Vacuum-Assisted Interfacial Polymerization Technique for Enhanced Pervaporation Separation Performance of Thin-Film Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified PAN (mPAN) Porous Membrane Supports
2.3. Fabrication of TFC Membranes
2.4. Evaluation of Diamine Monomer Distribution in Porous Support
2.5. Membrane Characterization
2.6. Membrane Performance Test
3. Results and Discussion
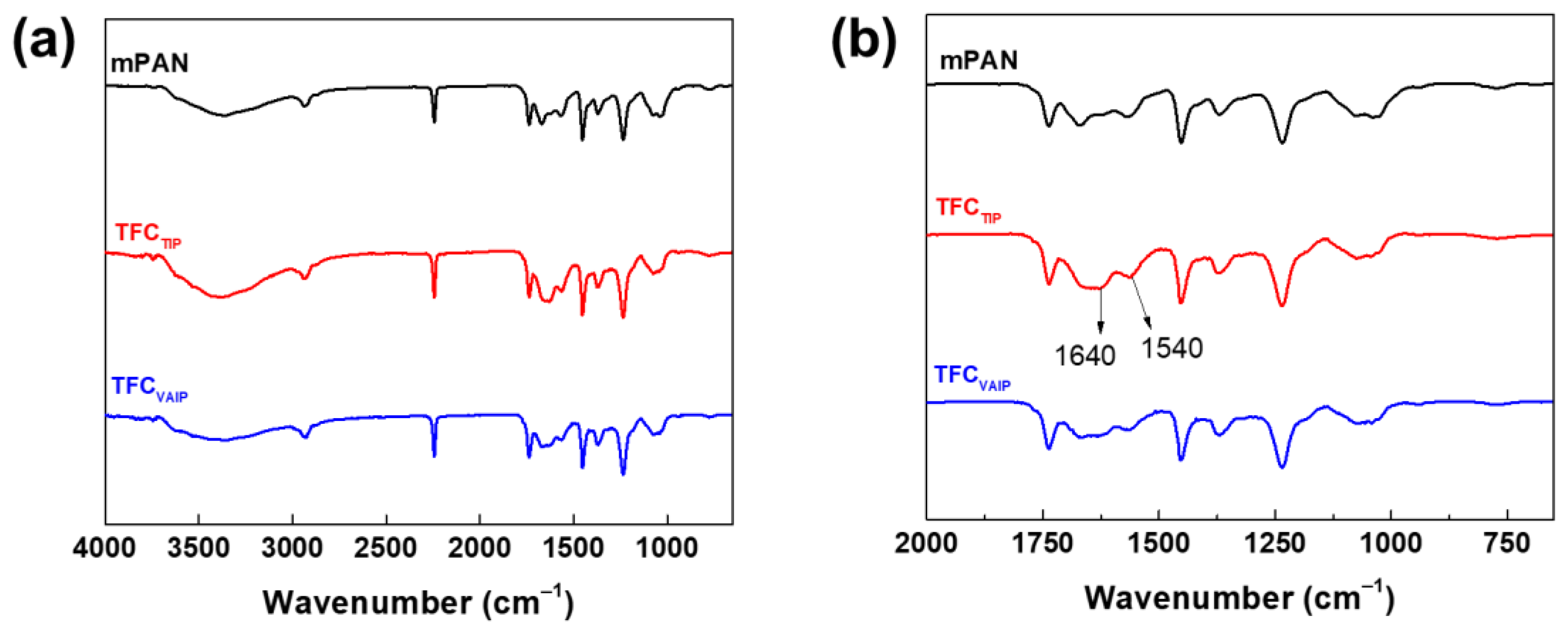
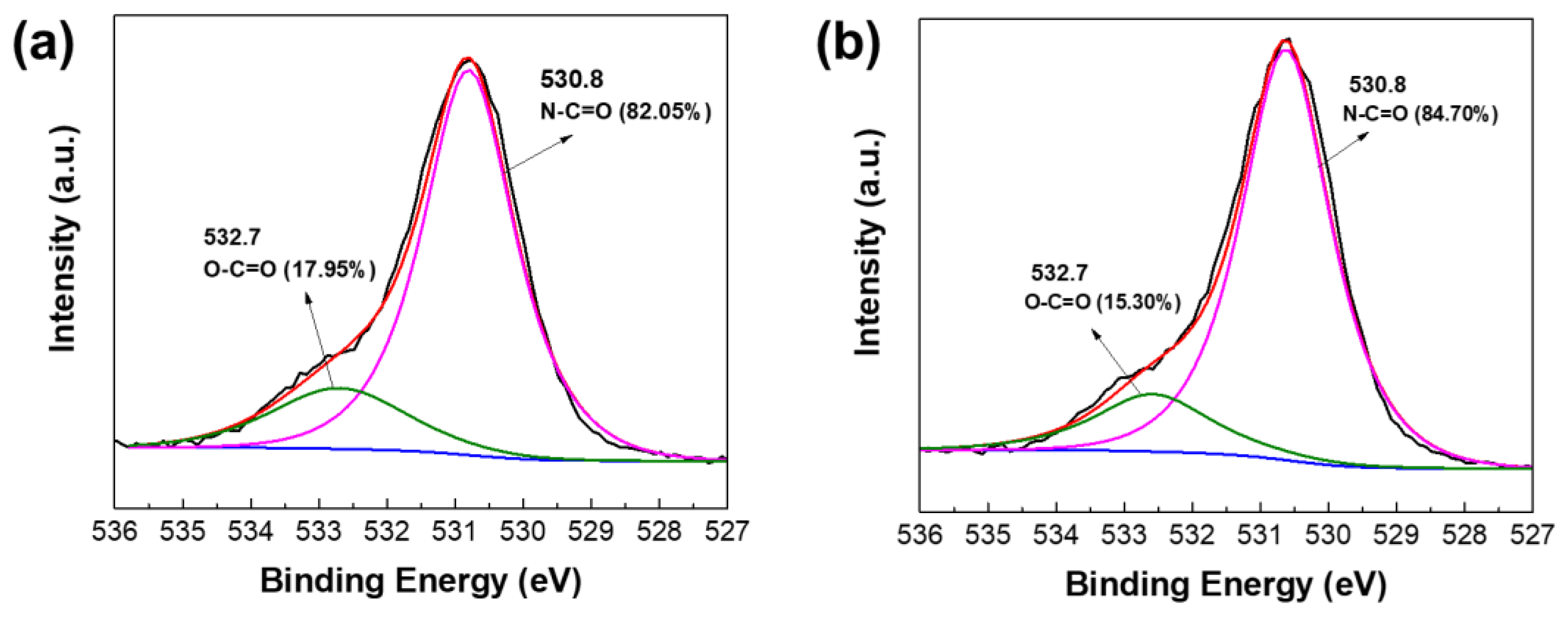
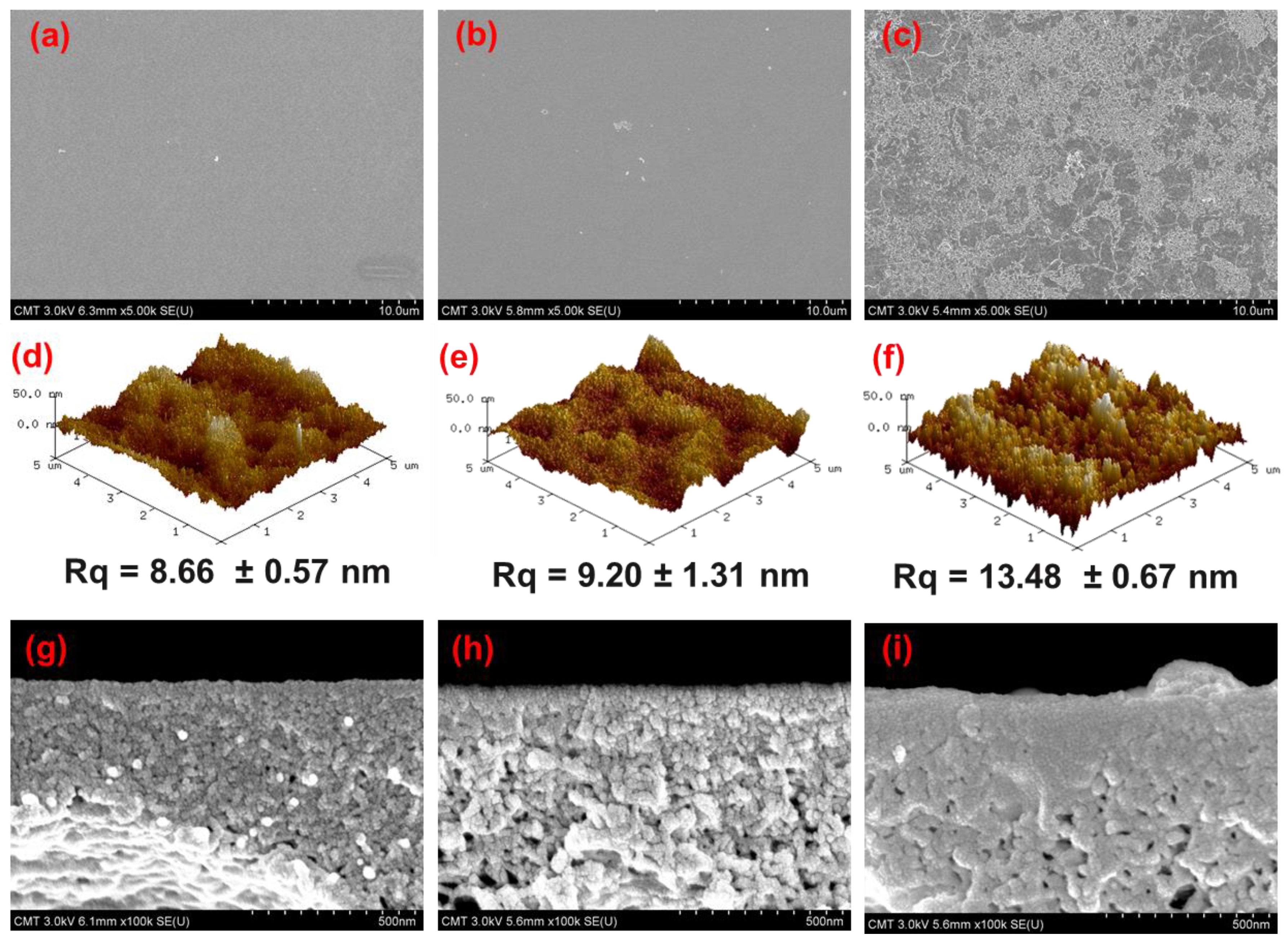
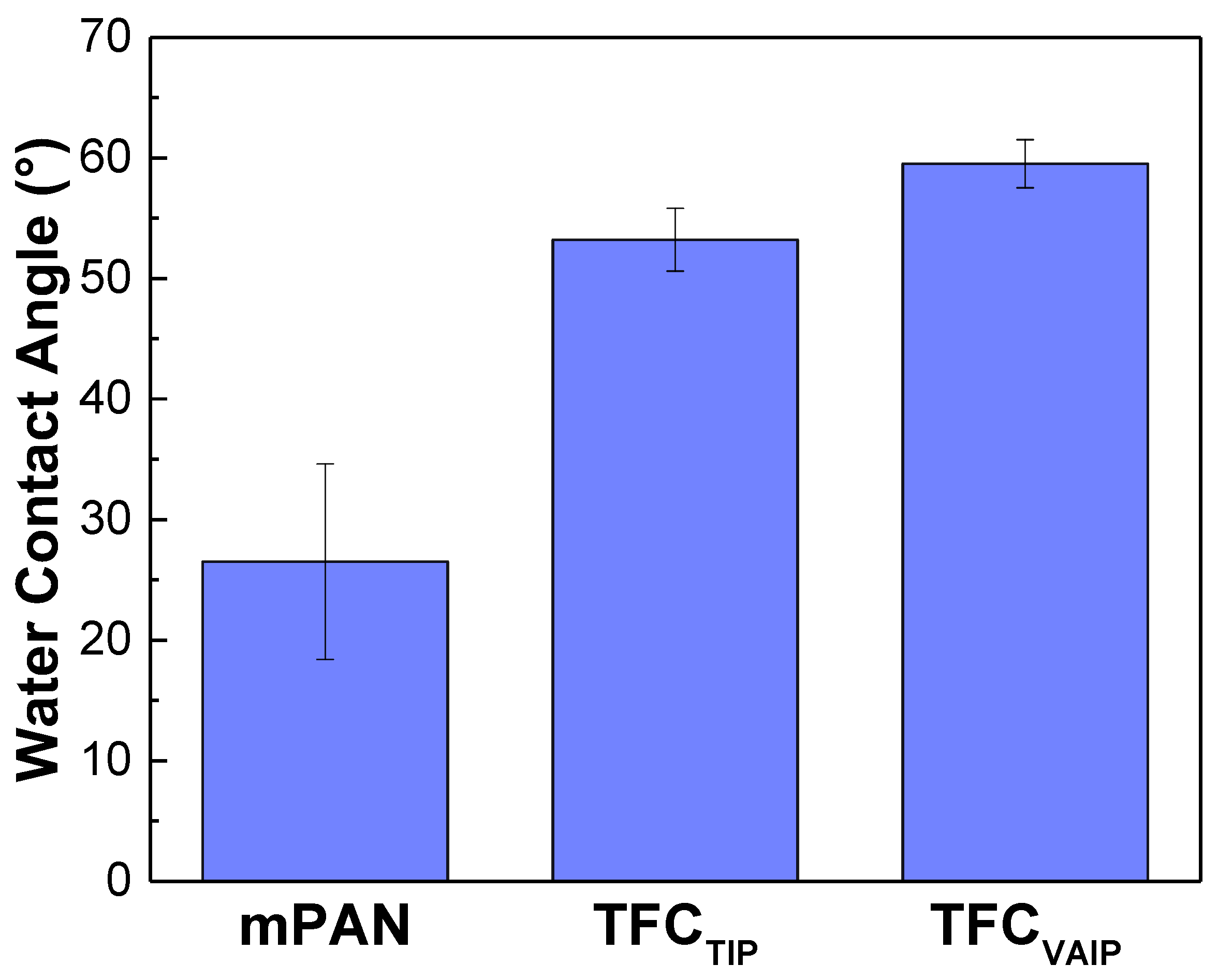
3.1. Chemical Structure and Morphology of TFC Membranes
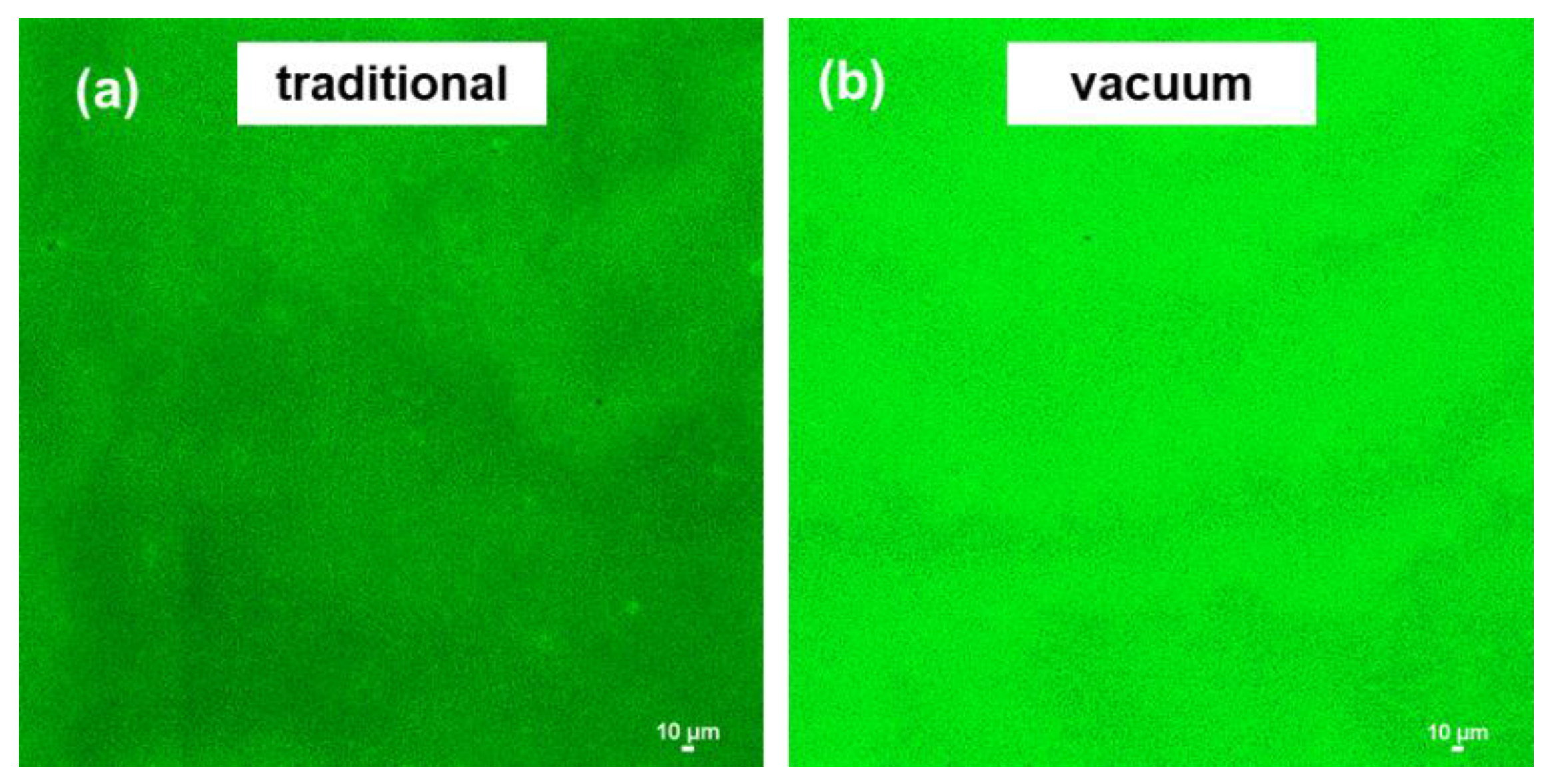
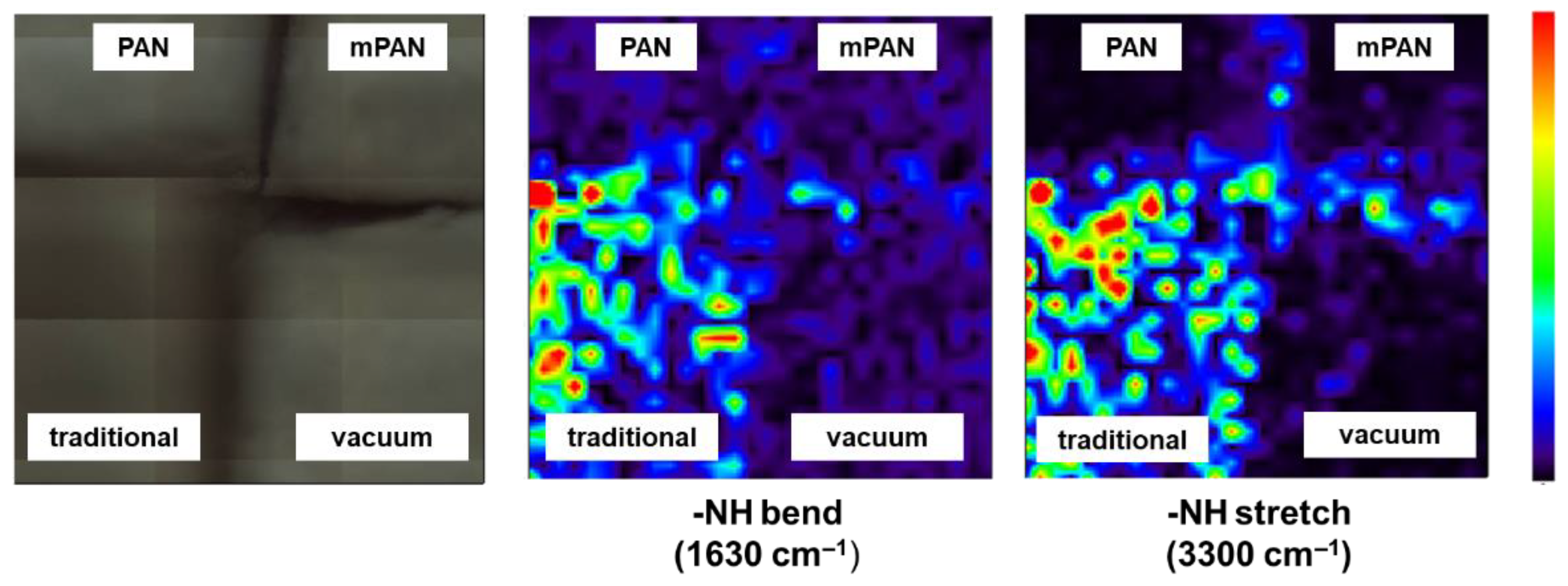
3.2. Diamine Monomer Spreading on mPAN Support
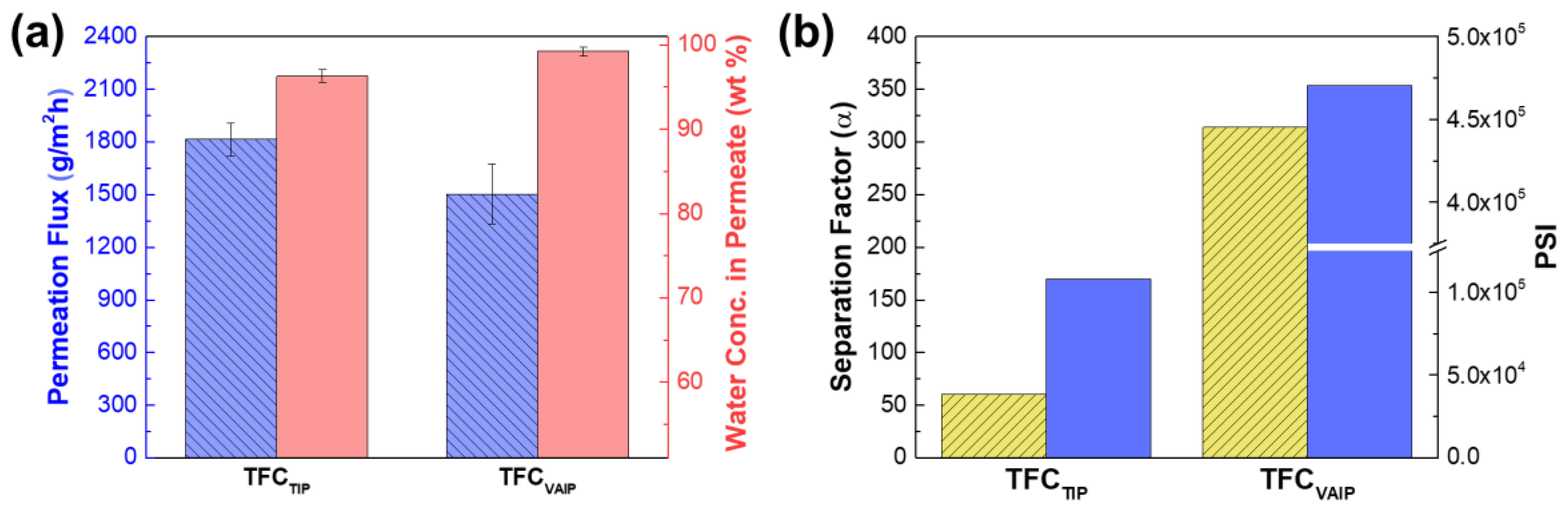
3.3. Pervaporation Performance of TFC Membranes
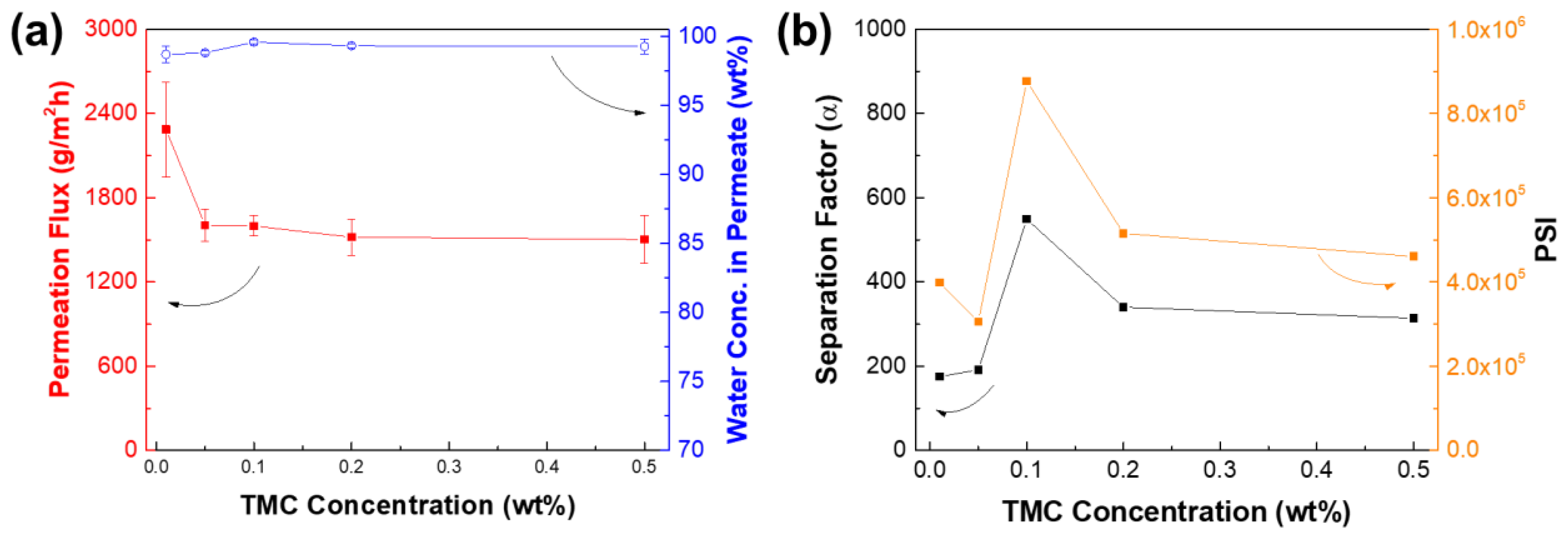
3.4. Optimization of IP Conditions for TFCVAIP Membranes
3.5. Membrane Stability Test and Comparison with Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Symbols
| J | permeation flux |
| Ji | initial permeation flux |
| α | separation factor |
| XW | water concentration in feed |
| XA | alcohol concentration in feed |
| YW | water concentration in permeate |
| YA | alcohol concentration in permeate |
References
- Cadoret, I.; Padovano, F. The political drivers of renewable energies policies. Energy Econ. 2016, 56, 261–269. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Connor, M.R.; Atsumi, S. Synthetic biology guides biofuel production. J. Biomed. Biotechnol. 2010, 2010, 541698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, K.; Koda, S.; Oshima, Y. Catalytic Hydration of Propylene with MoO3/Al2O3 in Supercritical Water. Ind. Eng. Chem. Res. 2002, 41, 3341–3344. [Google Scholar] [CrossRef]
- Jojima, T.; Inui, M.; Yukawa, H. Production of isopropanol by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2008, 77, 1219–1224. [Google Scholar] [CrossRef]
