 V (charging stage), ttot = 12 h | Synthetic wastewater: with 100 mM NH4C1, 50 mM CaCl2, and 50 mM MgCl2 | Product purity of ammonium sulfate increased from around 50% (standard CEM) to 85% (selective CEM). Selective recovery factor for NH4+ versus another cations: 2. Energy consumption: 2498 J mmol−1NH4+ (standard CEM), 887 mmol−1NH4+ (selective CEM) | Module design and process conditions require optimization | Selective recovery of NIII | [153] |
| ECS | ECS (electrochemical stripping) combines electrodialysis and membrane stripping in a three-chamber reactor: cathode//CEM//GPM/anode, where cation exchange membrane, CMI-7000 (Membranes International Inc., Ringwood, NJ) and gas permeable membrane, GPM (CLARCOR, Industrial Air, Overland Park, KS) were used. Catholyte was always 0.1 M NaCl. i = 10 mA cm2, U = 2.9 V t = 9 h | (NH4)2SO4 solution imitating municipal wastewater (30 mg (NIII) L−1), leather wastewater (300 mg (NIII) L−1), anaerobic digestate (3000 mg N L−1) | Process does not need adding strong base; constant NH3 recovery. NIII recovery efficiency: 65%; NIII removal efficiency: 73% | Back-diffusion of NH4+, a 2.5-fold decrease in the ammonium flux with an increase in the salinity of the feed solution from 300 to 3000 mg N L−1) | Selective recovery of NIII | [158] |
| ED | Cathode//CEM//AEM/anode, 1 pair cell with CEM and AEM (Membrane International Inc., Ringwood, NJ, USA). U = 5 V, t = 6 h | Real centrate: 1417 ± 29 mg L−1 (TAN), 103 ± 6 mg L−1 (PO43−), 393 ± 27 mg L−1 (Na+), 236 ± 21 mg L−1 (K+), 308 ± 23 mg L−1 (Ca2+), 1175 ± 48 mg L−1 (Cl−), 2707 ± 186 mg L−1 (TSS), 1663 ± 0.37 mg L−1 (COD) | Removal efficiency: 74 ± 4% (NIII), 60 ± 2% (PV). Energy consumption: 17.7 ± 0.6 kWh kg−1(NIII)or 291.3 ± 13.3 kWh kg−1 (PV) | Loss of almost 30% Cl− due to oxidation at the anode | Recovery of NIII and PV; reagentless pH shift due to electrode reactions | [159] |
| ED | Conventional ED stack consisting of 1 pair cell with Fujifilm Type 10 CEM and Fujifilm Type 10 AEM (Fujifilm, Netherlands) or self-produced CEM, AEM membranes. The solution volume in the dilute and concentrate circuits were equal to 1.0 L and 0.3 L, respectively. U = 50 V, t = 360 min | Sewage sludge ash leached by 0.05 M H2SO4 with PO43− concentration 2.95 g L−1 | Synthesized membranes demonstrated the same results as commercial one. Recovery factor: 14.75 (PO43−) achieved during 30 min | No data available for other components | Recovery and concentration of PV | [160] |
| ED | Conventional ED stack consisting of 4 pair cell with CEM and AEM (Mega, Czech Republic). S = 64 cm2 per membrane; W = L h−1; U = 6.6 V. The solution volume in the dilute and concentrate circuits equal to 2 and 0.5 L; batch mode; t = 120 h | The real municipal wastewater in the secondary clarifier tank of the CAS system: 67.8 mg L−1 (Cl−), 100 mg L−1 (NO3−), (113.3 mg L−1 (SO42−), 68.22 mg L−1 (Na+), 33.55 mg L−1 (K+), 52.4 mg L−1 (Ca2+), 10.19 mg L−1 (TOC), 500 mg L−1 (TDS), 340 mg L−1 (total salinity) | The high water recovery capacity of ED. NO3− concentration factor: 4.6 (single-stage); 19.2 (two-stage). Energy consumption: 1.44 kWh kg−1 (NO3−) (single-stage); 4.34 kWh kg−1 (NO3−) (two-stage). | heavy fouling AEMs by organic compounds, compare to CEMs | Recovery and concentration of NV | [161] |
| ED | Conventional ED stack consisting of 5 pair cell with IONSEP-HC-C and IONSEP-HC-A (Iontech, China) membranes. i = 25 mA cm−2 (1.25ilimexp) t = 4 h | A solution with 0.116 g L−1 Na2HPO4·7H2O, 0.085 g L−1 NaH2PO4·H2O, and 5.2 g L−1 Na2SO4 | Electrodialysis in overlimiting current modes provides the separation of sulfates and phosphates. SO42− are transferred through the AEM, while phosphates are converted into phosphoric acid molecules and accumulate in the diluate circuit | AEM degradation: the appearance of macropores between the ion-exchange polymer and the inert binder, loss of mechanical strength, decrease in electrical conductivity and selectivity, etc. | Selective recovery of PV | [162,163] |
| ED | Conventional ED stack consisting of 10 pair cell with PCA SA and PCA SK standard membranes as well as two PCA SC cation exchange end membranes. S = 64 cm2. The current density is dynamically adjusted in agreement with the decreasing ion concentration of the diluate, without exceeding the limiting current density | Synthetic solution of the sludge reject water: 6.6 g L−1 (NH4HCO3) | Removal efficiency: 90% (NIII); Concentration 10 g L−1 of NH4+ is reached. Energy consumption: 5.4M J kg−1(NIII) NH3 using as fuel in the solid oxide fuel cell which produces energy13 M J kg−1 (NIII) | Osmosis from the diluate compartment to the concentration compartment and ammonium reverse diffusion take place. About 5% of ammonium accumulating in electrode compartments (using end AEM might prevent it) | Recovery of NIII and energy production | [164] |
| SED | The electrodialysis stack contained five repeating units consisting of 5 PC-MVK membranes, 5 PC-MVA membranes, 5 PC-SA membranes, 4 PC-SK membranes and 2 PC-SC end membranes. From the anode to the cathode, a PC-SK membrane, a PC- MVK membrane, a PC-MVA membrane and a PC-SA membrane were installed in order. All membranes were provided by PolymerchemicAltmeier, GmbH, Heusweiler, Germany. S = 64 cm2; U = 7.8 V, W = 10.62 cm s−1, Operating time = 140 min | Simulated swine wastewater: 40 mg-P L-1 (NaH2PO4·H2O), 500 mg-N L−1 (NH4Cl), 100 mg-SO4 L−1 (Na2SO4), 400 mg-K L−1 (KCl), 60 mg-Mg L−1 (MgCl2) and 100 mg-Ca L (CaCl2) | 28.38 kWh/kg PO4–P energy consumption (89.6% recovery); energy consumption at 0.783 kWh/kg NH4-N (63.2% recovery). Recovered Mg2+ and Ca2+ during the process can be used for next phosphate precipitation (with dosing 2 mol L−1 NaOH) | Current efficiency 30.23% (NH4-N), 4.16% (PO4–P) | Selective recovery of PV and NIII | [165] |
| BMED | Base-BMED stack consisting of 7 pair cells with bipolar (electrically fused AR103 and CR61) and monopolar (CR67) membranes (SUEZ Water Technologies & Solutions, Canada) An AEM (AR 204, SUEZ Water Technologies & Solutions, Canada) was placed next to the cathode while an extra CEM (CR67, SUEZ Water Technologies & Solutions, Canada) was placed to the anode. S = 36,7 cm2; U = 30 V, W = 180 mL min−1, operating time, t= 60 min | Dewatering centrate: 1188.85 ± 31.5 mg L−1 (NH3-N); 120.66 ± 3.46 mg L−1 (Ca2+); 81.66 ± 2.42 mg L−1 (Mg2+); 101.58 ± 4.24 mg L−1(K+); 275.21 ± 7.66 mg L−1 (Na+); pH 7.63 ± 0.08 | Ammonia recovery: 60%; removal efficiency: 86,5% (NH4+); 95.1% (K+); 84,0% (Ca2+); 63,2% (Mg2+); energy consumption: 15.0 kW h kg−1N Dewatering centrate as the feed to BMED system did not need an extra pretreatment (e.g., filtration) because AEMs, that are vulnerable to organic fouling, were excluded from the BMED stack design (except for the electrode rinse cell) | 5.2% of ammonia was lost during operation; the negligible amount (0.01 g L−1) of ammonia was transferred to the electrode rinse solution through AEM located next to the cathode; 82.6–91.8% of Ca2+ and 62.6–76.0% of Mg2+ (compared with the mass of Ca2+ and Mg2+ in the feed dewatering centrate) were precipitated on the CEM | Reagentless pH shift for selective recovery of NIII | [166] |
| BMED | Tree-compartment-BMED stack consisting of triple cells with bipolar (PCA) and monopolar (PCA SK, PCA Acid-60) membranes (PCCell GmbH, Heusweiler, Germany). S = 62 cm2 | Synthetic residual streams: sludge reject water or certain industrial condensates: 6.6 g L−1 (NH4HCO3) | TAN removal efficiency: from 85 to 91%; the energy consumption: 19 MJ kg−1 (NIII). Replacing the CEMs by AEMs in the BMED membrane stack decreasing NH4+ loses | Leakage of hydroxide, diffusion of dissolved ammonia and ionic species from the base compartment to the diluate, which cause the current efficiency decreased from 69 to 54% during batch BMED. 27% of the NH4+ passes from the diluate solution to the electrode compartment trough CEM | Reagentless pH shift for selective recovery of NIII | [167] |
| BMED | Tree-compartment-BMED stack consisting of 1 triple cell with bipolar (BPM-1, BPM-2 self-produced) and monopolar (Fujifilm Type 10, synthesized AEM membranes. The electrode solution: 0.3 M Na2SO4. i = 10 mA cm−2, t = 300 min | Sewage sludge ash leached by 0.05 M H2SO4: 2.95 g L−1 (PO43−) | Achieved concentration of phosphoric acid is 0.104 M for BPM-2. (Improving of phosphoric acid production up to 45%). Synthesized membranes demonstrated the same results as commercial | Low phosphoric acid production | Reagentless pH shift for selective recovery of PV | [160] |
| BMED | Tree-compartment-BMED: BPM//AEM//CEM//BPM, base-BMED: BPM//CEM//BPM, acid-BMED: BPM//AEM//BPM. S = 180 cm2, I = 3A, Umax < 60 V, t = 330 min | Synthetic wastewater imitating the liquid fraction of animal manure after separation into solid and liquid phases: 4.28 g (NH4Cl), 9.90 g L−1 ((NH4)2SO4), 2.64 g L−1 NaH2PO4), 5.39 g L−1 (CH3COONH4), 1.33 mL L−1 (H3PO4), 2.64 mL L−1 (butyric acid), 2.04 mL L−1 (valeric acid) | Consistent application of the base-BMED and the acid-BMED reduced NH3 losses. NH3 was concentrated up to 16 g L−1 in the base solution (close to 99%) but energy consumption was risen to 2.73 MJ against 1.20 MJ for three-compartment- BMED | Tree-compartment-BMED: recovery rate: 44.5% (NH4+), 81.6% (Cl−) 96.0% (PO43−); about 18% of NH3 passes from the base compartment to the acid one; 70% of energy is consumed by the solution resistance, undesired NH3 flux, and concentration polarization phenomena | Reagentless pH shift for NIII and PV selective recovery | [168] |
| BMED + HFMC | Tree-compartment-BMED stack consisting of 4 triple cells with bipolar (BP-IE) and monopolar (CMX, AMX) membranes (Astom, Japan). Each membrane area S =189 cm2 HFMC module (Pureseaspring, China). The average flow velocity, V, of the basified wastewater and the acid solution are 2 cm s−1 and 1 cm s−1), respectively. I= 20 mA cm−2 | The synthetic wastewater: NH4C1 (5000 mg L−1), NaCl (2000 mg L−1), Na2SO4 (2000 mg L−1) in deionized water | BMED energy consumption: 119.88 kj mol−1NH4+ – N; current efficiency: 80.0%. BMED–HFMC NIII capture ratio: >99%; energy consumption: 111.26 kj mol−1 (NIII) NH4+ concentration in the wastewater was decreased to <10 mg L−1, the achieved concentration of by-product (NH4)2SO4 139.1 g L−1 | NH3 undergoes leakage from the acid compartment to the salt compartment via AEM owing to coion transport and concentration diffusion; membrane fouling of the complex organic and/or inorganic components in the real wastewater should be overcome | BMED alkalized the wastewater and transform NH4+ to NH3; the MCDI is used to remove ammonia | [169] |
| BMED+ MCDI | Tree-compartment-BMED stack consisting of triple cell with bipolar (Fumasep FBM, Fuma-Tech Co., Japan) and monopolar (CMX, AMX, Astom, Japan) membranes. S = 17.5 cm2. Synthetic seawater (sea salt concentration of 35 g L−1.) in the acidic chamber to increase the electrical conductivity. T = 8 h, U = 1.4 V | Synthetic wastewater with 2.5 mM PO43− and 12.5 mM NH4+ | Removing∼89% of phosphorus and∼77% of NH4+, recovering ∼81% of wastewater. Energy consumption: 3.22 kWh kg−1 N. Simultaneously getting struvite and NH4+ concentrating | Adding MgCl2 × 6H2O for struvite precipitation | BMED alkalized the wastewater to facilitate struvite precipitation; the MCDI is used to remove NH4+ | [154] |
3.7.2. Reagent-Free pH Control for Nutrient Recovery and Conversion
3.7.3. Integrated Electromembrane Processes
4. Bottlenecks in Nutrient Recovery Processes Using Ion-Exchange Membranes
4.1. Low Mass Transfer Characteristics and High Energy Consumption
- (1)
- (2)
- (3)
4.2. Membrane Fouling and Degradation
5. Fundamentals of Phosphates and Ammonia Transport in Electromembrane Systems
5.1. Phosphate Containing Solutions
- (1)
- The radii of large and strongly hydrated phosphoric acid anions exceed the radii of other anions; therefore, phosphates have more steric hindrances during their transport in AEMs.
- (2)
- A multicomponent nutrient solution with pH 6.2–7.5 contains H2PO4− anions, which are deprotonated in standard AEMs and transferred as doubly charged anions. The initial solution with pH 8.0 and higher is enriched in doubly charged HPO42– anions, which move through the AEM without deprotonation. Therefore, the current efficiency increases, and the pH of the solutions in the desalination and concentration compartments does not undergo significant changes, in contrast to more acidic feed solutions.
5.2. Ammonium Containing Solutions
5.3. Membrane Degradation
6. Innovations in Nutrient Recovery Processes with Ion-Exchange Membranes
6.1. Enhancement Nutrient Mass Transfer
6.2. Prevention of Fouling and Membrane Degradation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Nomenclature
| AD | Acid dissociation |
| AEM | Anion-exchange membrane |
| AnD | Anaerobic biochemical digestion |
| AnMBR | Anaerobic membrane bioreactor |
| aSED | Anion selectrodialysis |
| BMED | Bipolar membrane electrodialysis |
| BPM | Bipolar membrane |
| bSED | Biselectrodialysis |
| CC | Concentration compartment |
| CEM | Cation-exchange membrane |
| COD | Chemical oxygen demand |
| DBL | Diffusion boundary layer |
| DC | Desalination compartment |
| DD | Donnan dialysis |
| DOM | Dissolved organic matter |
| EC | Electroconvection |
| ED | Electrodialysis |
| FC | Freeze concentration |
| FO | Forward osmosis |
| GSM | Gas separation membrane |
| HFM | Hollow fiber membrane |
| HFMC | Hollow fiber membrane contactor |
| IEM | Ion-exchange membrane |
| LLMC | Liquid-liquid membrane contactor |
| MBR | Membrane bioreactor |
| MCDI | Membrane capacitive deionization |
| MD | Membrane distillation |
| MF | Microfiltration |
| MFCDI | Membrane capacitive deionization with flow electrodes |
| MFC | Microbiological fuel cell |
| MMFC | Membrane fuel cell |
| MVA | Anion-exchange membrane selective for monocharged anions |
| MVC | Cation-exchange membrane selective for monocharged cations |
| NF | Nanofiltration |
| OMFC | Osmotic microbiological cell |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethylene |
| PVC | Polyvinyl chloride |
| PVDF | Polyvinylidene fluoride |
| RO | Reverse osmosis |
| SED | Selectrodialysis |
| SOFC | Solid oxide fuel cell |
| TAN | Total ammonia nitrogen |
| TKN | Total Kjeldahl nitrogen |
| TSS | Total suspended solids |
| UF | Ultrafiltration |
| VMS | Vacuum membrane stripping |
| WS | Water splitting |
| WWTP | Wastewater treatment plant |
References
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E. World Population Growth. Available online: https://ourworldindata.org/world-population-growth (accessed on 7 April 2022).
