Influence of the Zeolite ZSM-22 Precursor on a UF-PES Selective Substrate Layer for Salts Rejection
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.3. Characterization
2.4. Performance Evaluation
3. Results
3.1. X-ray Diffraction (XRD) Analysis
3.2. The Attenuated Total Reflectance Fourier-Transform Infrared (ATR-FTIR) Spectroscopy Analysis
3.3. Analysis of Membranes Morphology
3.3.1. Scanning Electron Microscopy (SEM) Surface Analysis
3.3.2. Scanning Electron Microscopy (SEM) Cross-Sectional Analysis
3.3.3. Elemental Analysis
3.4. Hydrophobicity and Hydrophilicity Analysis
3.5. Membrane Performance Evaluation
3.5.1. Flux Analysis
3.5.2. Membranes Fouling Analysis
Antifouling Analysis
Flux Recovery and Reversible and Irreversible Fouling
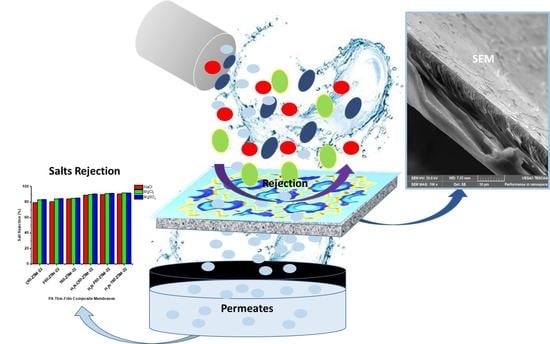
3.5.3. Saltwater Rejection Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rosentreter, H.; Walther, M.; Lerch, A. Partial Desalination of Saline Groundwater: Comparison of Nanofiltration, Reverse Osmosis and Membrane Capacitive Deionisation. Membranes 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, T.; Lee, J.; Park, J.; Choi, S.; Yu, J.-Y.; Ok, S.; Park, S.-H. Thin-Film Composite Nanofiltration Membranes for Non-Polar Solvents. Membranes 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; Al Ghaferi, A.; AlMarzooqi, F. Brine management in desalination industry: From waste to resources generation. Desalination 2019, 472, 114187. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [Green Version]
- Kucera, J. Desalination: Water from Water; John Wiley & Sons: New York, NY, USA, 2019. [Google Scholar]
- Cabassud, C.; Wirth, D. Membrane distillation for water desalination: How to chose an appropriate membrane? Desalination 2003, 157, 307–314. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Functional materials in desalination: A review. Desalination 2019, 468, 114077. [Google Scholar] [CrossRef]
- Lee, T.H.; Roh, J.S.; Yoo, S.Y.; Roh, J.M.; Choi, T.H.; Park, H.B. High-Performance Polyamide Thin-Film Nanocomposite Membranes Containing ZIF-8/CNT Hybrid Nanofillers for Reverse Osmosis Desalination. Ind. Eng. Chem. Res. 2019, 59, 5324–5332. [Google Scholar] [CrossRef]
- Yang, E.; Chae, K.-J.; Choi, M.-J.; He, Z.; Kim, I.S. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination 2018, 452, 40–67. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Francis, M.; Cunnington, M.; Su, S. Application of integrated forward and reverse osmosis for coal mine wastewater desalination. Sep. Purif. Technol. 2016, 163, 181–188. [Google Scholar] [CrossRef]
- Bagheri, M.; Akbari, A.; Mirbagheri, S.A. Advanced control of membrane fouling in filtration systems using artificial intelligence and machine learning techniques: A critical review. Process Saf. Environ. Prot. 2019, 123, 229–252. [Google Scholar] [CrossRef]
- Akhondi, E.; Zamani, F.; Tng, K.H.; Leslie, G.; Krantz, W.B.; Fane, A.G.; Chew, J.W. The Performance and Fouling Control of Submerged Hollow Fiber (HF) Systems: A Review. Appl. Sci. 2017, 7, 765. [Google Scholar] [CrossRef]
- Kucera, J. Biofouling of polyamide membranes: Fouling mechanisms, current mitigation and cleaning strategies, and future prospects. Membranes 2019, 9, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Deng, C.; Du, C.; Yang, B.; Tian, Q. Fouling mechanism and cleanability of ultrafiltration membranes modified with polydopamine-graft-PEG. Water SA 2015, 41, 448. [Google Scholar] [CrossRef] [Green Version]
- Gkotsis, P.K.; Banti, D.C.; Peleka, E.N.; Zouboulis, A.I.; Samaras, P.E. Fouling issues in membrane bioreactors (MBRs) for wastewater treatment: Major mechanisms, prevention and control strategies. Processes 2014, 2, 795–866. [Google Scholar] [CrossRef]
- Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-Conductive Membranes for Permeation Enhancement and Fouling Mitigation: A Short Review. Membranes 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, M.; Humplik, T.; Bevilacqua, A.; Tsapatsis, M.; Chiavazzo, E.; Wang, E.N.; Asinari, P. Interplay between hydrophilicity and surface barriers on water transport in zeolite membranes. Nat. Commun. 2016, 7, 12762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, J.; Theato, P.; Yoon, J. Measuring hydrophilicity of RO membranes by contact angles via sessile drop and captive bubble method: A comparative study. Desalination 2012, 303, 23–28. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Velu, S.; Muruganandam, L. Performance enhancement of polysulfone ultrafiltration membrane by blending with polyurethane hydrophilic polymer. J. Polym. Eng. 2020, 31, 125–131. [Google Scholar] [CrossRef]
- Hao, Y.; Zhou, L.; Su, Y.; Jiang, Z. Incorporating dual-defense mechanism with functionalized graphene oxide and perfluorosulfonic acid for anti-fouling membranes. Sep. Purif. Technol. 2019, 234, 116082. [Google Scholar] [CrossRef]
- Bai, L.; Wu, H.; Ding, J.; Ding, A.; Zhang, X.; Ren, N.; Li, G.; Liang, H. Cellulose nanocrystal-blended polyethersulfone membranes for enhanced removal of natural organic matter and alleviation of membrane fouling. Chem. Eng. J. 2019, 382, 122919. [Google Scholar] [CrossRef]
- Chauke, N.M.; Moutloali, R.M.; Ramontja, J. Development of ZSM-22/Polyethersulfone Membrane for Effective Salt Rejection. Polymers 2020, 12, 1446. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.G.G. Synthesis and Applications of Low Silica Zeolites from Bolivian Clay and Diatomaceous Earth. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2017. [Google Scholar]
- Li, R.; Chawla, A.; Linares, N.; Sutjianto, J.G.; Chapman, K.W.; Martínez, J.G.; Rimer, J.D. Diverse Physical States of Amorphous Precursors in Zeolite Synthesis. Ind. Eng. Chem. Res. 2018, 57, 8460–8471. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Knight, C.T.G.; Wang, J.; Kinrade, S.D. Do zeolite precursor species really exist in aqueous synthesis media? Phys. Chem. Chem. Phys. 2006, 8, 3099–3103. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Volli, V.; Munagapati, V.S.; Wen, J.-C.; Shu, C.-M. Synthesis of novel ZSM-22 zeolite from Taiwanese coal fly ash for the selective separation of Rhodamine 6G. J. Mater. Res. Technol. 2020, 9, 15381–15393. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Cao, J.-P.; Ren, X.-Y.; Feng, X.-B.; Zhao, X.-Y.; Huang, Y.; Wei, X.-Y. Synthesis of ZSM-5 using different silicon and aluminum sources nature for catalytic conversion of lignite pyrolysis volatiles to light aromatics. Fuel 2020, 268, 117286. [Google Scholar] [CrossRef]
- Júnior, L.V.D.S.; Ribeiro, T.R.S.; da Silva, B.J.B.; Quintela, P.H.L.; Alencar, S.L.; Filho, J.G.D.A.P.; da Silva, A.O.S. Different approaches to the synthesis of ZSM-22 zeolite with application in n-heptane cracking. Res. Soc. Dev. 2022, 11, e6411326070. [Google Scholar] [CrossRef]
- Musyoka, N.; Petrik, L.F.; Gitari, W.M.; Balfour, G.; Hums, E. Optimization of hydrothermal synthesis of pure phase zeolite Na-P1 from South African coal fly ashes. J. Environ. Sci. Health Part A 2012, 47, 337–350. [Google Scholar] [CrossRef]
- Lima, R.C.; Bieseki, L.; Melguizo, P.V.; Pergher, S.B.C. Zeolite Synthesis: General Aspects. In Environmentally Friendly Zeolites; Springer: Cham, Switzerland, 2019; pp. 21–63. [Google Scholar]
- Yoldi, M.; Fuentes-Ordoñez, E.; Korili, S.; Gil, A. Zeolite synthesis from industrial wastes. Microporous Mesoporous Mater. 2019, 287, 183–191. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, H.; Sun, H.; Li, L.; Zhu, X.; Ren, S.; Guo, Q.; Shen, B. Highly mesoporous IM-5 zeolite prepared by alkaline treatment and its catalytic cracking performance. Microporous Mesoporous Mater. 2018, 273, 297–306. [Google Scholar] [CrossRef]