- Baker, R.; Cussler, E.; Eykamp, W.; Koros, W.; Riley, R.; Strathmann, H. Membrane Separation Systems-Recent Developments and Future Directions; William Andrew Inc.: Park Ridge, NJ, USA, 1991. [Google Scholar]
- Nunes, S.P.; Peinemann, K.-V. Membrane Technology; Wiley Online Library: Hoboken, NJ, USA, 2001. [Google Scholar]
- Jiang, L.Y.; Wang, Y.; Chung, T.-S.; Qiao, X.Y.; Lai, J.-Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Polym. Sci. 2009, 34, 1135–1160. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, X.; Ma, T.; Xue, C.; Wu, M.; Ji, M.; Li, Y. Green recovery of hazardous acetonitrile from high-salt chemical wastewater by pervaporation. J. Cleaner Prod. 2018, 197, 742–749. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y.M. Liquid Separation by Membrane Pervaporation: A Review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Ghazali, M.; Nawawi, M.; Huang, R.Y. Pervaporation dehydration of isopropanol with chitosan membranes. J. Membr. Sci. 1997, 124, 53–62. [Google Scholar] [CrossRef]
- Ge, J.; Cui, Y.; Yan, Y.; Jiang, W. The effect of structure on pervaporation of chitosan membrane. J. Membr. Sci. 2000, 165, 75–81. [Google Scholar] [CrossRef]
- Anjali Devi, D.; Smitha, B.; Sridhar, S.; Aminabhavi, T.M. Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J. Membr. Sci. 2005, 262, 91–99. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Savon, N.A.; Missyul, A.B.; Mazur, A.S.; Kuzminova, A.I.; Zolotarev, A.A.; Mikhailovskii, V.; Lahderanta, E.; Markelov, D.A.; et al. Novel mixed-matrix membranes based on polyvinyl alcohol modified by carboxyfullerene for pervaporation dehydration. Sep. Purif. Technol. 2018, 204, 1–12. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, R.Y.M.; Feng, X. Preparation and properties of trimesoyl chloride crosslinked poly(vinyl alcohol) membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2006, 286, 245–254. [Google Scholar] [CrossRef]