- FAO. World Fertilizer Trends and Outlook to 2019; Food & Agriculture Org.: Rome, Italy, 2019. [Google Scholar]
- Mike, T.M. Phosphate Rock. Min. Eng. 2017, 69, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the World’s Phosphorus for A Food Secure Future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Kalashnik, A.I. Industrial and Environmental Safety in the Extractive Natural Resource Management for the Needs of Agriculture. IOP Conf. Ser. Earth Environ. Sci. 2021, 941, 012018. [Google Scholar] [CrossRef]
- Deng, Z.; van Linden, N.; Guillen, E.; Spanjers, H.; van Lier, J.B. Recovery and Applications of Ammoniacal Nitrogen from Nitrogen-Loaded Residual Streams: A Review. J. Environ. Manag. 2021, 295, 113096. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.A. Renaissance of Ammonia Synthesis for Sustainable Production of Energy and Fertilizers. Curr. Opin. Green Sustain. Chem. 2021, 29, 100466. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of Electrochemical Ammonia Production Technologies and Materials. Int. J. Hydrog. Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Lipman, A.; Shah, T. Ammonia as an Alternative Energy Storage Medium for Hydrogen Fuel Cells: Scientific and Technical Review for Near-Term Stationary Power Demonstration Projects, Final Report; Transportation Sustainability Research Center: Berkeley, CA, USA, 2007. [Google Scholar]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Philibert, C. Renewable Energy for Industry; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Recent Advances in Nutrient Removal and Recovery in Biological and Bioelectrochemical Systems. Bioresour. Technol. 2016, 215, 173–185. [Google Scholar] [CrossRef]
- Eurostat Livestock Population in Numbers. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20200923-1 (accessed on 7 April 2022).
- Dadrasnia, A.; de Bona Muñoz, I.; Yáñez, E.H.; Lamkaddam, I.U.; Mora, M.; Ponsá, S.; Ahmed, M.; Argelaguet, L.L.; Williams, P.M.; Oatley-Radcliffe, D.L. Sustainable Nutrient Recovery from Animal Manure: A Review of Current Best Practice Technology and the Potential for Freeze Concentration. J. Clean. Prod. 2021, 315, 128106. [Google Scholar] [CrossRef]
- Van Staden, T.L.; Van Meter, K.J.; Basu, N.B.; Parsons, C.T.; Akbarzadeh, Z.; Van Cappellen, P. Agricultural Phosphorus Surplus Trajectories for Ontario, Canada (1961–2016), and Erosional Export Risk. Sci. Total Environ. 2022, 818, 151717. [Google Scholar] [CrossRef]
- Appl, M. Ammonia: Principles and Industrial Practice; Wiley Online Library, Wiley-VCH: New York, NY, USA, 2007. [Google Scholar]
- Shepsko, C.S.; Dong, H.; Sengupta, A.K. Treated Municipal Wastewater Reuse: A Holistic Approach Using Hybrid Ion Exchange (HIX) with Concurrent Nutrient Recovery and CO2 Sequestration. ACS Sustain. Chem. Eng. 2019, 7, 9671–9679. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic Granular Sludge Technology: Mechanisms of Granulation and Biotechnological Applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef]
- Iskander, S.M.; Brazil, B.; Novak, J.T.; He, Z. Resource Recovery from Landfill Leachate Using Bioelectrochemical Systems: Opportunities, Challenges, and Perspectives. Bioresour. Technol. 2016, 201, 347–354. [Google Scholar] [CrossRef]
- Pervov, A.G.; Shirkova, T.N.; Tikhonov, K.V. Calculation of Reverse Osmosis and Nanofiltration Plants Used for the Treatment of Filtrate of Solid Domestic Waste. Membr. Membr. Technol. 2020, 10, 309–324. [Google Scholar] [CrossRef]
- Canfield, D.E.; Kristensen, E.; Thamdrup, B. Preface. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 48, pp. xi–xii. [Google Scholar]
- Xia, X.; Zhang, S.; Li, S.; Zhang, L.; Wang, G.; Zhang, L.; Wang, J.; Li, Z. The Cycle of Nitrogen in River Systems: Sources, Transformation, and Flux. Environ. Sci. Process. Impacts 2018, 20, 863–891. [Google Scholar] [CrossRef]
- Trimmer, J.T.; Miller, D.C.; Guest, J.S. Resource Recovery from Sanitation to Enhance Ecosystem Services. Nat. Sustain. 2019, 2, 681–690. [Google Scholar] [CrossRef]
- Wright, P.A.; Wood, C.M. Seven Things Fish Know about Ammonia and We Don’t. Respir. Physiol. Neurobiol. 2012, 184, 231–240. [Google Scholar] [CrossRef]
- Galloway, J.N.; Cowling, E.B. Reactive Nitrogen and the World: 200 Years of Change. Ambio 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2020; EPA 430-R-22-003; U.S. Environmental Protection Agency: Washington, DC, USA, 2022.
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach towards Circular Economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous Removal and Recovery from Urban Wastewater: Current Practices and New Directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Chen, J.; Hu, Y.; Li, H.; Li, H.; Jiang, S.; Peng, H.; Yuan, X.; Huang, H. Valorization of the Aqueous Phase Produced from Wet and Dry Thermochemical Processing Biomass: A Review. J. Clean. Prod. 2021, 294, 126238. [Google Scholar] [CrossRef]
- Iqbal, M.; Nauman, S.; Ghafari, M.; Parnianifard, A.; Gomes, A.; Gomes, C. Treatment of Wastewater for Agricultural Applications in Regions of Water Scarcity. Biointerface Res. Appl. Chem. 2022, 12, 6336–6360. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A Critical Review on Anaerobic Digestion of Microalgae and Macroalgae and Co-Digestion of Biomass for Enhanced Methane Generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic Co-Digestion of Sludge and Organic Food Waste-Performance, Inhibition, and Impact on the Microbial Community. Energies 2018, 11, 2325. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Verde, I.; Regueiro, L.; Lema, J.M.; Carballa, M. Blending Based Optimisation and Pretreatment Strategies to Enhance Anaerobic Digestion of Poultry Manure. Waste Manag. 2018, 71, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A Critical Review on Anaerobic Co-Digestion Achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Surendra, K.C.; Angelidaki, I.; Khanal, S.K. Bioconversion of Waste-to-Resources (BWR-2021): Valorization of Industrial and Agro-Wastes to Fuel, Feed, Fertilizer, and Biobased Products. Bioresour. Technol. 2022, 347, 126739. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Orner, K.D.; Camacho-Céspedes, F.; Cunningham, J.A.; Mihelcic, J.R. Assessment of Nutrient Fluxes and Recovery for a Small-Scale Agricultural Waste Management System. J. Environ. Manag. 2020, 267, 110626. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Logan, M.; Visvanathan, C. Management Strategies for Anaerobic Digestate of Organic Fraction of Municipal Solid Waste: Current Status and Future Prospects. Waste Manag. Res. 2019, 37, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Lü, Z.; Guan, H.; Li, L.; Jia, W. Isolation and Identifaction of Acidithiobacillus Thiooxidans with Strong Phosphorous Ore Bioleaching Ability. Chinese J. Appl. Environ. Biol. 2011, 17, 326–329. [Google Scholar] [CrossRef]
- Lin, H.; Gan, J.; Rajendran, A.; Reis, C.E.R.; Hu, B. Phosphorus Removal and Recovery from Digestate after Biogas Production. In Biofuels—Status and Perspective; Gan, J., Ed.; IntechOpen: Rijeka, Croatia, 2015; p. 24. [Google Scholar]
- Xu, Y.; Zhou, Q.; Wang, X.; Yang, M.; Fang, Y.; Lu, Y. An Efficient Strategy of Phosphorus Recovery: Electrochemical Pretreatment Enhanced the Anaerobic Fermentation of Waste Activated Sludge. Chemosphere 2021, 268, 129391. [Google Scholar] [CrossRef]
- Orner, K.D.; Smith, S.J.; Breunig, H.M.; Scown, C.D.; Nelson, K.L. Fertilizer Demand and Potential Supply through Nutrient Recovery from Organic Waste Digestate in California. Water Res. 2021, 206, 117717. [Google Scholar] [CrossRef]
- Oladejo, A.O.; Ma, H.; Qu, W.; Zhou, C.; Wu, B.; Uzoejinwa, B.B.; Onwude, D.I.; Yang, X. Application of Pretreatment Methods on Agricultural Products Prior to Frying: A Review. J. Sci. Food Agric. 2018, 98, 456–466. [Google Scholar] [CrossRef]
- Desloover, J.; Vlaeminck, S.E.; Clauwaert, P.; Verstraete, W.; Boon, N. Strategies to Mitigate N 2O Emissions from Biological Nitrogen Removal Systems. Curr. Opin. Biotechnol. 2012, 23, 474–482. [Google Scholar] [CrossRef]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilisation of Poultry Litter as an Energy Feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, M.; Zanoletti, A.; Benassi, L.; Federici, S.; Depero, L.E.; Bontempi, E. Stabilized Biomass Ash as a Sustainable Substitute for Commercial P-Fertilizers. Land Degrad. Dev. 2018, 29, 2199–2207. [Google Scholar] [CrossRef]
- Bourdin, S.; Raulin, F.; Josset, C. On the (Un)Successful Deployment of Renewable Energies: Territorial Context Matters. A Conceptual Framework and an Empirical Analysis of Biogas Projects. Energy Stud. Rev. 2020, 24, 1. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Digestate Mechanical Separation: Efficiency Profiles Based on Anaerobic Digestion Feedstock and Equipment Choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef]
- Sancho, I.; Lopez-Palau, S.; Arespacochaga, N.; Cortina, J.L. New Concepts on Carbon Redirection in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2019, 647, 1373–1384. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-Liquid Separation of Animal Slurry in Theory and Practice. A Review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S. Urine Drying with Ash and Lime at Temperatures 20–60 °C—Nutrient Recovery from Source Separated Urine; Swedish University of Agricultural Science: Uppsala, Sweden, 2012. [Google Scholar]
- Liu, R.; Liu, G.; Yousaf, B.; Abbas, Q. Operating Conditions-Induced Changes in Product Yield and Characteristics during Thermal-Conversion of Peanut Shell to Biochar in Relation to Economic Analysis. J. Clean. Prod. 2018, 193, 479–490. [Google Scholar] [CrossRef]
- Patel, A.; Mungray, A.A.; Mungray, A.K. Technologies for the Recovery of Nutrients, Water and Energy from Human Urine: A Review. Chemosphere 2020, 259, 127372. [Google Scholar] [CrossRef]
- Cantero, D.; Jara, R.; Navarrete, A.; Pelaz, L.; Queiroz, J.; Rodríguez-Rojo, S.; Cocero, M.J. Pretreatment Processes of Biomass for Biorefineries: Current Status and Prospects. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 289–310. [Google Scholar] [CrossRef] [Green Version]
- Ganrot, Z.; Dave, G.; Nilsson, E. Recovery of N and P from Human Urine by Freezing, Struvite Precipitation and Adsorption to Zeolite and Active Carbon. Bioresour. Technol. 2007, 98, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Chipako, T.L.; Randall, D.G. Urine Treatment Technologies and the Importance of PH. J. Environ. Chem. Eng. 2020, 8, 103622. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.S.; Guo, M.Z.; Cheeseman, C.R.; Tsang, D.C.W.; Donatello, S.; Poon, C.S. Phosphorus Recovery and Leaching of Trace Elements from Incinerated Sewage Sludge Ash (ISSA). Chemosphere 2018, 193, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Chagnes, A.; Cathelineau, M.; Boiron, M.C. Conditioning of Poultry Manure Ash for Subsequent Phosphorous Separation and Assessment for a Process Design. Sustain. Mater. Technol. 2022, 31, e00377. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E. Extracting Phosphorous from Incinerated Sewage Sludge Ash Rich in Iron or Aluminum. Chemosphere 2013, 91, 963–969. [Google Scholar] [CrossRef]
- Li, X.; Shen, S.; Xu, Y.; Guo, T.; Dai, H.; Lu, X. Application of Membrane Separation Processes in Phosphorus Recovery: A Review. Sci. Total Environ. 2021, 767, 144346. [Google Scholar] [CrossRef]
- Larsen, T.A.; Riechmann, M.E.; Udert, K.M. State of the Art of Urine Treatment Technologies: A Critical Review. Water Res. X 2021, 13, 100114. [Google Scholar] [CrossRef]
- Yakovleva, A.A.; Yakusheva, N.I.; Fedotova, O.A. Methods for Obtaining Struvite from Wastewater. PNRPU Bull. Chem. Technol. Biotechnol. 2019, 4, 62–72. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Dey, B.; Unpaprom, Y.; Ramaraj, R.; Maniam, G.P.; Govindan, N.; Jayaraman, S.; Arunachalam, T.; Paramasivan, B. Engineering Principles and Process Designs for Phosphorus Recovery as Struvite: A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 105579. [Google Scholar] [CrossRef]
- Ostara Nutrient Management Solutions. Available online: http://ostara.com/nutrient-management-solutions/ (accessed on 7 April 2022).
- Wu, Y.; Luo, J.; Zhang, Q.; Aleem, M.; Fang, F.; Xue, Z.; Cao, J. Potentials and Challenges of Phosphorus Recovery as Vivianite from Wastewater: A Review. Chemosphere 2019, 226, 246–258. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-Based Processes for Wastewater Nutrient Recovery: Technology, Challenges, and Future Direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Priambodo, R.; Shih, Y.J.; Huang, Y.H. Phosphorus Recovery as Ferrous Phosphate (Vivianite) from Wastewater Produced in Manufacture of Thin Film Transistor-Liquid Crystal Displays (TFT-LCD) by a Fluidized Bed Crystallizer (FBC). RSC Adv. 2017, 7, 40819–40828. [Google Scholar] [CrossRef] [Green Version]
- Delgadillo-Velasco, L.; Hernández-Montoya, V.; Montes-Morán, M.A.; Gómez, R.T.; Cervantes, F.J. Recovery of Different Types of Hydroxyapatite by Precipitation of Phosphates of Wastewater from Anodizing Industry. J. Clean. Prod. 2020, 242, 118564. [Google Scholar] [CrossRef]
- Simoes, F.; Vale, P.; Stephenson, T.; Soares, A. The Role of PH on the Biological Struvite Production in Digested Sludge Dewatering Liquors. Sci. Rep. 2018, 8, 7225. [Google Scholar] [CrossRef] [Green Version]
- Leverenz, H.; Adams, R.; Hazard, J.; Tchobanoglous, G. Continuous Thermal Stripping Process for Ammonium Removal from Digestate and Centrate. Sustainability 2021, 13, 2185. [Google Scholar] [CrossRef]
- Zeng, L.; Mangan, C.; Li, X. Ammonia Recovery from Anaerobically Digested Cattle Manure by Steam Stripping. Water Sci. Technol. 2006, 54, 137–145. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Anaergia Nutrients and Digestate Management: Create Marketable Products from Biosolids and Eliminate Disposal Costs. Available online: https://www.anaergia.com/what-we-do/wastewater-resource-recovery/nutrient-recovery-and-biosolids-management (accessed on 7 April 2022).