- Jamil, A.; Muraza, O.; Ahmed, M.H.; Zainalabdeen, A.; Muramoto, K.; Nakasaka, Y.; Yamani, Z.H.; Yoshikawa, T.; Masuda, T. Hydrothermally stable acid-modified ZSM-22 zeolite for selective propylene production via steam-assisted catalytic cracking of n-hexane. Microporous Mesoporous Mater. 2018, 260, 30–39. [Google Scholar] [CrossRef]
- Hedström, A. Ion Exchange of Ammonium in Zeolites: A Literature Review. J. Environ. Eng. 2001, 127, 673–681. [Google Scholar] [CrossRef]
- Fletcher, P.; Franklin, K.R.; Townsend, R.P.; Fletcher, P.; Ivers, D.J.; James, R.W. Thermodynamics of binary and ternary ion exchange in zeolites: The exchange of sodium, ammonium and potassium ions in mordenite. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1984, 312, 141–178. [Google Scholar]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.R.; Chang, C.-H.; Wu, K.C.-W.; Tung, K.-L.; Lue, S.J. Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Shelyakina, M.K.; Soldatkin, O.O.; Arkhypova, V.M.; Kasap, B.O.; Akata, B.; Dzyadevych, S.V. Study of zeolite influence on analytical characteristics of urea biosensor based on ion-selective field-effect transistors. Nanoscale Res. Lett. 2014, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, A.M.P.; Beving, D.; Wang, A.; Chen, W.; Yan, Y. Hydrophilic and Antimicrobial Zeolite Coatings for Gravity-Independent Water Separation. Adv. Funct. Mater. 2005, 15, 336–340. [Google Scholar] [CrossRef]
- Gao, X.; Gao, B.; Liu, H.; Zhang, C.; Zhang, Y.; Jiang, J.; Gu, X. Fabrication of stainless steel hollow fiber supported NaA zeolite membrane by self-assembly of submicron seeds (in English). Sep. Purif. Technol. 2019, 234, 116121. [Google Scholar] [CrossRef]
- Wenten, I.G.; Dharmawijaya, P.T.; Aryanti, P.T.P.; Mukti, R.R.; Khoiruddin, K. LTA zeolite membranes: Current progress and challenges in pervaporation. RSC Adv. 2017, 7, 29520–29539. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Zhang, X.; Zhao, Z.; Song, B.; Zhang, Z.; Song, W. Pervaporation performance of surface-modified zeolite/PU mixed matrix membranes for separation of phenol from water. Iran. Polym. J. 2017, 26, 193–203. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, L.; Jiang, J.; Gu, X. Mass transfer model, preparation and applications of zeolite membranes for pervaporation dehydration: A review. Chin. J. Chem. Eng. 2017, 25, 1627–1638. [Google Scholar] [CrossRef]
- Shahverdi, M.; Baheri, B.; Rezakazemi, M.; Motaee, E.; Mohammadi, T. Pervaporation study of ethylene glycol dehydration through synthesized (PVA–4A)/polypropylene mixed matrix composite membranes. Polym. Eng. Sci. 2013, 53, 1487–1493. [Google Scholar] [CrossRef]
- García-Viñuales, S.; Rubio, C.; Martínez-Izquierdo, L.; Zornoza, B.; Piera, E.; Caballero, M.; Téllez, C. Study of Melamine-Formaldehyde/Phase Change Material Microcapsules for the Preparation of Polymer Films by Extrusion. Membranes 2022, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Kamińska, G.; Bohdziewicz, J. Preparation of Polymer Membranes by In Situ Interfacial Polymerization. Int. J. Polym. Sci. 2019, 2019, 6217924. [Google Scholar] [CrossRef]
- Widhyahrini, K.; Handayani, N.; Wahyuningrum, D.; Radiman, C.L. The synthesis of sulfonated polyethersulfone (SPES) and the preparation of its membranes as matrix in the immobilization of Candida antarctica lipase B (Cal-B). Polym. Bull. 2019, 77, 3735–3748. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Koyuncu, I.; Sengur, R.; Turken, T.; Guclu, S.; Pasaoglu, M.E. Advances in water treatment by microfiltration, ultrafiltration, and nanofiltration. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 83–128. [Google Scholar]
- Reif, O.W.W. Microfiltration membranes: Characteristics and manufacturing. In Sterile Filtration; Springer: New York, NY, USA, 2006; pp. 73–103. [Google Scholar]