- Yeom, C.-K.; Lee, K.-H. Pervaporation separation of water-acetic acid mixtures through poly(vinyl alcohol) membranes crosslinked with glutaraldehyde. J. Membr. Sci. 1996, 109, 257–265. [Google Scholar] [CrossRef]
- Zhao, Q.; An, Q.F.; Ji, Y.; Qian, J.; Gao, C. Polyelectrolyte complex membranes for pervaporation, nanofiltration and fuel cell applications. J. Membr. Sci. 2011, 379, 19–45. [Google Scholar] [CrossRef]
- Liu, T.; An, Q.-F.; Zhao, Q.; Lee, K.-R.; Zhu, B.-K.; Qian, J.-W.; Gao, C.-J. Preparation and characterization of polyelectrolyte complex membranes bearing alkyl side chains for the pervaporation dehydration of alcohols. J. Membr. Sci. 2013, 429, 181–189. [Google Scholar] [CrossRef]
- Kalyani, S.; Smitha, B.; Sridhar, S.; Krishnaiah, A. Pervaporation separation of ethanol–water mixtures through sodium alginate membranes. Desalination 2008, 229, 68–81. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Different viscosity grade sodium alginate and modified sodium alginate membranes in pervaporation separation of water + acetic acid and water + isopropanol mixtures. J. Membr. Sci. 2004, 228, 199–208. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.-P.; Xu, Z.-L.; Cheng, F.-Y.; Ma, X.-H.; Xu, X.-R. Thin-film composite membranes fabricated directly on a large-porous ceramic support using poly (4-styrenesulfonic acid) as a scaffold for ethanol dehydration. J. Membr. Sci. 2021, 619, 118775. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.-S. Design and synthesis of a fluoro-silane amine monomer for novel thin film composite membranes to dehydrate ethanol via pervaporation. J. Mater. Chem. A 2013, 1, 9814–9826. [Google Scholar] [CrossRef]