- Jamaludin, Z.; Rollings-Scattergood, S.; Lutes, K.; Vaneeckhaute, C. Evaluation of Sustainable Scrubbing Agents for Ammonia Recovery from Anaerobic Digestate. Bioresour. Technol. 2018, 270, 596–602. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J.; et al. New Frontiers from Removal to Recycling of Nitrogen and Phosphorus from Wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.Y.; Zhang, Q.; Liu, H. Overview of Recent Developments of Resource Recovery from Wastewater via Electrochemistry-Based Technologies. Sci. Total Environ. 2021, 757, 143901. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yin, W.; Yu, Z.; Chen, J.; Huang, R.; Zhou, X. Membrane Technologies in Toilet Urine Treatment for Toilet Urine Resource Utilization: A Review. RSC Adv. 2021, 11, 35525–35535. [Google Scholar] [CrossRef] [PubMed]
- Elmaadawy, K.; Liu, B.; Hassan, G.K.; Wang, X.; Wang, Q.; Hu, J.; Hou, H.; Yang, J.; Wu, X. Microalgae-Assisted Fixed-Film Activated Sludge MFC for Landfill Leachate Treatment and Energy Recovery. Process Saf. Environ. Prot. 2022, 160, 221–231. [Google Scholar] [CrossRef]
- Pourcelly, G.; Nikonenko, V.V.; Pismenskaya, N.D.; Yaroslavtsev, A.B. Applications of Charged Membranes in Separation, Fuel Cells, and Emerging Processes. In Ionic Interactions in Natural and Synthetic Macromolecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 761–815. [Google Scholar]
- Jiang, N.; Huang, L.; Huang, M.; Cai, T.; Song, J.; Zheng, S.; Guo, J.; Kong, Z.; Chen, L. Electricity Generation and Pollutants Removal of Landfill Leachate by Osmotic Microbial Fuel Cells with Different Forward Osmosis Membranes. Sustain. Environ. Res. 2021, 31, 22. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N.; Alentiev, A. Perfluorinated Polymers as Materials of Membranes for Gas and Vapor Separation. J. Membr. Sci. 2020, 598, 117779. [Google Scholar] [CrossRef]
- Vidhyeswari, D.; Surendhar, A.; Bhuvaneshwari, S. Enhanced Performance of Novel Carbon Nanotubes—Sulfonated Poly Ether Ether Ketone (Speek) Composite Proton Exchange Membrane in Mfc Application. Chemosphere 2022, 293, 133560. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A Comprehensive Review on the Synthesis and Applications of Ion Exchange Membranes. Chemosphere 2021, 282, 130817. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion Exchange Membranes: New Developments and Applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Bhagat, M.S.; Mungray, A.K.; Mungray, A.A. Comparative Investigation of Solenoid Magnetic Field Direction on the Performance of Osmotic Microbial Fuel Cell. Mater. Today Chem. 2022, 24, 100778. [Google Scholar] [CrossRef]
- Xue, W.; He, Y.; Yumunthama, S.; Udomkittayachai, N.; Hu, Y.; Tabucanon, A.S.; Zhang, X.; Kurniawan, T.A. Membrane Cleaning and Performance Insight of Osmotic Microbial Fuel Cell. Chemosphere 2021, 285, 131549. [Google Scholar] [CrossRef]
- Nassar, L.; Hegab, H.M.; Khalil, H.; Wadi, V.S.; Naddeo, V.; Banat, F.; Hasan, S.W. Development of Green Polylactic Acid Asymmetric Ultrafiltration Membranes for Nutrient Removal. Sci. Total Environ. 2022, 824, 153869. [Google Scholar] [CrossRef]
- Arun, S.; Ramasamy, S.; Pakshirajan, K.; Pugazhenthi, G. Bioelectricity Production and Shortcut Nitrogen Removal by Microalgal-Bacterial Consortia Using Membrane Photosynthetic Microbial Fuel Cell. J. Environ. Manag. 2022, 301, 113871. [Google Scholar] [CrossRef]
- Mehrotra, S.; Kiran Kumar, V.; Man mohan, K.; Gajalakshmi, S.; Pathak, B. Bioelectrogenesis from Ceramic Membrane-Based Algal-Microbial Fuel Cells Treating Dairy Industry Wastewater. Sustain. Energy Technol. Assess. 2021, 48, 101653. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Pawlowski, S.; Galinha, C.F.; Otero, V.; García-Calvo, E.; Velizarov, S.; Crespo, J.G. Nitrate Removal by Donnan Dialysis and Anion-Exchange Membrane Bioreactor Using Upcycled End-of-Life Reverse Osmosis Membranes. Membranes 2022, 12, 101. [Google Scholar] [CrossRef]
- Tay, M.F.; Lee, S.; Xu, H.; Jeong, K.; Liu, C.; Cornelissen, E.R.; Wu, B.; Chong, T.H. Impact of Salt Accumulation in the Bioreactor on the Performance of Nanofiltration Membrane Bioreactor (NF-MBR)+ Reverse Osmosis (RO) Process for Water Reclamation. Water Res. 2020, 170, 115352. [Google Scholar] [CrossRef]
- Hacıfazlıoğlu, M.C.; Tomasini, H.R.; Kabay, N.; Bertin, L.; Pek, T.; Kitiş, M.; Yiğit, N.; Yüksel, M. Effect of Pressure on Desalination of MBR Effluents with High Salinity by Using NF and RO Processes for Reuse in Irrigation. J. Water Process Eng. 2018, 25, 22–27. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Khanzada, N.K.; Farid, M.U.; Kim, J.; Jeong, S.; An, A.K. Membrane Distillation Bioreactor (MDBR) for Wastewater Treatment, Water Reuse, and Resource Recovery: A Review. J. Water Process Eng. 2022, 47, 102687. [Google Scholar] [CrossRef]
- Robles, Á.; Ruano, M.V.; Charfi, A.; Lesage, G.; Heran, M.; Harmand, J.; Seco, A.; Steyer, J.P.; Batstone, D.J.; Kim, J.; et al. A Review on Anaerobic Membrane Bioreactors (AnMBRs) Focused on Modelling and Control Aspects. Bioresour. Technol. 2018, 270, 612–626. [Google Scholar] [CrossRef] [Green Version]
- Pretel, R.; Moñino, P.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. Economic and Environmental Sustainability of an AnMBR Treating Urban Wastewater and Organic Fraction of Municipal Solid Waste. J. Environ. Manag. 2016, 179, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Amarasiri, M.; Kitajima, M.; Nguyen, T.H.; Okabe, S.; Sano, D. Bacteriophage Removal Efficiency as a Validation and Operational Monitoring Tool for Virus Reduction in Wastewater Reclamation: Review. Water Res. 2017, 121, 258–269. [Google Scholar] [CrossRef]
- Harb, M.; Hong, P.Y. Anaerobic Membrane Bioreactor Effluent Reuse: A Review of Microbial Safety Concerns. Fermentation 2017, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Ting, Y.P. Direct Phosphorus Recovery from Municipal Wastewater via Osmotic Membrane Bioreactor (OMBR) for Wastewater Treatment. Bioresour. Technol. 2014, 170, 221–229. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Minier-Matar, J.; Adham, S.; Nasser, M.S.; Judd, S.J. The Status of Forward Osmosis Technology Implementation. Desalination 2019, 461, 10–21. [Google Scholar] [CrossRef]
- Viet, N.D.; Jang, A. Fertilizer Draw Solution Index in Osmotic Membrane Bioreactor for Simultaneous Wastewater Treatment and Sustainable Agriculture. Chemosphere 2022, 296, 134002. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Pramanik, B.K.; Halder, P.; Patel, S.; Ramezani, M.; Khairul, M.A.; Marzbali, M.H.; Paz-Ferreiro, J.; Crosher, S.; Short, G.; et al. Source and Central Level Recovery of Nutrients from Urine and Wastewater: A State-of-Art on Nutrients Mapping and Potential Technological Solutions. J. Environ. Chem. Eng. 2022, 10, 107146. [Google Scholar] [CrossRef]
- Ibrahim, R.S.B.; Zainon Noor, Z.; Baharuddin, N.H.; Ahmad Mutamim, N.S.; Yuniarto, A. Microbial Fuel Cell Membrane Bioreactor in Wastewater Treatment, Electricity Generation and Fouling Mitigation. Chem. Eng. Technol. 2020, 43, 1908–1921. [Google Scholar] [CrossRef]
- Jiménez-Benítez, A.; Ferrer, F.J.; Greses, S.; Ruiz-Martínez, A.; Fatone, F.; Eusebi, A.L.; Mondéjar, N.; Ferrer, J.; Seco, A. AnMBR, Reclaimed Water and Fertigation: Two Case Studies in Italy and Spain to Assess Economic and Technological Feasibility and CO2 Emissions within the EU Innovation Deal Initiative. J. Clean. Prod. 2020, 270, 122398. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Z.; Zhou, L.; Li, T.; Liao, C.; Yan, X.; Li, N.; Wang, X. Bioelectrochemical Ammoniation Coupled with Microbial Electrolysis for Nitrogen Recovery from Nitrate in Wastewater. Environ. Sci. Technol. 2020, 54, 3002–3011. [Google Scholar] [CrossRef]
- Han, C.; Yuan, X.; Ma, S.; Li, Y.; Feng, Y.; Liu, J. Simultaneous Recovery of Nutrients and Power Generation from Source-Separated Urine Based on Bioelectrical Coupling with the Hydrophobic Gas Permeable Tube System. Sci. Total Environ. 2022, 824, 153788. [Google Scholar] [CrossRef]
- Hou, D.; Iddya, A.; Chen, X.; Wang, M.; Zhang, W.; Ding, Y.; Jassby, D.; Ren, Z.J. Nickel-Based Membrane Electrodes Enable High-Rate Electrochemical Ammonia Recovery. Environ. Sci. Technol. 2018, 52, 8930–8938. [Google Scholar] [CrossRef]
- Christiaens, M.E.R.; Udert, K.M.; Arends, J.B.A.; Huysman, S.; Vanhaecke, L.; McAdam, E.; Rabaey, K. Membrane Stripping Enables Effective Electrochemical Ammonia Recovery from Urine While Retaining Microorganisms and Micropollutants. Water Res. 2019, 150, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Sabin, J.M.; Leverenz, H.; Bischel, H.N. Microbial Fuel Cell Treatment Energy-Offset for Fertilizer Production from Human Urine. Chemosphere 2022, 294, 133594. [Google Scholar] [CrossRef]
- Yang, K.; Qin, M. The Application of Cation Exchange Membranes in Electrochemical Systems for Ammonia Recovery from Wastewater. Membranes 2021, 11, 494. [Google Scholar] [CrossRef]
- Chen, X.; Gao, Y.; Hou, D.; Ma, H.; Lu, L.; Sun, D.; Zhang, X.; Liang, P.; Huang, X.; Ren, Z.J. The Microbial Electrochemical Current Accelerates Urea Hydrolysis for Recovery of Nutrients from Source-Separated Urine. Environ. Sci. Technol. Lett. 2017, 4, 305–310. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, D.; Wang, H.; Lu, L.; Ma, H.; Wang, L.; Ren, Z.J.; Liang, P.; Zhang, X.; Chen, X.; et al. Urine-Powered Synergy of Nutrient Recovery and Urine Purification in a Microbial Electrochemical System. Environ. Sci. Water Res. Technol. 2018, 4, 1427–1438. [Google Scholar] [CrossRef]
- Lu, S.; Li, H.; Tan, G.; Wen, F.; Flynn, M.T.; Zhu, X. Resource Recovery Microbial Fuel Cells for Urine-Containing Wastewater Treatment without External Energy Consumption. Chem. Eng. J. 2019, 373, 1072–1080. [Google Scholar] [CrossRef]
- Blatter, M.; Vermeille, M.; Furrer, C.; Pouget, G.; Fischer, F. Mechanisms and Model Process Parameters in Bioelectrochemical Wet Phosphate Recovery from Iron Phosphate Sewage Sludge. ACS Sustain. Chem. Eng. 2019, 7, 5856–5866. [Google Scholar] [CrossRef]
- Ye, B.; Liang, T.; Nong, Z.; Qin, C.; Lin, S.; Lin, W.; Liu, H.; Li, H. Simultaneous Desalination and Ammonia Recovery Using Microbial Electrolysis Desalination and Chemical-Production Cell: A Feasibility Study of Alkaline Soil Washing Wastewater. Desalination 2021, 520, 115372. [Google Scholar] [CrossRef]
- Lin, L.; Tam, L.h.; Xia, X.; Li, X. Yan Electro-Fermentation of Iron-Enhanced Primary Sedimentation Sludge in a Two-Chamber Bioreactor for Product Separation and Resource Recovery. Water Res. 2019, 157, 145–154. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Park, S.G.; Pandit, S.; Yang, E.; Ali Abdelkareem, M.; Jang, J.K.; Chae, K.J. Scalability of Microbial Electrochemical Technologies: Applications and Challenges. Bioresour. Technol. 2022, 345, 126498. [Google Scholar] [CrossRef]
- Teoh, G.H.; Jawad, Z.A.; Ooi, B.S.; Low, S.C. Simultaneous Water Reclamation and Nutrient Recovery of Aquaculture Wastewater Using Membrane Distillation. J. Water Process Eng. 2022, 46, 102573. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination Using Membrane Distillation: Experimental Studies on Full Scale Spiral Wound Modules. J. Membr. Sci. 2011, 375, 104–112. [Google Scholar] [CrossRef]
- Fumasoli, A.; Etter, B.; Sterkele, B.; Morgenroth, E.; Udert, K.M. Operating a Pilot-Scale Nitrification/Distillation Plant for Complete Nutrient Recovery from Urine. Water Sci. Technol. 2016, 73, 215–222. [Google Scholar] [CrossRef]
- Van Linden, N.; Spanjers, H.; van Lier, J.B. Fuelling a Solid Oxide Fuel Cell with Ammonia Recovered from Water by Vacuum Membrane Stripping. Chem. Eng. J. 2022, 428, 131081. [Google Scholar] [CrossRef]
- Rivera, F.; Muñoz, R.; Prádanos, P.; Hernández, A.; Palacio, L. A Systematic Study of Ammonia Recovery from Anaerobic Digestate Using Membrane-Based Separation. Membranes 2022, 12, 19. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of Liquid-Liquid Membrane Contactors and Electrodialysis for Ammonium Recovery and Concentration as a Liquid Fertilizer. Chemosphere 2020, 245, 125606. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Bhushan, B.; Gibert, O.; Valderrama, C.; Cortina, J.L. Liquid Fertilizer Production by Ammonia Recovery from Treated Ammonia-Rich Regenerated Streams Using Liquid-Liquid Membrane Contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Commission, E. Reference Document on Best Available Techniques (BAT) for the Manufacture of Large Volume Inorganic Chemicals, Ammonia, Acids and Fertilisers. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2022-03/LVIC-AAF.pdf (accessed on 3 April 2022).