- Sewerin, T.; Elshof, M.G.; Matencio, S.; Boerrigter, M.; Yu, J.; de Grooth, J. Advances and Applications of Hollow Fiber Nanofiltration Membranes: A Review. Membranes 2021, 11, 890. [Google Scholar] [CrossRef]
- Siddique, T.; Dutta, N.K.; Choudhury, N.R. Mixed-Matrix Membrane Fabrication for Water Treatment. Membranes 2021, 11, 557. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, Q. Fabrication of hierarchical ZSM-22 hollow sphere. Mater. Lett. 2019, 244, 96–99. [Google Scholar] [CrossRef]
- Sousa, L.V.; Silva, A.O.S.; Silva, B.J.; Teixeira, C.M.; Arcanjo, A.P.; Frety, R.; Pacheco, J.G. Fast synthesis of ZSM-22 zeolite by the seed-assisted method of crystallization with methanol. Microporous Mesoporous Mater. 2017, 254, 192–200. [Google Scholar] [CrossRef]
- Motsa, M.M.; Msagati, T.A.M.; Thwala, J.M.; Mamba, B.B. Polypropylene–zeolite polymer composites for water purification: Synthesis, characterisation and application. Desalination Water Treat. 2015, 53, 2604–2612. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Huang, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO2 particles on membrane surface to enhance membrane antifouling performance for water treatment. J. Colloid Interface Sci. 2020, 570, 273–285. [Google Scholar] [CrossRef]
- Ferreira, R.D.S.B.; Oliveira, S.S.L.; Salviano, A.F.; Araújo, E.M.; Leite, A.M.D.; Lira, H.D.L. Polyethersulfone Hollow Fiber Membranes Developed for Oily Emulsion Treatment. Mater. Res. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Vilakati, G.D.; Hoek, E.M.; Mamba, B. Probing the mechanical and thermal properties of polysulfone membranes modified with synthetic and natural polymer additives. Polym. Test. 2014, 34, 202–210. [Google Scholar] [CrossRef]
- Turgman-Cohen, S.; Araque, J.C.; Hoek, E.M.V.; Escobedo, F.A. Molecular Dynamics of Equilibrium and Pressure-Driven Transport Properties of Water through LTA-Type Zeolites. Langmuir 2013, 29, 12389–12399. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, M.T.M.; Nygaard, J.M.; Ghosh, A.K.; Hoek, E.M. Using nanocomposite materials technology to understand and control reverse osmosis membrane compaction. Desalination 2010, 261, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Flyagina, I.S.; Mahdi, E.M.; Titov, K.; Tan, J.-C. Thermo-mechanical properties of mixed-matrix membranes encompassing zeolitic imidazolate framework-90 and polyvinylidine difluoride: ZIF-90/PVDF nanocomposites. APL Mater. 2017, 5, 086104. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-H.; Yang, H.; Ji, S.-F.; Gao, C.-M.; Fang, H.; Xing, Y.-Q.; Han, N.-X.; Ding, G.-D.; Jia, L. Fabricating PES/SPSF membrane via reverse thermally induced phase separation (RTIPS) process to enhance permeability and hydrophilicity. RSC Adv. 2019, 9, 26807–26816. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-Y.; Luo, Z.-Y.; Yin, M.-J.; Wang, N.; Qin, Z.; Lee, K.-R.; An, Q.-F. A comprehensive study on phase inversion behavior of a novel polysulfate membrane for high-performance ultrafiltration applications. J. Membr. Sci. 2020, 610, 118404. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Hendrix, K.; Vaneynde, M.; Koeckelberghs, G.; Vankelecom, I.F. Synthesis of modified poly(ether ether ketone) polymer for the preparation of ultrafiltration and nanofiltration membranes via phase inversion. J. Membr. Sci. 2013, 447, 96–106. [Google Scholar] [CrossRef]
- Nady, N.; Salem, N.; Kandil, S. Preparation and Characterization of a Novel Poly (vinylidene fluoride-co-hexafluoropropylene)/Poly (ethersulfone) Blend Membrane Fabricated Using an Innovative Method of Mixing Electrospinning and Phase Inversion. Polymers 2021, 13, 790. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.-P.; Bouyer, D.; Drioli, E.; Kiadeh, N.T.H. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2019, 597, 117601. [Google Scholar] [CrossRef]