- Freger, V. Kinetics of film formation by interfacial polycondensation. Langmuir 2005, 21, 1884–1894. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Marquez, J.A.D.; Huang, S.H.; Lee, K.R. A recent review of developmental trends in fabricating pervaporation membranes through interfacial polymerization and future prospects. J. Ind. Eng. Chem. 2021, 97, 129–141. [Google Scholar] [CrossRef]
- An, Q.F.; Ang, M.B.M.Y.; Huang, Y.H.; Huang, S.H.; Chia, Y.H.; Lai, C.L.; Tsai, H.A.; Hun, W.S.; Hu, C.C.; Wu, Y.P.; et al. Microstructural characterization and evaluation of pervaporation performance of thin-film composite membranes fabricated through interfacial polymerization on hydrolyzed polyacrylonitrile substrate. J. Membr. Sci. 2019, 583, 31–39. [Google Scholar] [CrossRef]
- Zuo, J.; Lai, J.Y.; Chung, T.S. In-situ synthesis and cross-linking of polyamide thin film composite (TFC) membranes for bioethanol applications. J. Membr. Sci. 2014, 458, 47–57. [Google Scholar] [CrossRef]
- Kang, X.; Liu, X.; Liu, J.; Wen, Y.; Qi, J.; Li, X. Spin-assisted interfacial polymerization strategy for graphene oxide-polyamide composite nanofiltration membrane with high performance. Appl. Surf. Sci. 2020, 508, 145198. [Google Scholar] [CrossRef]
- Alibakhshian, F.; Pourafshari Chenar, M.; Asghari, M. Thin film composite membranes with desirable support layer for MeOH/MTBE pervaporation. J. Appl. Polym. Sci. 2019, 136, 47519. [Google Scholar] [CrossRef]
- Kong, B.S.; Geng, J.; Jung, H.T. Layer-by-layer assembly of graphene and gold nanoparticles by vacuum filtration and spontaneous reduction of gold ions. Chem. Commun. 2009, 16, 2174–2176. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Zeng, W.-J.; Jiang, T.-T.; Chen, X.; Zhang, X.-L. Incorporating attapulgite nanorods into graphene oxide nanofiltration membranes for efficient dyes wastewater treatment. Sep. Purif. Technol. 2019, 214, 21–30. [Google Scholar] [CrossRef]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Tseng, H.H.; Wahab, R.A.; Ismail, A.F. Progress of Interfacial Polymerization Techniques for Polyamide Thin Film (Nano)Composite Membrane Fabrication: A Comprehensive Review. Polymers 2020, 12, 2817. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Li, H.-N.; Yang, J.; Li, J.-J.; Ye, J.-R.; Xu, Z.-K. Vacuum-assisted diamine monomer distribution for synthesizing polyamide composite membranes by interfacial polymerization. J. Membr. Sci. 2020, 616, 118557. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Tan, Y.H.; Ng, B.C.; Ismail, A.F. A novel interfacial polymerization approach towards synthesis of graphene oxide-incorporated thin film nanocomposite membrane with improved surface properties. Arabian J. Chem. 2019, 12, 75–87. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Tan, Y.H.; Chong, C.Y.; Krause-Rehberg, R.; Awad, S. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem. Eng. J. 2018, 344, 524–534. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Ang, M.; Huang, S.H.; Hu, F.C.; Chiao, Y.H.; Tsai, H.A.; Lee, K.R. Cosolvent-Driven Interfacial Polymerization for Superior Separation Performance of Polyurea-Based Pervaporation Membrane. Polymers 2021, 13, 1179. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Yang, J.; Zhu, C.-Y.; Zhang, L.; Xu, Z.-K. Nanofiltration membranes with hydrophobic microfiltration substrates for robust structure stability and high water permeation flux. J. Membr. Sci. 2020, 593, 117444. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Gallardo, M.R.; Dizon, G.V.C.; De Guzman, M.R.; Tayo, L.L.; Huang, S.H.; Lai, C.L.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; et al. Graphene oxide functionalized with zwitterionic copolymers as selective layers in hybrid membranes with high pervaporation performance. J. Membr. Sci. 2019, 587, 117188. [Google Scholar] [CrossRef]