- Volpin, F.; Heo, H.; Hasan Johir, M.A.; Cho, J.; Phuntsho, S.; Shon, H.K. Techno-Economic Feasibility of Recovering Phosphorus, Nitrogen and Water from Dilute Human Urine via Forward Osmosis. Water Res. 2019, 150, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, Z.; Liang, P.; Huang, X. Direct Concentration of Municipal Sewage by Forward Osmosis and Membrane Fouling Behavior. Bioresour. Technol. 2018, 247, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Phosphorus Recovery from Digested Sludge Centrate Using Seawater-Driven Forward Osmosis. Sep. Purif. Technol. 2016, 163, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward Osmosis as a Platform for Resource Recovery from Municipal Wastewater—A Critical Assessment of the Literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Toward Resource Recovery from Wastewater: Extraction of Phosphorus from Digested Sludge Using a Hybrid Forward Osmosis-Membrane Distillation Process. Environ. Sci. Technol. Lett. 2014, 1, 191–195. [Google Scholar] [CrossRef]
- Hancock, N.T.; Xu, P.; Roby, M.J.; Gomez, J.D.; Cath, T.Y. Towards Direct Potable Reuse with Forward Osmosis: Technical Assessment of Long-Term Process Performance at the Pilot Scale. J. Membr. Sci. 2013, 445, 34–46. [Google Scholar] [CrossRef]
- Almoalimi, K.; Liu, Y.-Q. Enhancing Ammonium Rejection in Forward Osmosis for Wastewater Treatment by Minimizing Cation Exchange. J. Membr. Sci. 2022, 648, 120365. [Google Scholar] [CrossRef]
- Kramer, F.C.; Shang, R.; Rietveld, L.C.; Heijman, S.J.G. Influence of PH, Multivalent Counter Ions, and Membrane Fouling on Phosphate Retention during Ceramic Nanofiltration. Sep. Purif. Technol. 2019, 227, 115675. [Google Scholar] [CrossRef] [Green Version]
- Adam, G.; Mottet, A.; Lemaigre, S.; Tsachidou, B.; Trouvé, E.; Delfosse, P. Fractionation of Anaerobic Digestates by Dynamic Nanofiltration and Reverse Osmosis: An Industrial Pilot Case Evaluation for Nutrient Recovery. J. Environ. Chem. Eng. 2018, 6, 6723–6732. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Ammonia Recovery and Fouling Mitigation of Hydrolyzed Human Urine Treated by Nanofiltration and Reverse Osmosis. Environ. Sci. Water Res. Technol. 2022, 8, 429–442. [Google Scholar] [CrossRef]
- Courtney, C.; Randall, D.G. Concentrating Stabilized Urine with Reverse Osmosis: How Does Stabilization Method and Pre-Treatment Affect Nutrient Recovery, Flux, and Scaling? Water Res. 2022, 209, 117970. [Google Scholar] [CrossRef]
- Deemter, D.; Salmerón, I.; Oller, I.; Amat, A.M.; Malato, S. Valorization of UWWTP Effluents for Ammonium Recovery and MC Elimination by Advanced AOPs. Sci. Total Environ. 2022, 823, 153693. [Google Scholar] [CrossRef]
- Schütte, T.; Niewersch, C.; Wintgens, T.; Yüce, S. Phosphorus Recovery from Sewage Sludge by Nanofiltration in Diafiltration Mode. J. Membr. Sci. 2015, 480, 74–82. [Google Scholar] [CrossRef]
- Lazarev, S.I.; Kovalev, S.V.; Konovalov, D.N.; Lua, P. Electrochemical and Transport Characteristics of Membrane Systems in the Electronanofiltration Separation of Solutions Containing Ammonium Nitrate and Potassium Sulfate. Russ. J. Electrochem. 2021, 57, 607–624. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, G.; van der Meer, W.G.J. One-Step Reverse Osmosis Based on Riverbank Filtration for Future Drinking Water Purification. Engineering 2022, 9, 27–34. [Google Scholar] [CrossRef]
- Grossi, L.B.; Magalhães, N.C.; Araújo, B.M.; De Carvalho, F.; Andrade, L.H.; Amaral, M.C.S. Water Conservation in Mining Industry by Integrating Pressure-Oriented Membrane Processes for Nitrogen-Contaminated Wastewater Treatment: Bench and Pilot-Scale Studies. J. Environ. Chem. Eng. 2021, 9, 104779. [Google Scholar] [CrossRef]
- Samanta, P.; Schönettin, H.M.; Horn, H.; Saravia, F. MF–NF Treatment Train for Pig Manure: Nutrient Recovery and Reuse of Product Water. Membranes 2022, 12, 165. [Google Scholar] [CrossRef]
- Gui, S.; Mai, Z.; Fu, J.; Wei, Y.; Wan, J. Transport Models of Ammonium Nitrogen in Wastewater from Rare Earth Smelteries by Reverse Osmosis Membranes. Sustainability 2020, 12, 6230. [Google Scholar] [CrossRef]
- Wang, Y.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically Mediated Precipitation of Phosphate Minerals for Phosphorus Removal and Recovery: Progress and Perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef]
- Bagastyo, A.Y.; Anggrainy, A.D.; Khoiruddin, K.; Ursada, R.; Warmadewanthi, I.D.A.A.; Wenten, I.G. Electrochemically-Driven Struvite Recovery: Prospect and Challenges for the Application of Magnesium Sacrificial Anode. Sep. Purif. Technol. 2022, 288, 120653. [Google Scholar] [CrossRef]
- Reza, A.; Chen, L. Electrochemical Treatment of Livestock Waste Streams. A Review. Environ. Chem. Lett. 2022, 1–33. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Wu, Y.; Wang, C.; Zheng, J.; Xu, K.; Li, J. Recovery of Potassium from Landfill Leachate Concentrates Using a Combination of Cation-Exchange Membrane Electrolysis and Magnesium Potassium Phosphate Crystallization. Sep. Purif. Technol. 2015, 144, 1–7. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, K.; He, C.; Wang, K. Ammonia Removal from Municipal Wastewater via Membrane Capacitive Deionization (MCDI) in Pilot-Scale. Sep. Purif. Technol. 2022, 286, 120469. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, X.; Wang, M.; Ma, J.; Collins, R.; Kinsela, A.; Zhang, Y.; Waite, T.D. Phosphate Recovery as Vivianite Using a Flow-Electrode Capacitive Desalination (FCDI) and Fluidized Bed Crystallization (FBC) Coupled System. Water Res. 2021, 194, 116939. [Google Scholar] [CrossRef]
- Fang, K.; He, W.; Peng, F.; Wang, K. Ammonia Recovery from Concentrated Solution by Designing Novel Stacked FCDI Cell. Sep. Purif. Technol. 2020, 250, 117066. [Google Scholar] [CrossRef]
- Gao, F.; Wang, L.; Wang, J.; Zhang, H.; Lin, S. Nutrient Recovery from Treated Wastewater by a Hybrid Electrochemical Sequence Integrating Bipolar Membrane Electrodialysis and Membrane Capacitive Deionization. Environ. Sci. Water Res. Technol. 2020, 6, 383–391. [Google Scholar] [CrossRef]
- Mohammadi, R.; Tang, W.; Sillanpää, M. A Systematic Review and Statistical Analysis of Nutrient Recovery from Municipal Wastewater by Electrodialysis. Desalination 2021, 498, 114626. [Google Scholar] [CrossRef]