- Zheng, Q.-Z.; Wang, P.; Yang, Y.-N. Rheological and thermodynamic variation in polysulfone solution by PEG introduction and its effect on kinetics of membrane formation via phase-inversion process. J. Membr. Sci. 2006, 279, 230–237. [Google Scholar] [CrossRef]
- Buchwald, Z.; Sandomierski, M.; Voelkel, A. Calcium-Rich 13X Zeolite as a Filler with Remineralizing Potential for Dental Composites. ACS Biomater. Sci. Eng. 2020, 6, 3843–3854. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Tellez, C.; Yeung, K.L.; Ho, K. The role of surface chemistry in zeolite membrane formation. J. Membr. Sci. 2000, 164, 257–275. [Google Scholar] [CrossRef]
- Qu, H.; Xiao, X.; Han, Z.; Hu, M.; Shen, S.; Yang, L.; Jia, F.; Wang, T.; Ye, Z.; Sun, W.; et al. Graphene Oxide Nanofiltration Membrane Based on Three-Dimensional Size-Controllable Metal–Organic Frameworks for Water Treatment. ACS Appl. Nano Mater. 2022, 5, 5196–5207. [Google Scholar] [CrossRef]
- Berned-Samatán, V.; Rubio, C.; Galán-González, A.; Muñoz, E.; Benito, A.M.; Maser, W.K.; Coronas, J.; Téllez, C. Single-walled carbon nanotube buckypaper as support for highly permeable double layer polyamide/zeolitic imidazolate framework in nanofiltration processes. J. Membr. Sci. 2022, 652, 120490. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes–recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Maghami, M.; Abdelrasoul, A. Zeolite Mixed Matrix Membranes (Zeolite-Mmms) for Sustainable Engineering. Zeolites Appl. 2018, 27, 115. [Google Scholar]
- Zhang, Z.; An, Q.; Ji, Y.; Qian, J.; Gao, C. Effect of zero shear viscosity of the casting solution on the morphology and permeability of polysulfone membrane prepared via the phase-inversion process. Desalination 2010, 260, 43–50. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Benelfellah, A.; Papadopoulos, L.; Terzopoulou, Z.; Malletzidou, L.; Vasileiadis, I.G.; Chrissafis, K.; Bikiaris, D.N.; Hocine, N.A. Influence of montmorillonite/carbon nanotube hybrid nanofillers on the properties of poly(lactic acid). Appl. Clay Sci. 2020, 201, 105925. [Google Scholar] [CrossRef]
- Pielichowski, K.; Michalowski, S. Nanostructured flame retardants: Performance, toxicity, and environmental impact. In Health and Environmental Safety of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 251–277. [Google Scholar]
- Król-Morkisz, K.; Pielichowska, K. Preparation and characterization of polyoxymethylene/functionalized hydroxyapatite nanocomposites. Eng. Biomater. 2017, 20, 56. [Google Scholar]
- Wang, Y.-N.; Wang, R. Reverse osmosis membrane separation technology. In Membrane Separation Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–45. [Google Scholar]
- Kertész, S.; De Freitas, T.B.; Hodúr, C. Characterization of polymer membranes by contact angle goniometer. Analecta Tech. Szeged. 2014, 8, 18–22. [Google Scholar] [CrossRef]
- Gholami, S.; López, J.; Rezvani, A.; Vatanpour, V.; Cortina, J.L. Fabrication of thin-film nanocomposite nanofiltration membranes incorporated with aromatic amine-functionalized multiwalled carbon nanotubes. Rejection performance of inorganic pollutants from groundwater with improved acid and chlorine resistance. Chem. Eng. J. 2019, 384, 123348. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Vatanpour, V. Polymer/carbon nanotubes mixed matrix membranes for water purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–110. [Google Scholar]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Zhao, F.; Liu, Y.; Liu, L.; Wang, L.; Geng, C.; Huang, P. Preparation of a hydrophilic and antibacterial dual function ultrafiltration membrane with quaternized graphene oxide as a modifier. J. Colloid Interface Sci. 2019, 562, 182–192. [Google Scholar] [CrossRef]
- PALL. Polyethersulfone Membrane (Hydrophilic). Available online: https://shop.pall.com/us/en/medical/infusion-therapy/oem-manufacturing/zidgri78lte (accessed on 15 April 2020).