- Valamohammadi, E.; Behdarvand, F.; Tofighy, M.A.; Mohammadi, T. Preparation of positively charged thin-film nanocomposite membranes based on the reaction between hydrolyzed polyacrylonitrile containing carbon nanomaterials and HPEI for water treatment application. Sep. Purif. Technol. 2020, 242, 116826. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, H.; Ji, S.; Liu, Z. Self-assembly of polyelectrolyte multilayer pervaporation membranes by a dynamic layer-by-layer technique on a hydrolyzed polyacrylonitrile ultrafiltration membrane. J. Membr. Sci. 2007, 292, 1–8. [Google Scholar] [CrossRef]
- Shen, L.; Hung, W.-s.; Zuo, J.; Zhang, X.; Lai, J.-Y.; Wang, Y. High-performance thin-film composite polyamide membranes developed with green ultrasound-assisted interfacial polymerization. J. Membr. Sci. 2019, 570–571, 112–119. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Li, S.; Su, B.; Han, L.; Mandal, B. Graphene oxide (GO)-interlayered thin-film nanocomposite (TFN) membranes with high solvent resistance for organic solvent nanofiltration (OSN). J. Mater. Chem. A 2019, 7, 13315–13330. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Liu, C.; Yang, J.; Guo, B.-B.; Li, H.-N.; Xu, Z.-K. Polyamide nanofilms with linearly-tunable thickness for high performance nanofiltration. J. Membr. Sci. 2021, 627, 119142. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Zhang, X.; Zhang, S. Polyamide thin-film composite membranes prepared from a novel triamine 3,5-diamino-N-(4-aminophenyl)-benzamide monomer and m-phenylenediamine. J. Membr. Sci. 2010, 353, 78–84. [Google Scholar] [CrossRef]
- Pawley, J. Handbook of Biological Confocal Microscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 236. [Google Scholar]
- Cheng, X.; Pan, F.; Wang, M.; Li, W.; Song, Y.; Liu, G.; Yang, H.; Gao, B.; Wu, H.; Jiang, Z. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of gases and vapors in glassy and rubbery polymers. In Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons: Chichester, UK, 2006; pp. 1–49. [Google Scholar]