- Xu, L.; Yu, C.; Tian, S.; Mao, Y.; Zong, Y.; Zhang, X.; Zhang, B.; Zhang, C.; Wu, D. Selective Recovery of Phosphorus from Synthetic Urine Using Flow-Electrode Capacitive Deionization (FCDI)-Based Technology. ACS Environ. Sci. Technol. Water 2021, 1, 175–184. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, X.; Lu, L.; Liang, P.; Ren, Z.J. Concurrent Nitrogen and Phosphorus Recovery Using Flow-Electrode Capacitive Deionization. ACS Sustain. Chem. Eng. 2019, 7, 7844–7850. [Google Scholar] [CrossRef]
- Liu, M.J.; Neo, B.S.; Tarpeh, W.A. Building an Operational Framework for Selective Nitrogen Recovery via Electrochemical Stripping. Water Res. 2020, 169, 115226. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, T.; He, Z. Minimizing Effects of Chloride and Calcium towards Enhanced Nutrient Recovery from Sidestream Centrate in a Decoupled Electrodialysis Driven by Solar Energy. J. Clean. Prod. 2020, 263, 121419. [Google Scholar] [CrossRef]
- Khadem Modarresi, Z.; Mowla, D.; Karimi, G. Electrodialytic Separation of Phosphate from Sewage Sludge Ash Using Electrospun Ion Exchange Membranes. Sep. Purif. Technol. 2021, 275, 119202. [Google Scholar] [CrossRef]
- Mohammadi, R.; Ramasamy, D.L.; Sillanpää, M. Enhancement of Nitrate Removal and Recovery from Municipal Wastewater through Single- and Multi-Batch Electrodialysis: Process Optimisation and Energy Consumption. Desalination 2021, 498, 114726. [Google Scholar] [CrossRef]
- Rotta, E.H.; Marder, L.; Pérez-Herranz, V.; Bernardes, A.M. Characterization of an Anion-Exchange Membrane Subjected to Phosphate and Sulfate Separation by Electrodialysis at Overlimiting Current Density Condition. J. Membr. Sci. 2021, 635, 119510. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus Recovery from Low Phosphate-Containing Solution by Electrodialysis. J. Membr. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Van Linden, N.; Spanjers, H.; van Lier, J.B. Application of Dynamic Current Density for Increased Concentration Factors and Reduced Energy Consumption for Concentrating Ammonium by Electrodialysis. Water Res. 2019, 163, 114856. [Google Scholar] [CrossRef]
- Ye, Z.L.; Ghyselbrecht, K.; Monballiu, A.; Pinoy, L.; Meesschaert, B. Fractionating Various Nutrient Ions for Resource Recovery from Swine Wastewater Using Simultaneous Anionic and Cationic Selective-Electrodialysis. Water Res. 2019, 160, 424–434. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, P.; Pavlovic, V.; Barber, J.; Kim, Y. Ammonium Sulfate Production from Wastewater and Low-Grade Sulfuric Acid Using Bipolar- and Cation-Exchange Membranes. J. Clean. Prod. 2021, 285, 124888. [Google Scholar] [CrossRef]
- Van Linden, N.; Bandinu, G.L.; Vermaas, D.A.; Spanjers, H.; van Lier, J.B. Bipolar Membrane Electrodialysis for Energetically Competitive Ammonium Removal and Dissolved Ammonia Production. J. Clean. Prod. 2020, 259, 120788. [Google Scholar] [CrossRef]
- Shi, L.; Xiao, L.; Hu, Z.; Zhan, X. Nutrient Recovery from Animal Manure Using Bipolar Membrane Electrodialysis: Study on Product Purity and Energy Efficiency. Water Cycle 2020, 1, 54–62. [Google Scholar] [CrossRef]
- Yan, H.; Wu, L.; Wang, Y.; Irfan, M.; Jiang, C.; Xu, T. Ammonia Capture from Wastewater with a High Ammonia Nitrogen Concentration by Water Splitting and Hollow Fiber Extraction. Chem. Eng. Sci. 2020, 227, 115934. [Google Scholar] [CrossRef]
- Melnikov, S.; Loza, S.; Sharafan, M.; Zabolotskiy, V. Electrodialysis Treatment of Secondary Steam Condensate Obtained during Production of Ammonium Nitrate. Technical and Economic Analysis. Sep. Purif. Technol. 2016, 157, 179–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Desmidt, E.; Van Looveren, A.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Phosphate Separation and Recovery from Wastewater by Novel Electrodialysis. Environ. Sci. Technol. 2013, 47, 5888–5895. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Wu, G.; Luo, J.; Wang, S. Development of a Selective Electrodialysis for Nutrient Recovery and Desalination during Secondary Effluent Treatment. Chem. Eng. J. 2017, 322, 224–233. [Google Scholar] [CrossRef]
- Kedwell, K.C.; Jørgensen, M.K.; Quist-Jensen, C.A.; Pham, T.D.; Van der Bruggen, B.; Christensen, M.L. Selective Electrodialysis for Simultaneous but Separate Phosphate and Ammonium Recovery. Environ. Technol. 2021, 42, 2177–2186. [Google Scholar] [CrossRef]
- Meesschaert, B.; Ghyselbrecht, K.; Monballiu, A.; Pinoy, L. Pilot Scale Anion Selectrodialysis for Water Reclamation and Nutrient Recovery from UASB Effluent after Nitrification, Ultrafiltration and UV C Treatment. Environ. Technol. Innov. 2021, 22, 101449. [Google Scholar] [CrossRef]
- Ghyselbrecht, K.; Jongbloet, A.; Pinoy, L.; Meesschaert, B. Optimization of the Configuration of the Anion Selectrodialysis Stack for Fractionation of Phosphate from UASB Effluent in Batch Mode on Lab Scale and Pilot Scale. J. Environ. Chem. Eng. 2020, 8, 104492. [Google Scholar] [CrossRef]
- Oliveira, V.; Dias-Ferreira, C.; González-García, I.; Labrincha, J.; Horta, C.; García-González, M.C. A Novel Approach for Nutrients Recovery from Municipal Waste as Biofertilizers by Combining Electrodialytic and Gas Permeable Membrane Technologies. Waste Manag. 2021, 125, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, S.S.; Mugtamov, O.A.; Zabolotsky, V.I. Study of Electrodialysis Concentration Process of Inorganic Acids and Salts for the Two-Stage Conversion of Salts into Acids Utilizing Bipolar Electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, Y.; Du, Y.; Feng, H.; Xu, T. Simultaneous Recovery of Ammonium and Phosphorus via the Integration of Electrodialysis with Struvite Reactor. J. Membr. Sci. 2015, 490, 65–71. [Google Scholar] [CrossRef]
- Ward, A.J.; Arola, K.; Thompson Brewster, E.; Mehta, C.M.; Batstone, D.J. Nutrient Recovery from Wastewater through Pilot Scale Electrodialysis. Water Res. 2018, 135, 57–65. [Google Scholar] [CrossRef]
- Arora, A.S.; Nawaz, A.; Qyyum, M.A.; Ismail, S.; Aslam, M.; Tawfik, A.; Yun, C.M.; Lee, M. Energy Saving Anammox Technology-Based Nitrogen Removal and Bioenergy Recovery from Wastewater: Inhibition Mechanisms, State-of-the-Art Control Strategies, and Prospects. Renew. Sustain. Energy Rev. 2021, 135, 110126. [Google Scholar] [CrossRef]
- Saltworks Awarded Funding to Commercialize Ammonia Splitter. Filtr. Ind. Anal. 2016, 2016, 4. [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar Membranes: A Review on Principles, Latest Developments, and Applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Saabas, D.; Lee, J. Recovery of Ammonia from Simulated Membrane Contactor Effluent Using Bipolar Membrane Electrodialysis. J. Memb. Sci. 2022, 644, 120081. [Google Scholar] [CrossRef]
- Ali, M.A.B.; Rakib, M.; Laborie, S.; Viers, P.; Durand, G. Coupling of Bipolar Membrane Electrodialysis and Ammonia Stripping for Direct Treatment of Wastewaters Containing Ammonium Nitrate. J. Membr. Sci. 2004, 244, 89–96. [Google Scholar] [CrossRef]
- Xu, L.; Dong, F.; Yang, J.; Liu, W.; Zhu, L.; He, Q.; Wang, X.; Li, H.; Wang, X. Electricity Generation and Acid and Alkaline Recovery from Pickled Waters/Wastewaters through Anaerobic Digestion, Bipolar Membrane Electrodialysis and Solid Oxide Fuel Cell Hybrid System. Energy Convers. Manag. 2022, 251, 114973. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Shi, S.; Cao, H.; Yip, N.Y.; Lin, S. Bipolar Membrane Electrodialysis for Ammonia Recovery from Synthetic Urine: Experiments, Modeling, and Performance Analysis. Environ. Sci. Technol. 2021, 55, 14886–14896. [Google Scholar] [CrossRef]
- Ding, R.; Ding, Z.; Chen, X.; Fu, J.; Zhou, Z.; Chen, X.; Zheng, X.; Jin, Y.; Chen, R. Integration of Electrodialysis and Donnan Dialysis for the Selective Separation of Ammonium from High-Salinity Wastewater. Chem. Eng. J. 2021, 405, 127001. [Google Scholar] [CrossRef]
- Monetti, J.; Ledezma, P.; Freguia, S. Optimised Operational Parameters for Improved Nutrient Recovery from Hydrolysed Urine by Bio-Electroconcentration. Sep. Purif. Technol. 2021, 279, 119793. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Bhushan, B.; López, J.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of Liquid–Liquid Membrane Contactors and Electrodialysis for Ammonia Recovery from Urban Wastewaters. In Frontiers in Water-Energy-Nexus; Advances in Science, Technology & Innovation; Springer: Berlin, Germany, 2020; pp. 359–361. [Google Scholar] [CrossRef] [Green Version]
- Kuntke, P.; Sleutels, T.H.J.A.; Rodríguez Arredondo, M.; Georg, S.; Barbosa, S.G.; ter Heijne, A.; Hamelers, H.V.M.; Buisman, C.J.N. (Bio)Electrochemical Ammonia Recovery: Progress and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 3865–3878. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Xie, S.; Hu, Z.; Wu, G.; Morrison, L.; Croot, P.; Hu, H.; Zhan, X. Nutrient Recovery from Pig Manure Digestate Using Electrodialysis Reversal: Membrane Fouling and Feasibility of Long-Term Operation. J. Membr. Sci. 2019, 573, 560–569. [Google Scholar] [CrossRef]
- Monetti, J.; Ledezma, P.; Virdis, B.; Freguia, S. Nutrient Recovery by Bio-Electroconcentration Is Limited by Wastewater Conductivity. ACS Omega 2019, 4, 2152–2159. [Google Scholar] [CrossRef]
- Sarapulova, V.; Nevakshenova, E.; Pismenskaya, N.; Dammak, L.; Nikonenko, V. Unusual Concentration Dependence of Ion-Exchange Membrane Conductivity in Ampholyte-Containing Solutions: Effect of Ampholyte Nature. J. Membr. Sci. 2015, 479, 28–38. [Google Scholar] [CrossRef]
- Melnikov, S.; Kolot, D.; Nosova, E.; Zabolotskiy, V. Peculiarities of Transport-Structural Parameters of Ion-Exchange Membranes in Solutions Containing Anions of Carboxylic Acids. J. Membr. Sci. 2018, 557, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Feng, X.; Cui, W.; Deng, H.; Ji, Z.; Zhao, Y.; Guo, X.; Yuan, J. Effects of Inorganic Ions on the Transfer of Weak Organic Acids and Their Salts in Electrodialysis Process. J. Membr. Sci. 2021, 624, 119109. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Sarapulova, V.; Nevakshenova, E.; Kononenko, N.; Fomenko, M.; Nikonenko, V. Concentration Dependencies of Diffusion Permeability of Anion-Exchange Membranes in Sodium Hydrogen Carbonate, Monosodium Phosphate, and Potassium Hydrogen Tartrate Solutions. Membranes 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Tadimeti, J.G.D.; Bhuvanesh, E.; Pathiwada, D.; Chattopadhyay, S. Switching Selectivity of Carboxylic Acids and Associated Physico-Chemical Changes with PH during Electrodialysis of Ternary Mixtures. Sep. Purif. Technol. 2018, 193, 327–344. [Google Scholar] [CrossRef]
- Rybalkina, O.; Tsygurina, K.; Melnikova, E.; Mareev, S.; Moroz, I.; Nikonenko, V.; Pismenskaya, N. Partial Fluxes of Phosphoric Acid Anions through Anion-Exchange Membranes in the Course of NaH2PO4 Solution Electrodialysis. Int. J. Mol. Sci. 2019, 20, 3593. [Google Scholar] [CrossRef] [Green Version]
- Martí-Calatayud, M.C.; Evdochenko, E.; Bär, J.; García-Gabaldón, M.; Wessling, M.; Pérez-Herranz, V. Tracking Homogeneous Reactions during Electrodialysis of Organic Acids via EIS. J. Membr. Sci. 2020, 595, 117592. [Google Scholar] [CrossRef]
- Belashova, E.D.; Kharchenko, O.A.; Sarapulova, V.V.; Nikonenko, V.V.; Pismenskaya, N.D. Effect of Protolysis Reactions on the Shape of Chronopotentiograms of a Homogeneous Anion-Exchange Membrane in NaH2PO4 Solution. Pet. Chem. 2017, 57, 1207–1218. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Moroz, I.A.; Gorobchenko, A.D.; Pismenskaya, N.D.; Nikonenko, V.V. Development of Electroconvection at the Undulate Surface of an Anion-Exchange Membrane in Sodium Chloride and Sodium Hydrogen Tartrate Solutions. Membr. Membr. Technol. 2022, 4, 31–38. [Google Scholar] [CrossRef]
- Gally, C.; García-Gabaldón, M.; Ortega, E.M.; Bernardes, A.M.; Pérez-Herranz, V. Chronopotentiometric Study of the Transport of Phosphoric Acid Anions through an Anion-Exchange Membrane under Different PH Values. Sep. Purif. Technol. 2020, 238, 116421. [Google Scholar] [CrossRef]
- Aminov, O.A.; Shaposhnik, V.A.; Guba, A.A.; Kutsenko, A.E. The Conjugate Transport of Ammonium Ions with Hydrogen and Hydroxyl Ions in Electrodialysis in the Region of Overlimiting Current Densities. Sorbtsionnye I Khromatographicheskie Protsessy 2013, 13, 816–822. [Google Scholar]