- Akhtar, F.H.; Kumar, M.; Vovusha, H.; Shevate, R.; Villalobos, L.F.; Schwingenschlögl, U.; Peinemann, K.-V. Scalable Synthesis of Amphiphilic Copolymers for CO2- and Water-Selective Membranes: Effect of Copolymer Composition and Chain Length. Macromolecules 2019, 52, 6213–6226. [Google Scholar] [CrossRef]
- Ji, Y.L.; Ang, M.B.M.Y.; Hung, H.C.; Huang, S.H.; An, Q.F.; Lee, K.R.; Lai, J.Y. Bio-inspired deposition of polydopamine on PVDF followed by interfacial cross-linking with trimesoyl chloride as means of preparing composite membranes for isopropanol dehydration. J. Membr. Sci. 2018, 557, 58–66. [Google Scholar] [CrossRef]
- Liao, Y.L.; Hu, C.C.; Lai, J.Y.; Liu, Y.L. Crosslinked polybenzoxazine based membrane exhibiting in-situ self promoted separation performance for pervaporation dehydration on isopropanol aqueous solutions. J. Membr. Sci. 2017, 531, 10–15. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Li, W.; Xing, W. Preparation and characterization of chitosan-poly (vinyl alcohol)/polyvinylidene fluoride hollow fiber composite membranes for pervaporation dehydration of isopropanol. Korean J. Chem. Eng. 2015, 32, 1369–1376. [Google Scholar] [CrossRef]
- Cheng, C.; Li, P.Y.; Shen, K.; Zhang, T.H.; Cao, X.Z.; Wang, B.Y.; Wang, X.F.; Hsiao, B.S. Integrated polyamide thin-film nanofibrous composite membrane regulated by functionalized interlayer for efficient water/isopropanol separation. J. Membr. Sci. 2018, 553, 70–81. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Zolotarev, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Ermakov, S.; Penkova, A. Novel Membranes Based on Hydroxyethyl Cellulose/Sodium Alginate for Pervaporation Dehydration of Isopropanol. Polymers 2021, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.A.; Chen, Y.L.; Huang, S.H.; Hu, C.C.; Hung, W.S.; Lee, K.R.; Lai, J.Y. Preparation of polyamide/polyacrylonitrile composite hollow fiber membrane by synchronous procedure of spinning and interfacial polymerization. J. Membr. Sci. 2018, 551, 261–272. [Google Scholar] [CrossRef]