- Kozaderova, O.A.; Niftaliev, S.I.; Kim, K.B. Ionic Transport in Electrodialysis of Ammonium Nitrate. Russ. J. Electrochem. 2018, 54, 363–367. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Tsygurina, K.A.; Melnikova, E.D.; Pourcelly, G.; Nikonenko, V.V.; Pismenskaya, N.D. Catalytic Effect of Ammonia-Containing Species on Water Splitting during Electrodialysis with Ion-Exchange Membranes. Electrochim. Acta 2019, 299, 946–962. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; García-Gabaldón, M.; Pérez-Herranz, V. Mass Transfer Phenomena during Electrodialysis of Multivalent Ions: Chemical Equilibria and Overlimiting Currents. Appl. Sci. 2018, 8, 1566. [Google Scholar] [CrossRef] [Green Version]
- Rybalkina, O.A.; Sharafan, M.V.; Nikonenko, V.V.; Pismenskaya, N.D. Two Mechanisms of H+/OH− Ion Generation in Anion-Exchange Membrane Systems with Polybasic Acid Salt Solutions. J. Membr. Sci. 2022, 651, 120449. [Google Scholar] [CrossRef]
- Belloň, T.; Polezhaev, P.; Vobecká, L.; Slouka, Z. Fouling of a Heterogeneous Anion-Exchange Membrane and Single Anion-Exchange Resin Particle by SsDNA Manifests Differently. J. Membr. Sci. 2019, 572, 619–631. [Google Scholar] [CrossRef]
- Belloň, T.; Slouka, Z. Overlimiting Behavior of Surface-Modified Heterogeneous Anion-Exchange Membranes. J. Membr. Sci. 2020, 610, 118291. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, J.H.; Yeon, K.H.; Moon, S.H. An Approach to Fouling Characterization of an Ion-Exchange Membrane Using Current-Voltage Relation and Electrical Impedance Spectroscopy. J. Colloid Interface Sci. 2006, 294, 129–138. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Chang, H.; Kwon, D.; Kim, J. Rejections and Membrane Fouling of Submerged Direct Contact Hollow-Fiber Membrane Distillation as Post-Treatment for Anaerobic Fluidized Bed Bioreactor Treating Domestic Sewage. Chemosphere 2022, 296, 133964. [Google Scholar] [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Bdiri, M.; Sarapulova, V.; Kozmai, A.; Fouilloux, J.; Baklouti, L.; Larchet, C.; Renard, E.; Dammak, L. A Review on Ion-Exchange Membranes Fouling during Electrodialysis Process in Food Industry, Part 2: Influence on Transport Properties and Electrochemical Characteristics, Cleaning and Its Consequences. Membranes 2021, 11, 811. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices. Int. J. Mol. Sci. 2020, 21, 7874. [Google Scholar] [CrossRef]
- Guo, H.; Kim, Y. Membrane Scaling in Electrodialysis Fed with High-Strength Wastewater. Environ. Eng. Sci. 2021, 38, 832–840. [Google Scholar] [CrossRef]
- Dammak, L.; Pismenskaya, N. In-Depth on the Fouling and Antifouling of Ion-Exchange Membranes. Membranes 2021, 11, 962. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Tsygurina, K.A.; Sarapulova, V.V.; Mareev, S.A.; Nikonenko, V.V.; Pismenskaya, N.D. Evolution of Current–Voltage Characteristics and Surface Morphology of Homogeneous Anion-Exchange Membranes during the Electrodialysis Desalination of Alkali Metal Salt Solutions. Membr. Membr. Technol. 2019, 1, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Pismenskaya, N.D.; Melnikova, E.D.; Rybalkina, O.A.; Nikonenko, V.V. The Impact of Long-Time Operation of an Anion-Exchange Membrane AMX-Sb in the Electrodialysis Desalination of Sodium Chloride Solution on the Membrane Current–Voltage Characteristic and the Water Splitting Rate. Membr. Membr. Technol. 2019, 1, 88–98. [Google Scholar] [CrossRef]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 385–427. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Van der Bruggen, B.; Pinoy, L.; Meesschaert, B. Separation of Nutrient Ions and Organic Compounds from Salts in RO Concentrates by Standard and Monovalent Selective Ion-Exchange Membranes Used in Electrodialysis. J. Membr. Sci. 2009, 332, 104–112. [Google Scholar] [CrossRef]
- Belashova, E.D.; Pismenskaya, N.D.; Nikonenko, V.V.; Sistat, P.; Pourcelly, G. Current-Voltage Characteristic of Anion-Exchange Membrane in Monosodium Phosphate Solution. Modelling and Experiment. J. Membr. Sci. 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Melnikova, E.D.; Pismenskaya, N.D.; Bazinet, L.; Mikhaylin, S.; Nikonenko, V.V. Effect of Ampholyte Nature on Current-Voltage Characteristic of Anion-Exchange Membrane. Electrochim. Acta 2018, 285, 185–191. [Google Scholar] [CrossRef]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Simons, R. Water Splitting in Ion Exchange Membranes. Electrochim. Acta 1985, 30, 275–282. [Google Scholar] [CrossRef]
- Titorova, V.D.; Mareev, S.A.; Gorobchenko, A.D.; Gil, V.V.; Nikonenko, V.V.; Sabbatovskii, K.G.; Pismenskaya, N.D. Effect of Current-Induced Coion Transfer on the Shape of Chronopotentiograms of Cation-Exchange Membranes. J. Membr. Sci. 2021, 624, 119036. [Google Scholar] [CrossRef]
- Newman, J.S. Electrochemical Systems; Prentice Hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Rybalkina, O.A.; Solonchenko, K.V.; Nikonenko, V.V.; Pismenskaya, N.D. Investigation of Causes of Low Current Efficiency in Electrodialysis of Phosphate-Containing Solutions. Membr. Membr. Technol. 2021, 3, 220–230. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Rybalkina, O.; Moroz, I.; Mareev, S.; Nikonenko, V. Influence of Electroconvection on Chronopotentiograms of an Anion-Exchange Membrane in Solutions of Weak Polybasic Acid Salts. Int. J. Mol. Sci. 2021, 22, 13518. [Google Scholar] [CrossRef] [PubMed]
- Pismenskaya, N.D.; Rybalkina, O.A.; Kozmai, A.E.; Tsygurina, K.A.; Melnikova, E.D.; Nikonenko, V.V. Generation of H+ and OH− Ions in Anion-Exchange Membrane/Ampholyte-Containing Solution Systems: A Study Using Electrochemical Impedance Spectroscopy. J. Membr. Sci. 2020, 601, 117920. [Google Scholar] [CrossRef]
- Kozaderova, O.A.; Kim, K.B.; Gadzhiyeva, C.S.; Niftaliev, S.I. Electrochemical Characteristics of Thin Heterogeneous Ion Exchange Membranes. J. Membr. Sci. 2020, 604, 118081. [Google Scholar] [CrossRef]
- Niftaliev, S.I.; Kozaderova, O.A.; Kim, K.B. Electrodialysis of Ammonium Nitrate Solution in Intensive Current Regimes. Int. J. Electrochem. Sci. 2016, 11, 9057–9066. [Google Scholar] [CrossRef]
- Melnikova, E.D.; Tsygurina, K.A.; Pismenskaya, N.D.; Nikonenko, V.V. Influence of Protonation–Deprotonation Reactions on the Diffusion of Ammonium Chloride through Anion-Exchange Membrane. Membr. Membr. Technol. 2021, 3, 324–333. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A Review of the Synthesis and Characterization of Anion Exchange Membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef] [Green Version]
- Pine, S.H. The Base-Promoted Rearrangements of Quaternary Ammonium Salts. Org. React. 2011, 18, 403–464. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Mizutani, Y.; Yamane, R.; Motomura, H. Studies of Ion Exchange Membranes. XXII. Semicontinuous Preparation of Ion Exchange Membranes by the “Paste Method”. Bull. Chem. Soc. Jpn. 1965, 38, 689–694. [Google Scholar] [CrossRef]
- Higa, M.; Tanaka, N.; Nagase, M.; Yutani, K.; Kameyama, T.; Takamura, K.; Kakihana, Y. Electrodialytic Properties of Aromatic and Aliphatic Type Hydrocarbon-Based Anion-Exchange Membranes with Various Anion-Exchange Groups. Polymer 2014, 55, 3951–3960. [Google Scholar] [CrossRef]
- Doi, S.; Yasukawa, M.; Kakihana, Y.; Higa, M. Alkali Attack on Anion Exchange Membranes with PVC Backing and Binder: Effect on Performance and Correlation between Them. J. Membr. Sci. 2019, 573, 85–96. [Google Scholar] [CrossRef]
- Doi, S.; Taniguchi, I.; Yasukawa, M.; Kakihana, Y.; Higa, M. Effect of Alkali Treatment on the Mechanical Properties of Anion-Exchange Membranes with a Poly(Vinyl Chloride) Backing and Binder. Membranes 2020, 10, 344. [Google Scholar] [CrossRef]
- Amato, L.; Gilbert, M.; Caswell, A. Degradation Studies of Crosslinked Polyethylene. II Aged in Water. Plast. Rubber Compos. 2005, 34, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Vasil’eva, V.I.; Pismenskaya, N.D.; Akberova, E.M.; Nebavskaya, K.A. Effect of Thermochemical Treatment on the Surface Morphology and Hydrophobicity of Heterogeneous Ion-Exchange Membranes. Russ. J. Phys. Chem. A 2014, 88, 1293–1299. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of Ion Exchange Membranes: A Review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Petrov, K.V.; Paltrinieri, L.; Poltorak, L.; de Smet, L.C.P.M.; Sudhölter, E.J.R. Modified Cation-Exchange Membrane for Phosphate Recovery in an Electrochemically Assisted Adsorption-Desorption Process. Chem. Commun. 2020, 56, 5046–5049. [Google Scholar] [CrossRef]
- Schug, K.A.; Lindner, W. Noncovalent Binding between Guanidinium and Anionic Groups: Focus on Biological- and Synthetic-Based Arginine/Guanidinium Interactions with Phosph[on]Ate and Sulf[on]Ate Residues. Chem. Rev. 2005, 105, 67–113. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Xie, S.; Wu, G.; Hu, Z.; Zhan, X. Recovery of Nutrients and Volatile Fatty Acids from Pig Manure Hydrolysate Using Two-Stage Bipolar Membrane Electrodialysis. Chem. Eng. J. 2018, 334, 134–142. [Google Scholar] [CrossRef]
- Petrus, H.B.; Li, H.; Chen, V.; Norazman, N. Enzymatic Cleaning of Ultrafiltration Membranes Fouled by Protein Mixture Solutions. J. Membr. Sci. 2008, 325, 783–792. [Google Scholar] [CrossRef]
- Bilad, M.R.; Baten, M.; Pollet, A.; Courtin, C.; Wouters, J.; Verbiest, T.; Vankelecom, I.F.J. A Novel In-Situ Enzymatic Cleaning Method for Reducing Membrane Fouling in Membrane Bioreactors (MBRs). Indones. J. Sci. Technol. 2016, 1, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Bdiri, M.; Bensghaier, A.; Chaabane, L.; Kozmai, A.; Baklouti, L.; Larchet, C. Preliminary Study on Enzymatic-Based Cleaning of Cation-Exchange Membranes Used in Electrodialysis System in Red Wine Production. Membranes 2019, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Khoiruddin, K.; Ariono, D.; Subagjo, S.; Wenten, I.G. Improved Anti-Organic Fouling of Polyvinyl Chloride-Based Heterogeneous Anion-Exchange Membrane Modified by Hydrophilic Additives. J. Water Process Eng. 2021, 41, 102007. [Google Scholar] [CrossRef]
- Xie, H.; Pan, J.; Wei, B.; Feng, J.; Liao, S.; Li, X.; Yu, Y. Anti-Fouling Anion Exchange Membrane for Electrodialysis Fabricated by in-Situ Interpenetration of the Ionomer to Gradient Cross-Linked Network of Ca-Na Alginate. Desalination 2021, 505, 115005. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Jin, D.; Van der Bruggen, B. Modification of an Anion Exchange Membrane Based on Rapid Mussel-Inspired Deposition for Improved Antifouling Performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126267. [Google Scholar] [CrossRef]
- Ribera-Pi, J.; Badia-Fabregat, M.; Espí, J.; Clarens, F.; Jubany, I.; Martínez-Lladó, X. Decreasing Environmental Impact of Landfill Leachate Treatment by MBR, RO and EDR Hybrid Treatment. Environ. Technol. 2021, 42, 3508–3522. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]
- Meng, J.; Shi, L.; Hu, Z.; Hu, Y.; Lens, P.; Wang, S.; Zhan, X. Novel Electro-Ion Substitution Strategy in Electrodialysis for Ammonium Recovery from Digested Sludge Centrate in Coastal Regions. J. Membr. Sci. 2022, 642, 120001. [Google Scholar] [CrossRef]
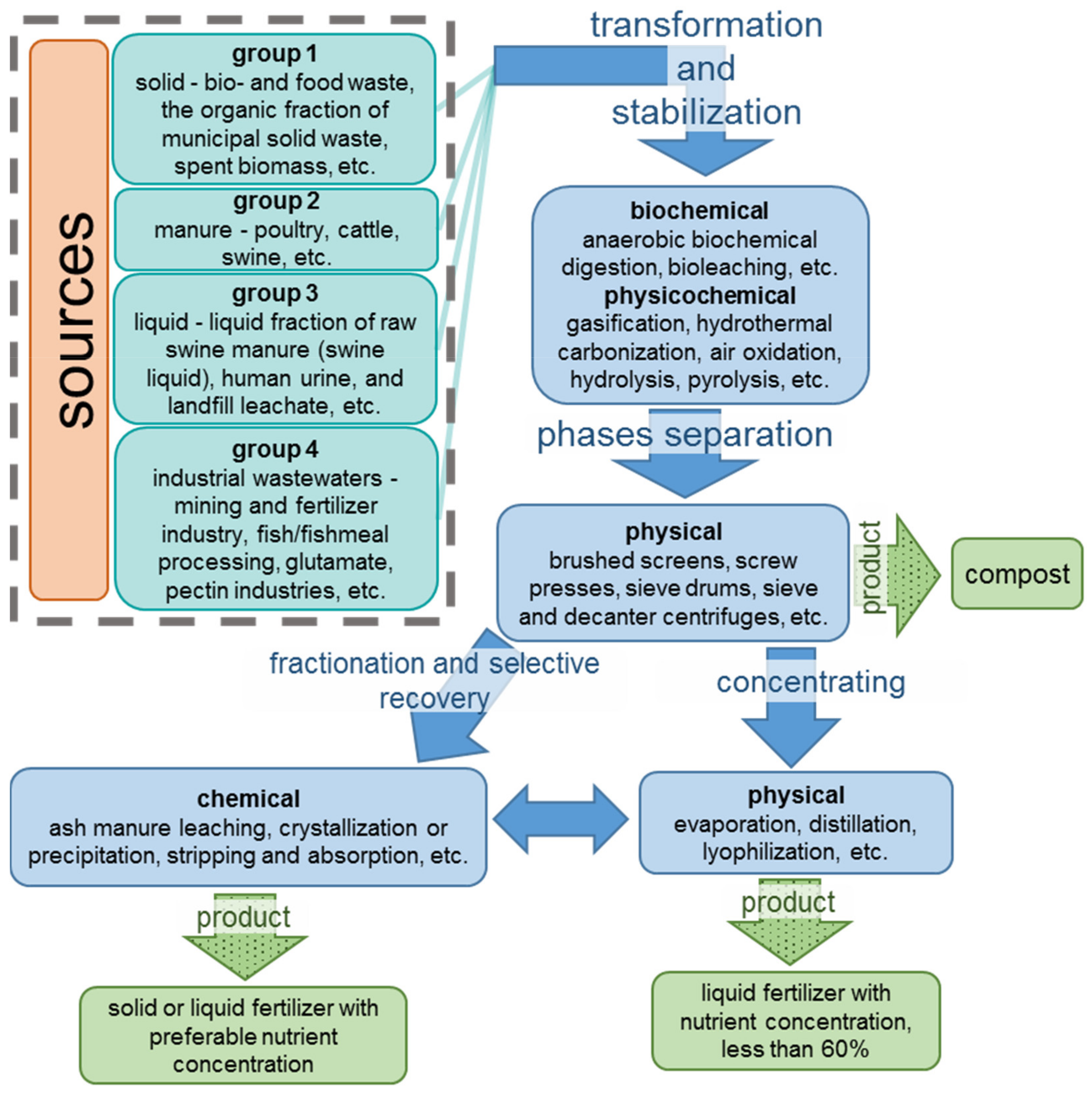
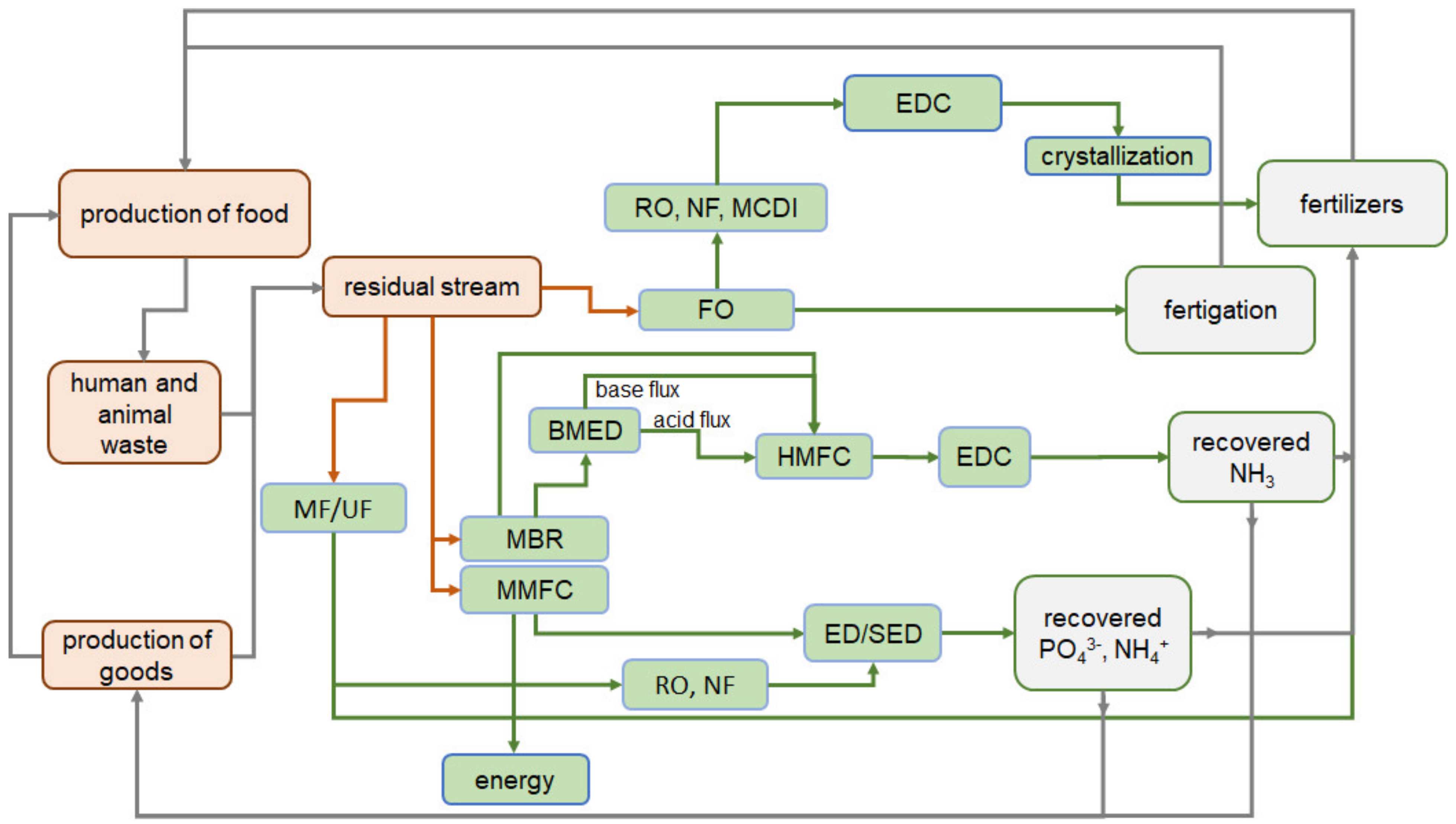
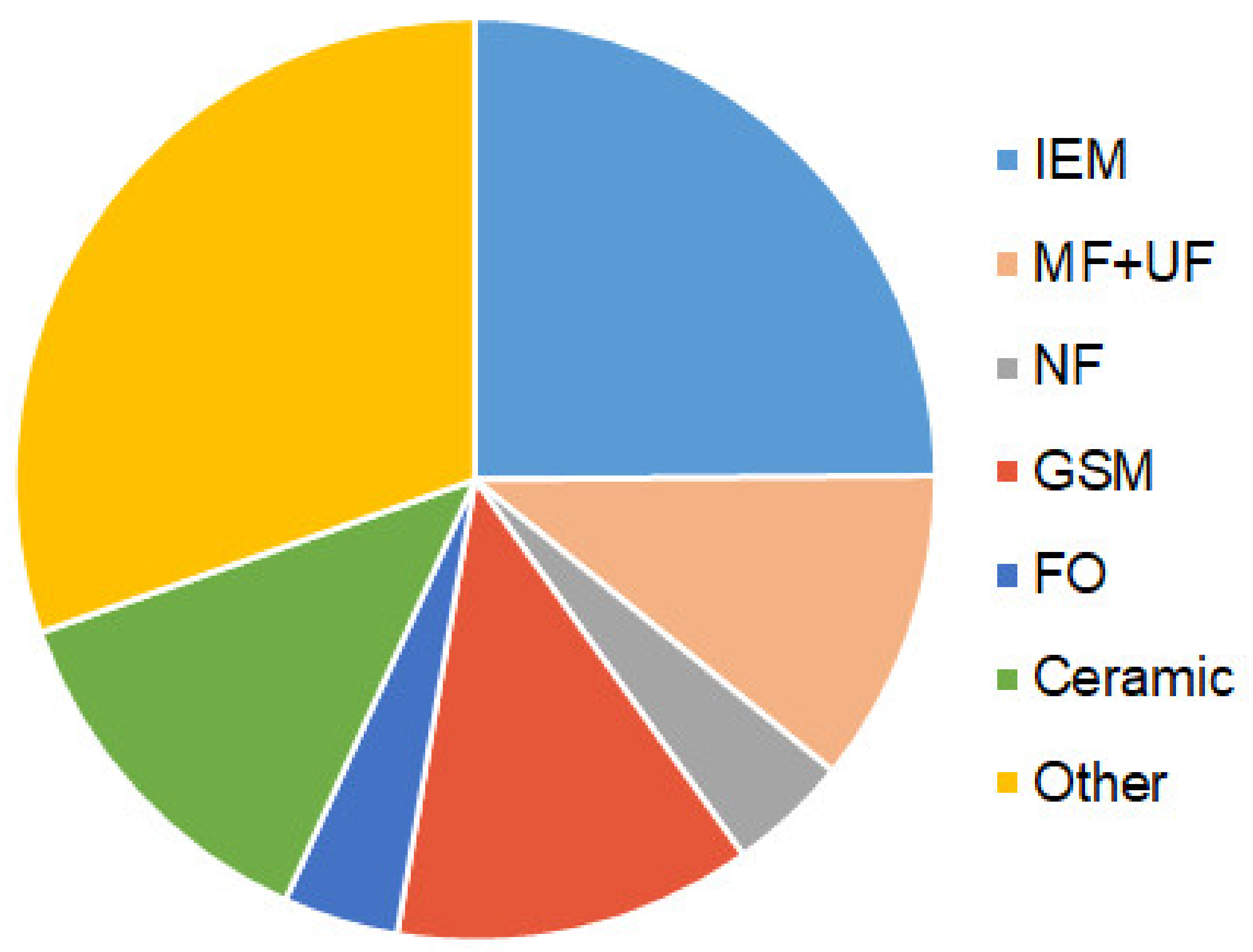

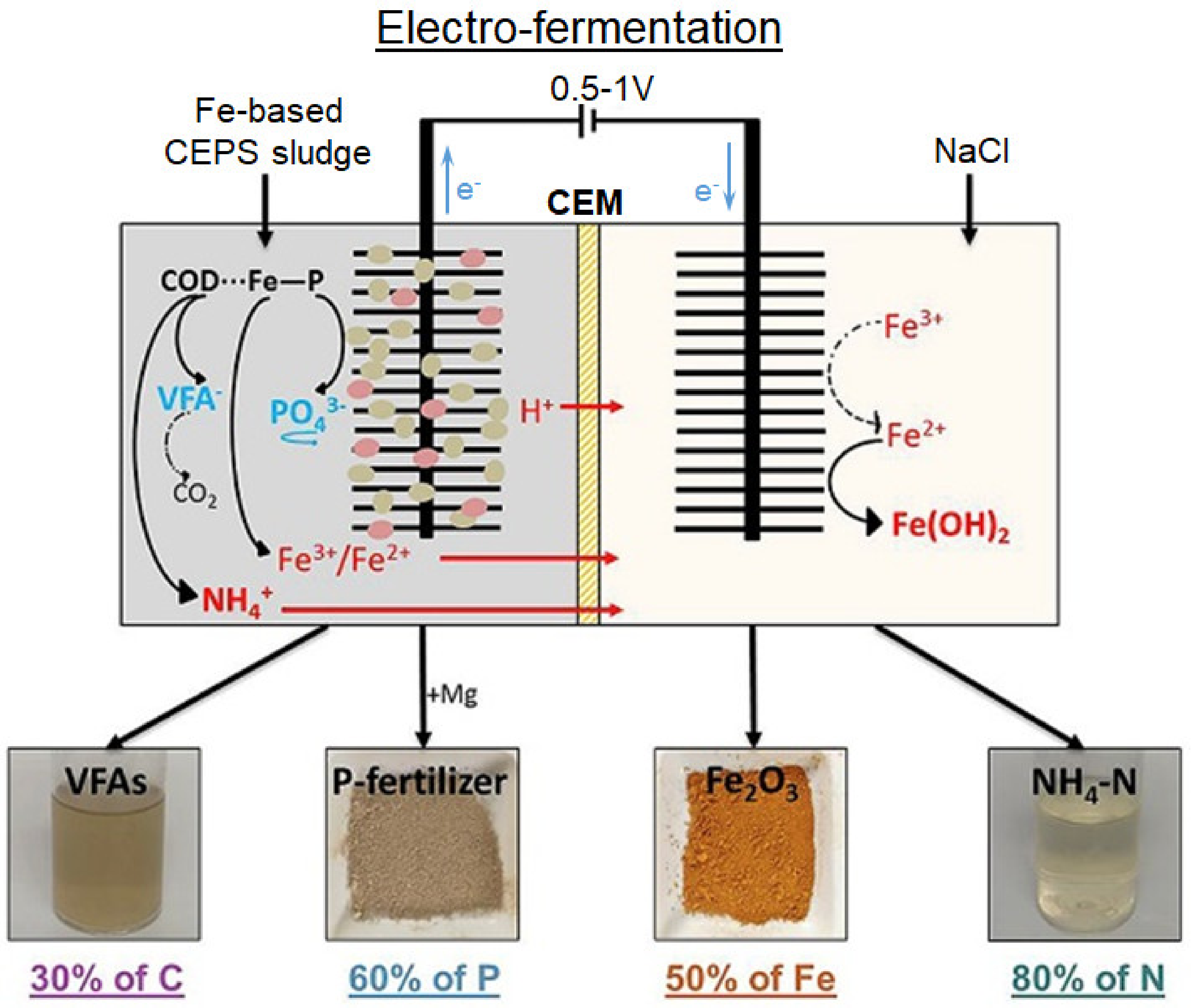
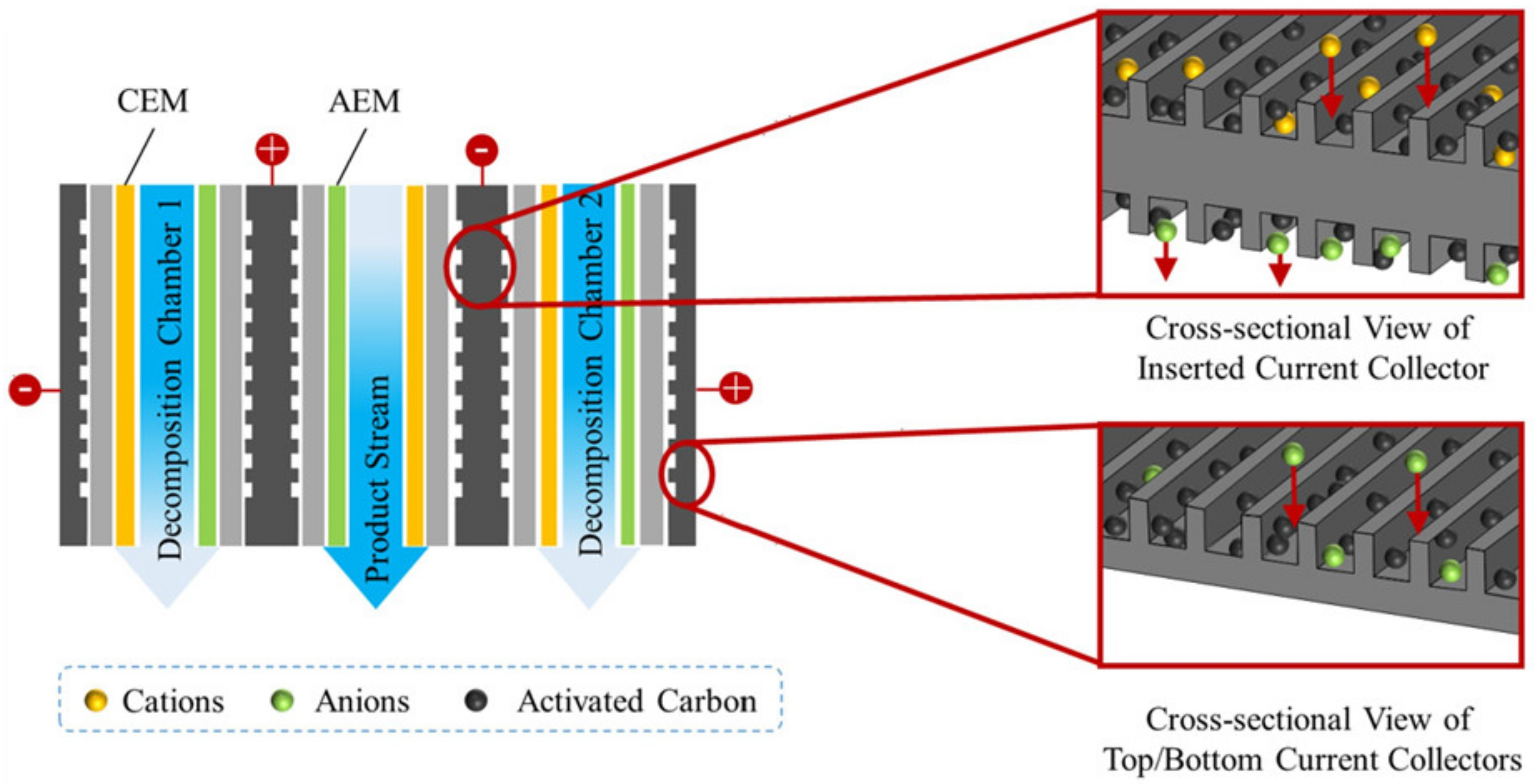



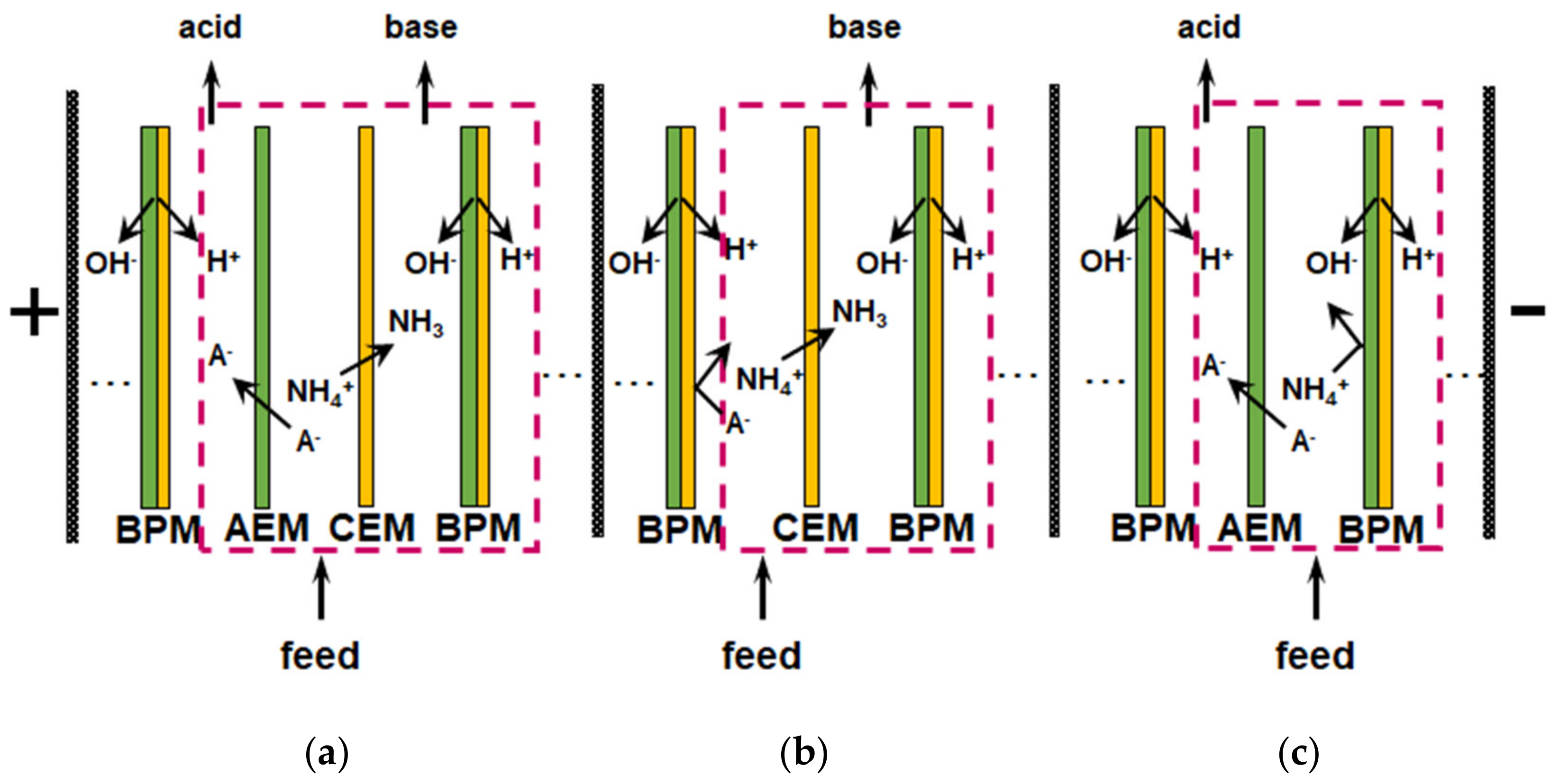
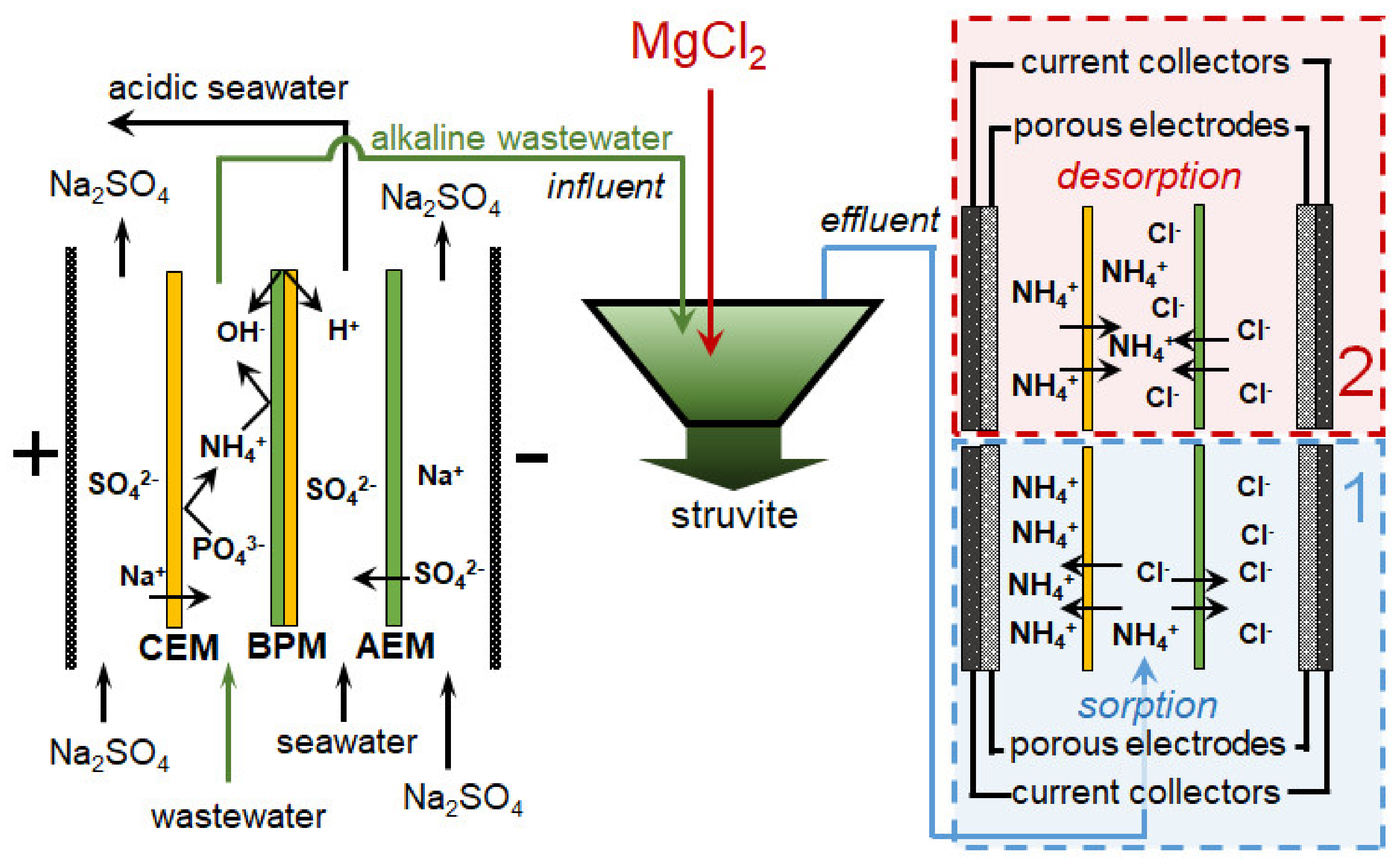

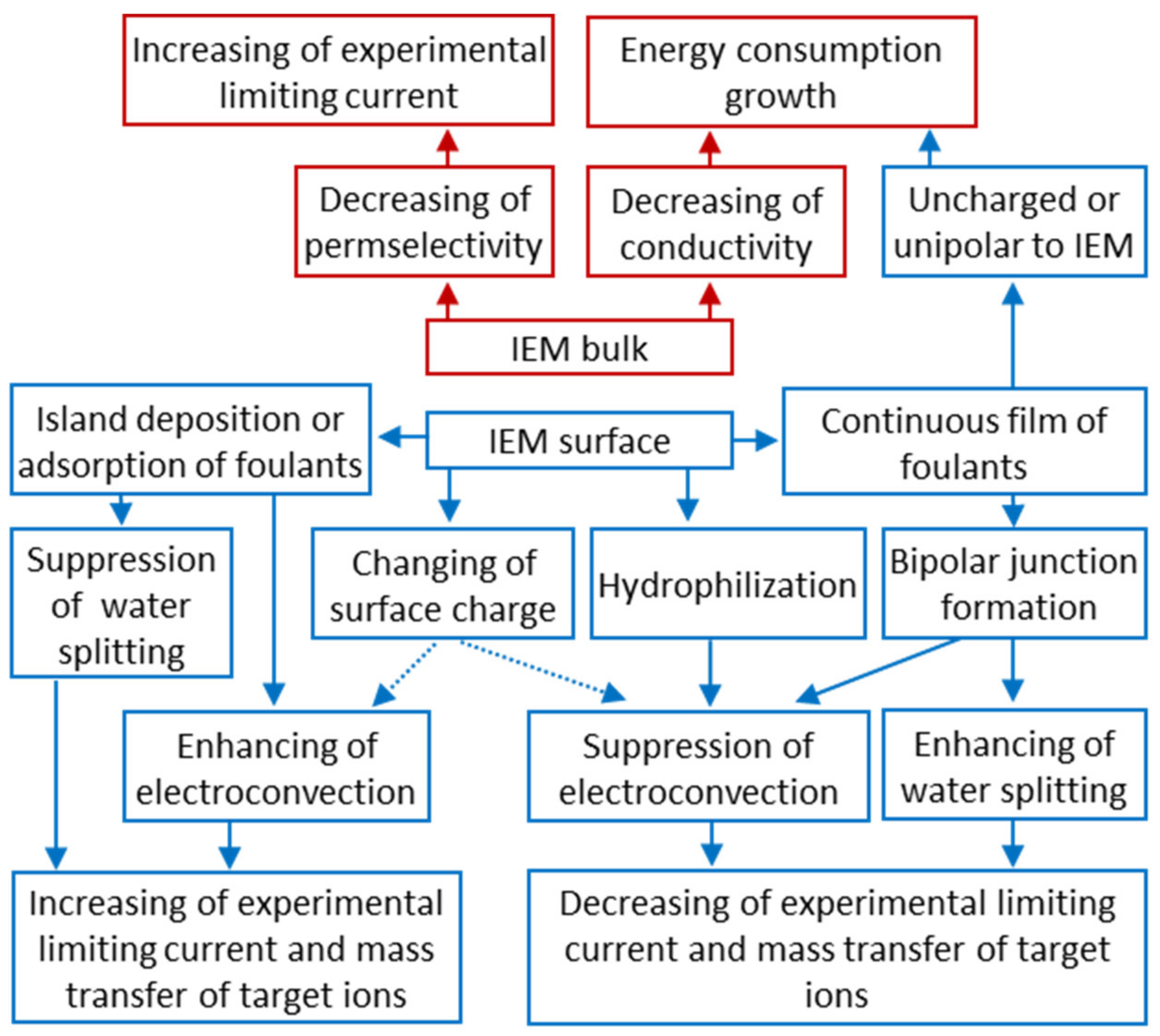
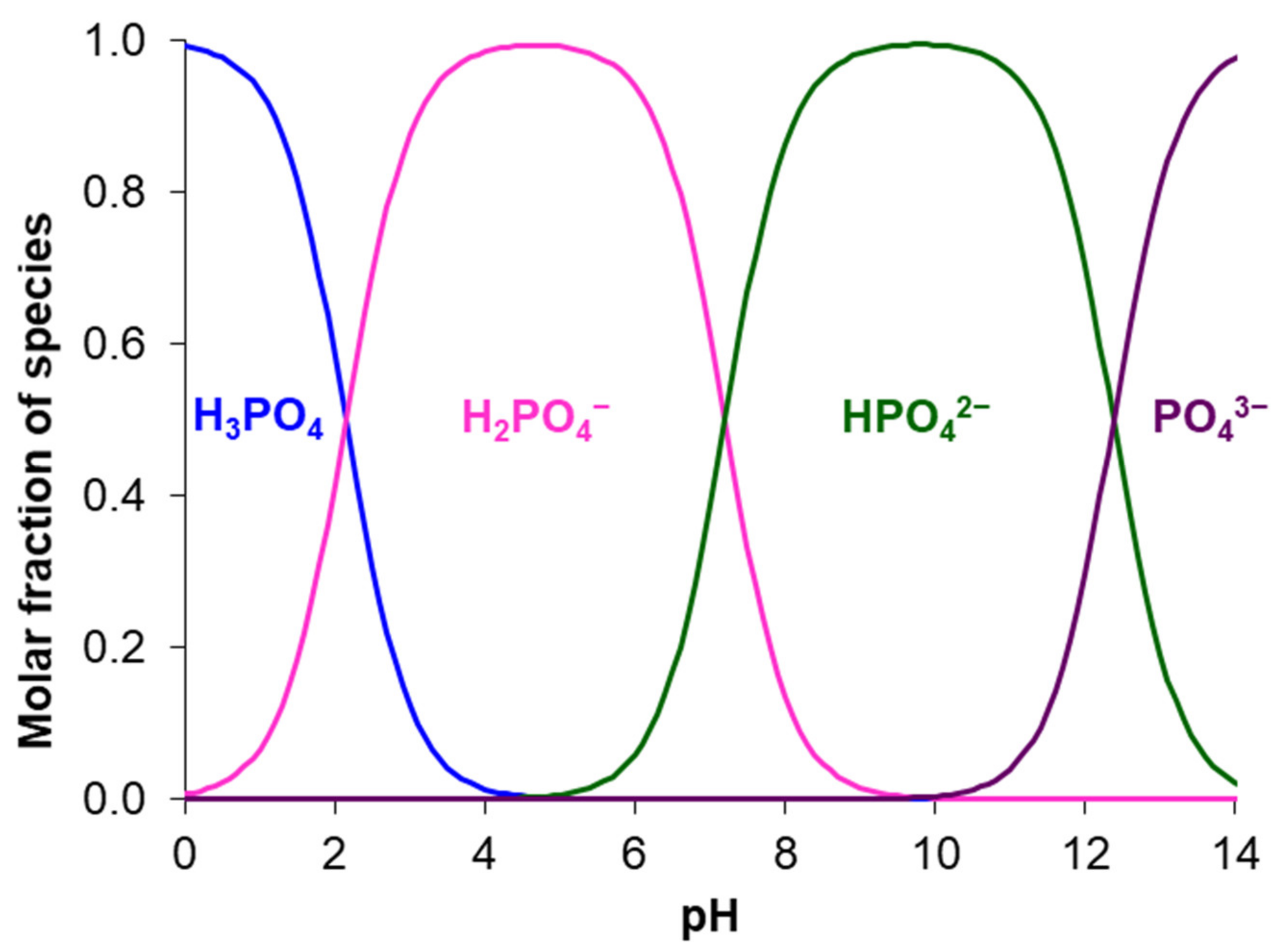


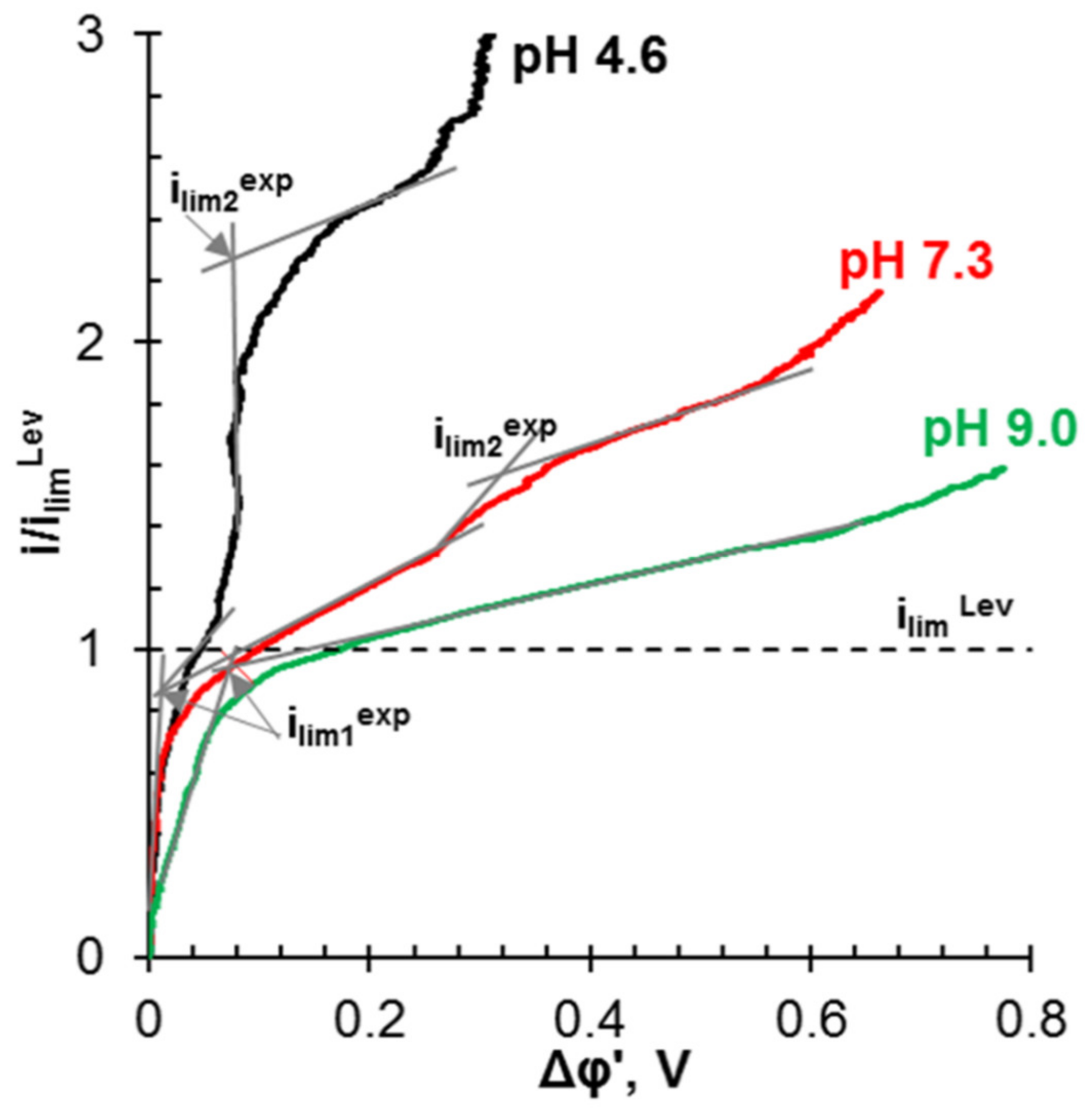

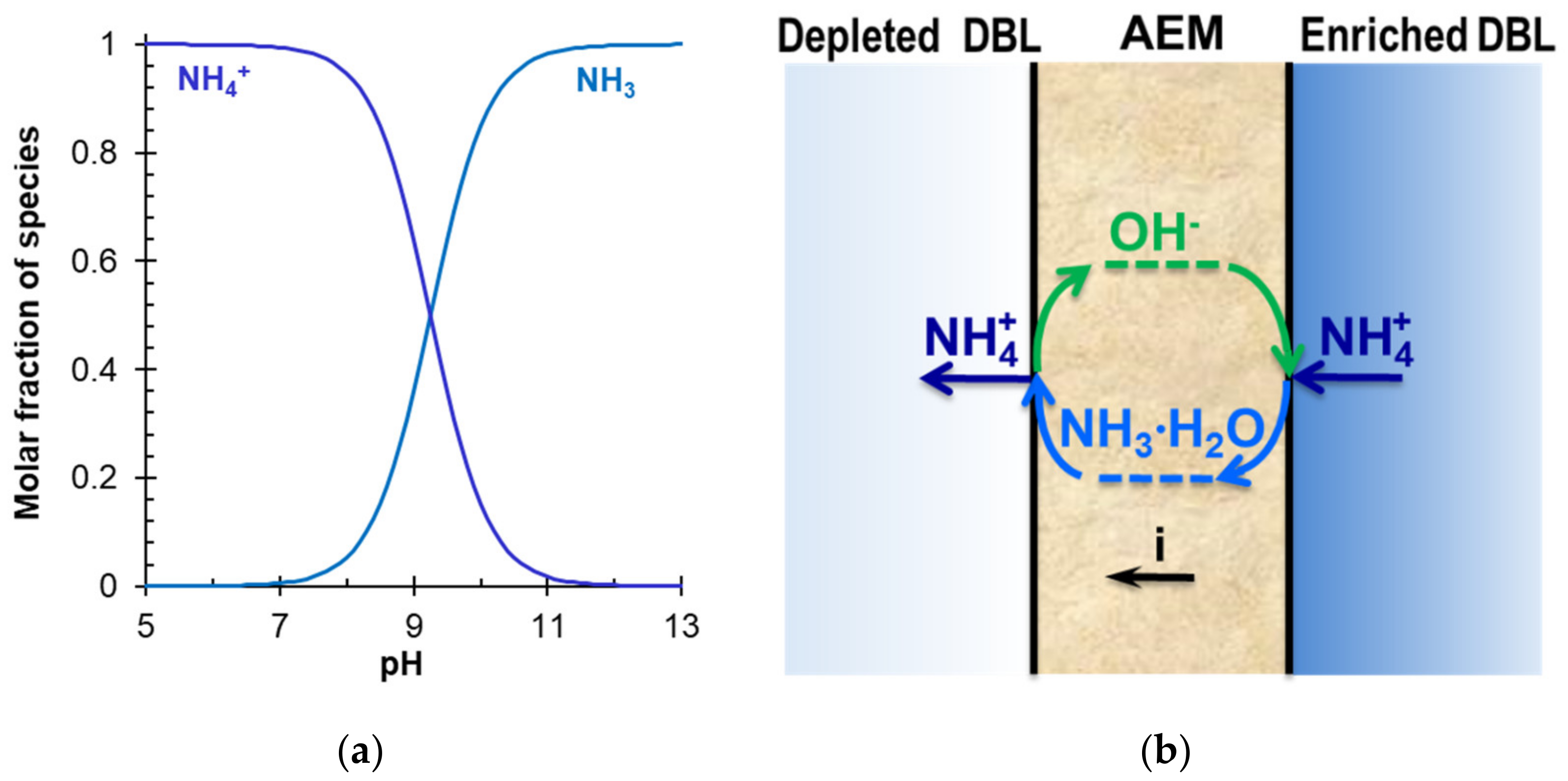
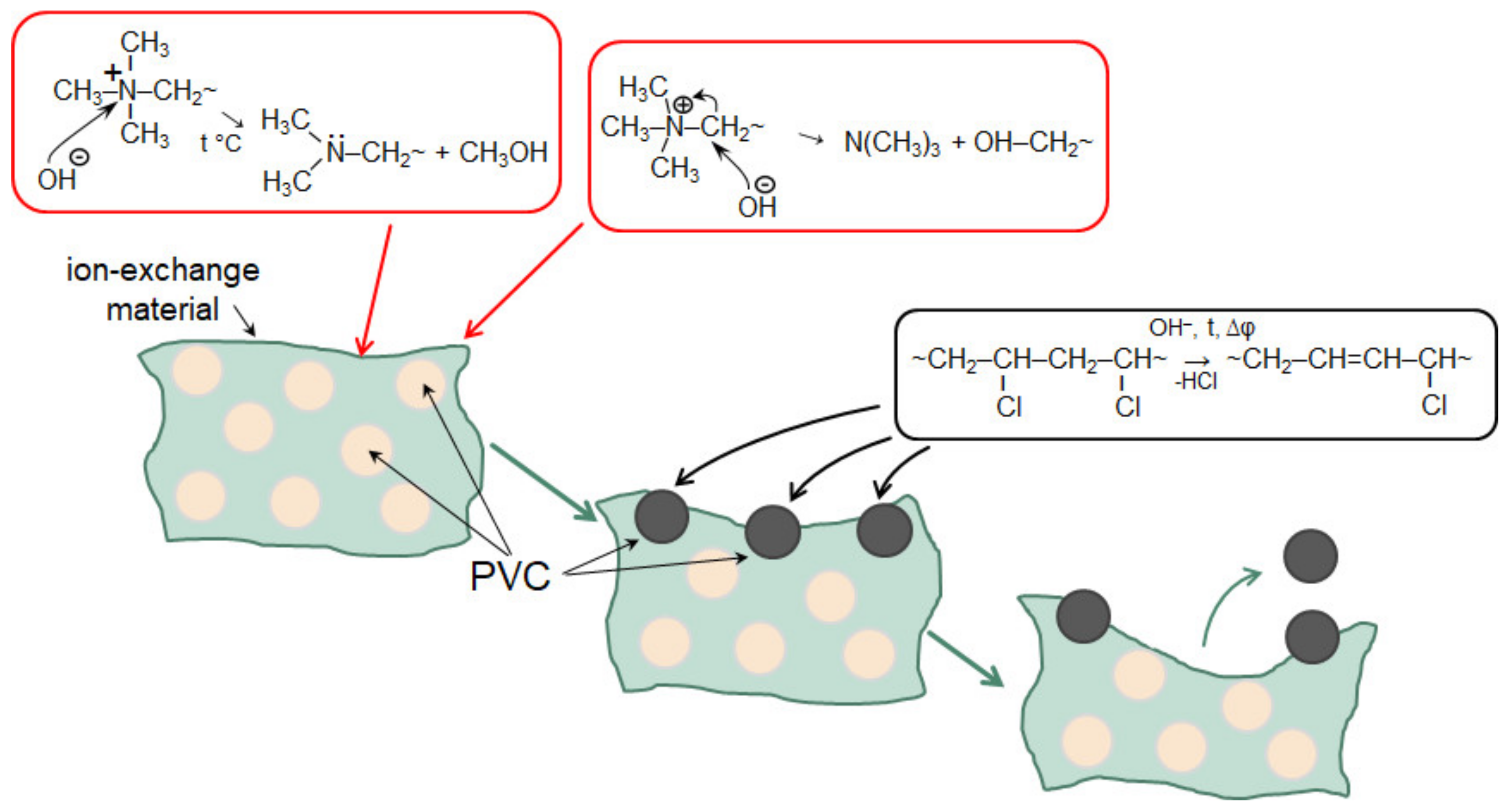
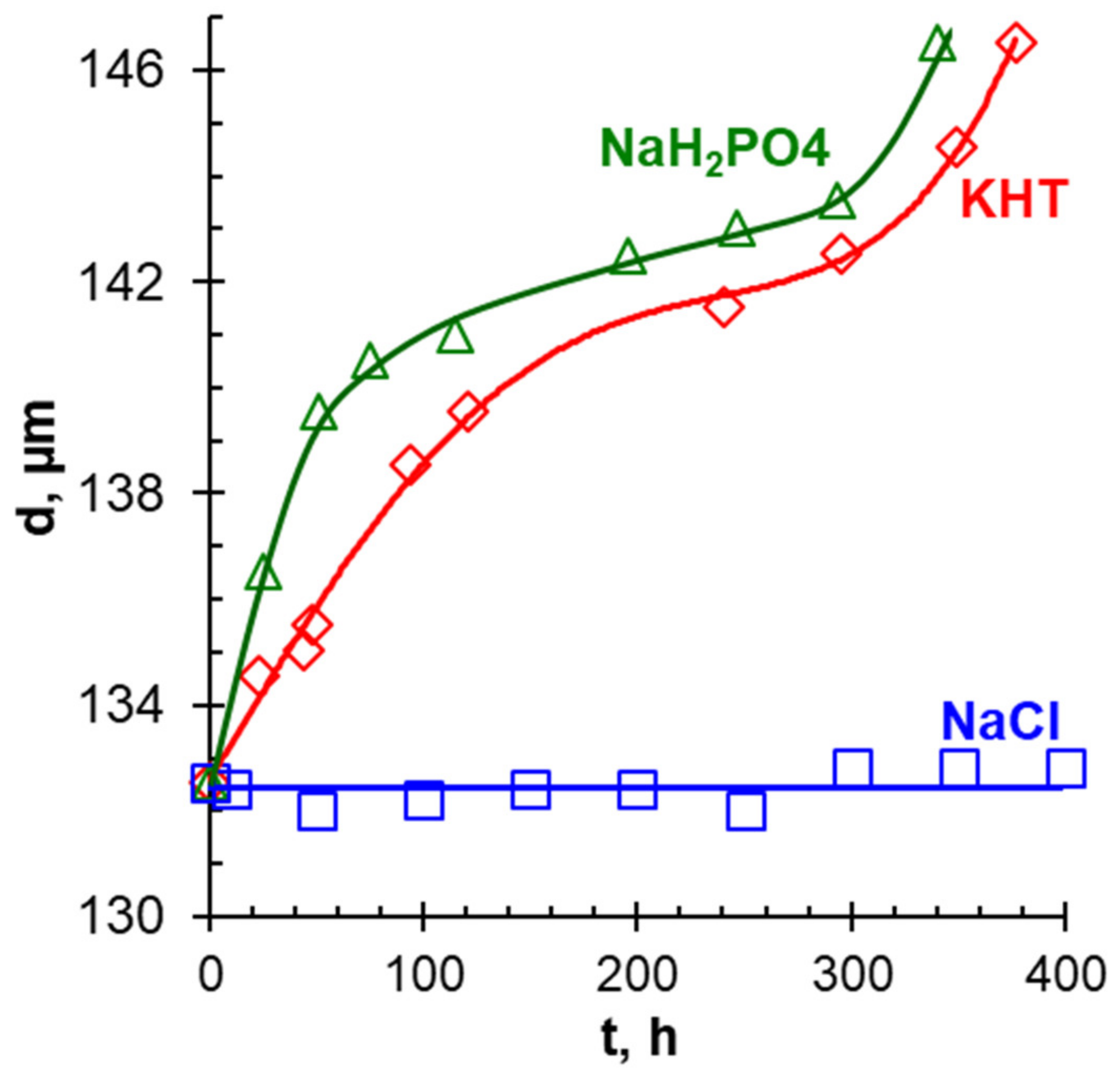

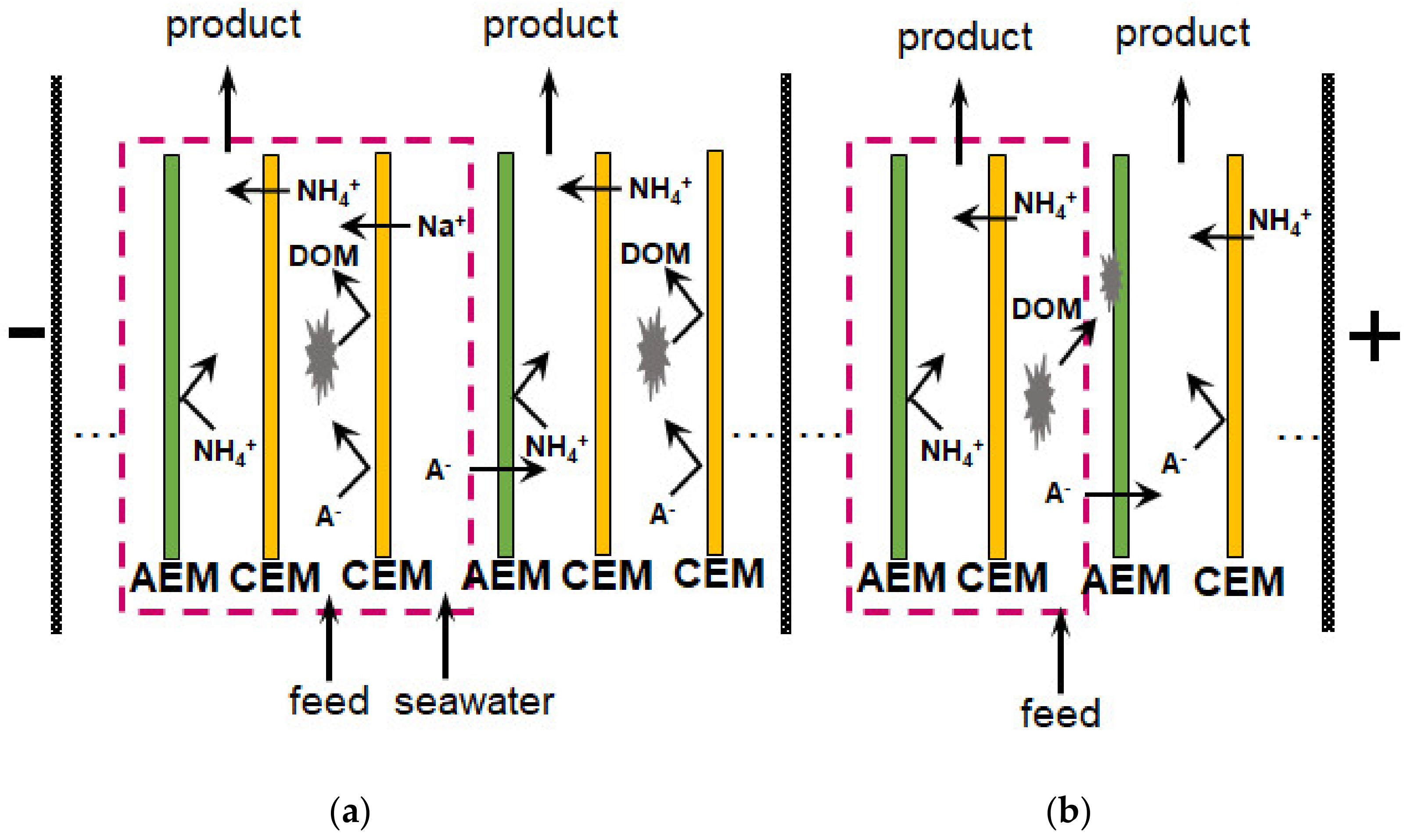
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pismenskaya, N.; Tsygurina, K.; Nikonenko, V. Recovery of Nutrients from Residual Streams Using Ion-Exchange Membranes: Current State, Bottlenecks, Fundamentals and Innovations. Membranes 2022, 12, 497. https://doi.org/10.3390/membranes12050497
Pismenskaya N, Tsygurina K, Nikonenko V. Recovery of Nutrients from Residual Streams Using Ion-Exchange Membranes: Current State, Bottlenecks, Fundamentals and Innovations. Membranes. 2022; 12(5):497. https://doi.org/10.3390/membranes12050497
Chicago/Turabian StylePismenskaya, Natalia, Kseniia Tsygurina, and Victor Nikonenko. 2022. "Recovery of Nutrients from Residual Streams Using Ion-Exchange Membranes: Current State, Bottlenecks, Fundamentals and Innovations" Membranes 12, no. 5: 497. https://doi.org/10.3390/membranes12050497
APA StylePismenskaya, N., Tsygurina, K., & Nikonenko, V. (2022). Recovery of Nutrients from Residual Streams Using Ion-Exchange Membranes: Current State, Bottlenecks, Fundamentals and Innovations. Membranes, 12(5), 497. https://doi.org/10.3390/membranes12050497