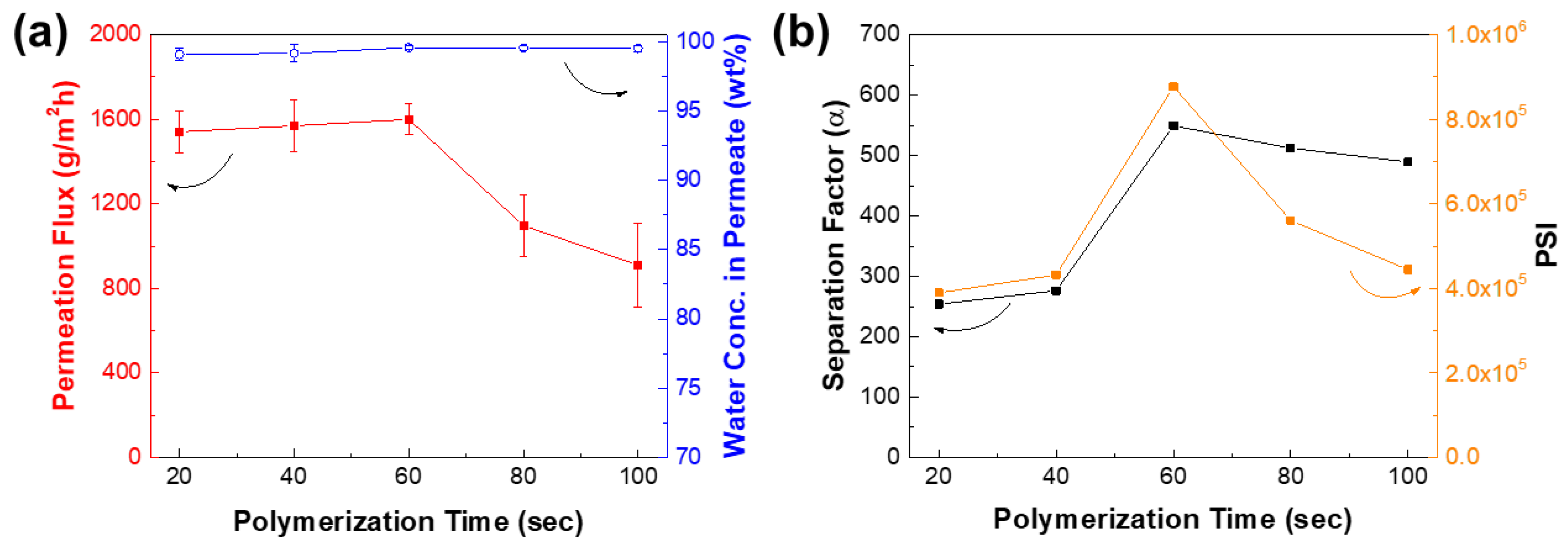
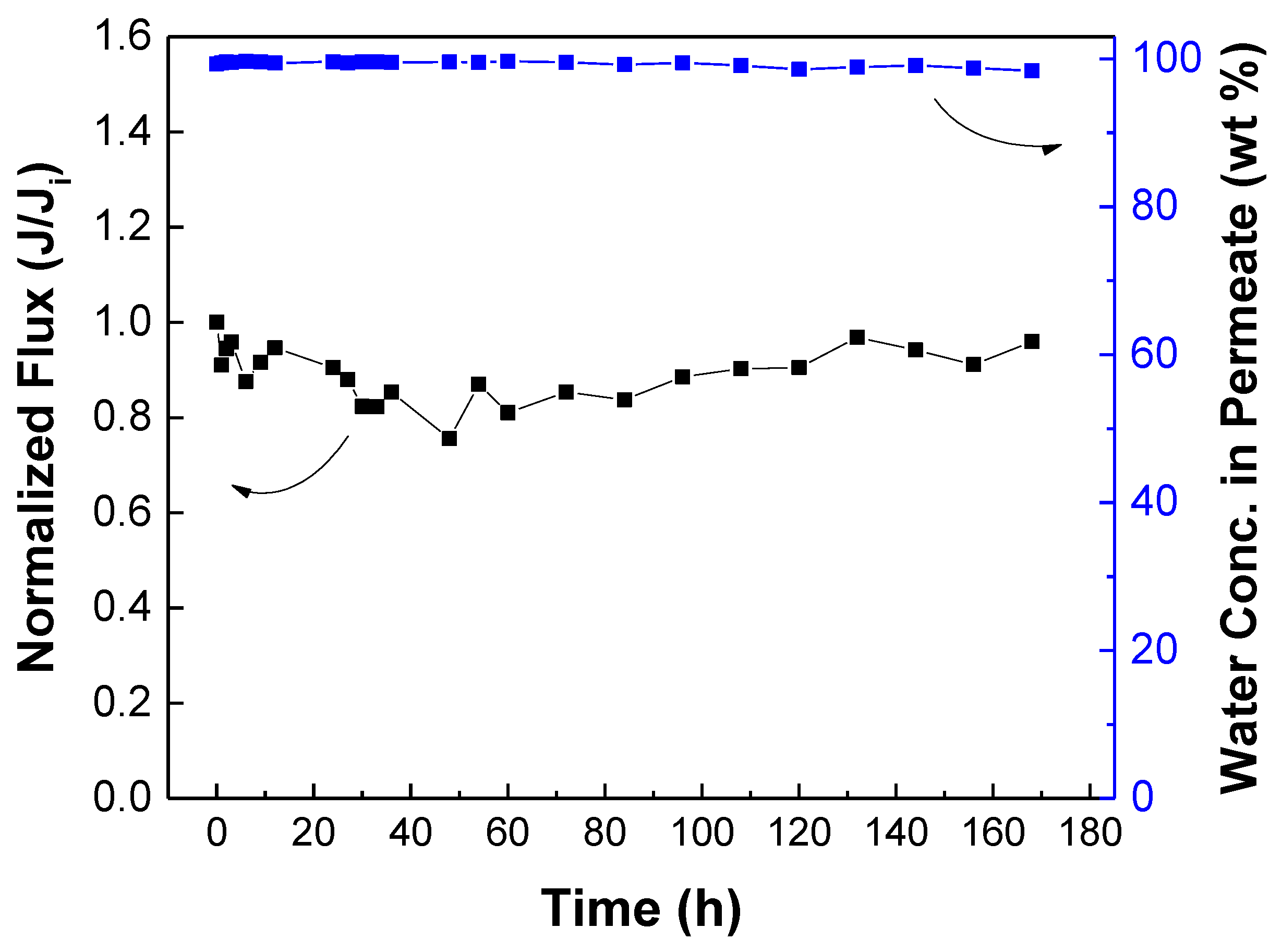
| Membrane | IPA in Feed (wt%) | Temperature (°C) | Permeation Flux (g∙m−2∙h−1) | Water Conc. in Permeate (wt%) | Reference |
|---|---|---|---|---|---|
| PDAA/PVDF | 70 | 25 | 95.7 | 2411 | [48] |
| CR-PBz/PEI | 70 | 30 | 357 | 100 | [49] |
| CS-PVA/PVDF HF | 90 | 30 | 70 | 98.7 | [50] |
| Chitosan-HMDI/PSf | 70 | 30 | 1600 | 97.1 | [11] |
| PA/eGO/PAN | 90 | 30 | 1670 | 99.15 | [51] |
| HEC/SA/PAN | 70 | 22 | 1212 | 95.54 | [52] |
| PA/PAN HF | 90 | 25 | 419 | 96.60 | [53] |
| TFCVAIP | 70 | 25 | 1600 | 99.58 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo, M.R.; Ang, M.B.M.Y.; Millare, J.C.; Huang, S.-H.; Tsai, H.-A.; Lee, K.-R. Vacuum-Assisted Interfacial Polymerization Technique for Enhanced Pervaporation Separation Performance of Thin-Film Composite Membranes. Membranes 2022, 12, 508. https://doi.org/10.3390/membranes12050508
Gallardo MR, Ang MBMY, Millare JC, Huang S-H, Tsai H-A, Lee K-R. Vacuum-Assisted Interfacial Polymerization Technique for Enhanced Pervaporation Separation Performance of Thin-Film Composite Membranes. Membranes. 2022; 12(5):508. https://doi.org/10.3390/membranes12050508
Chicago/Turabian StyleGallardo, Marwin R., Micah Belle Marie Yap Ang, Jeremiah C. Millare, Shu-Hsien Huang, Hui-An Tsai, and Kueir-Rarn Lee. 2022. "Vacuum-Assisted Interfacial Polymerization Technique for Enhanced Pervaporation Separation Performance of Thin-Film Composite Membranes" Membranes 12, no. 5: 508. https://doi.org/10.3390/membranes12050508
APA StyleGallardo, M. R., Ang, M. B. M. Y., Millare, J. C., Huang, S.-H., Tsai, H.-A., & Lee, K.-R. (2022). Vacuum-Assisted Interfacial Polymerization Technique for Enhanced Pervaporation Separation Performance of Thin-Film Composite Membranes. Membranes, 12(5), 508. https://doi.org/10.3390/membranes12050508