- Li, J.-F.; Xu, Z.-L.; Yang, H.; Feng, C.-P.; Shi, J.-H. Hydrophilic microporous PES membranes prepared by PES/PEG/DMAc casting solutions. J. Appl. Polym. Sci. 2007, 107, 4100–4108. [Google Scholar] [CrossRef]
- Jain, H.; Verma, A.K.; Dhupper, R.; Wadhwa, S.; Garg, M.C. Development of CA-TiO2-incorporated thin-film nanocomposite forward osmosis membrane for enhanced water flux and salt rejection. Int. J. Environ. Sci. Technol. 2021, 19, 5387–5400. [Google Scholar] [CrossRef]
- Malekizadeh, A.; Schenk, P.M. High flux water purification using aluminium hydroxide hydrate gels. Sci. Rep. 2017, 7, 17437. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhao, L.; Zhang, L.; Chen, H.; Gao, C.; Ho, W.W. High-flux reverse osmosis membranes incorporated with NaY zeolite nanoparticles for brackish water desalination. J. Membr. Sci. 2015, 476, 373–383. [Google Scholar] [CrossRef]
- Junaidi, N.F.D.; Othman, N.H.; Shahruddin, M.Z.; Alias, N.H.; Lau, W.J.; Ismail, A.F. Effect of graphene oxide (GO) and polyvinylpyrollidone (PVP) additives on the hydrophilicity of composite polyethersulfone (PES) membrane. Malays. J. Fundam. Appl. Sci. 2019, 15, 361–366. [Google Scholar] [CrossRef]
- Weichselbaum, E.; Österbauer, M.; Knyazev, D.; Batishchev, O.V.; Akimov, S.; Nguyen, T.H.; Zhang, C.; Knör, G.; Agmon, N.; Carloni, P.; et al. Origin of proton affinity to membrane/water interfaces. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Bosdriesz, E.; Wortel, M.T.; Haanstra, J.R.; Wagner, M.J.; Cortés, P.D.L.T.; Teusink, B. Low affinity uniporter carrier proteins can increase net substrate uptake rate by reducing efflux. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, X.; Gao, S.; Wang, X.; Zhang, R. Progress in Research and Application of Nanofiltration (NF) Technology for Brackish Water Treatment. Membranes 2021, 11, 662. [Google Scholar] [CrossRef]
- Hassanajili, S.; Khademi, M.; Keshavarz, P. Influence of various types of silica nanoparticles on permeation properties of polyurethane/silica mixed matrix membranes. J. Membr. Sci. 2014, 453, 369–383. [Google Scholar] [CrossRef]
- Li, S.; Ng, Y.H.; Lau, H.C.; Torsæter, O.; Stubbs, L.P. Experimental investigation of stability of silica nanoparticles at reservoir conditions for enhanced oil-recovery applications. Nanomaterials 2020, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ikhsan, S.W.; Yusof, N.; Nawi, N.M.; Bilad, M.; Shamsuddin, N.; Aziz, F.; Ismail, A. Halloysite Nanotube-Ferrihydrite Incorporated Polyethersulfone Mixed Matrix Membrane: Effect of Nanocomposite Loading on the Antifouling Performance. Polymers 2021, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lee, J.; Ma, J.; Elimelech, M. Antifouling Thin-Film Composite Membranes by Controlled Architecture of Zwitterionic Polymer Brush Layer. Environ. Sci. Technol. 2017, 51, 2161–2169. [Google Scholar] [CrossRef]
- Chen, J.C.; Li, Q.; Elimelech, M. In situ monitoring techniques for concentration polarization and fouling phenomena in membrane filtration. Adv. Colloid Interface Sci. 2003, 107, 83–108. [Google Scholar] [CrossRef]
- Samari, M.; Zinadini, S.; Zinatizadeh, A.A.; Jafarzadeh, M.; Gholami, F. Designing of a novel polyethersulfone (PES) ultrafiltration (UF) membrane with thermal stability and high fouling resistance using melamine-modified zirconium-based metal-organic framework (UiO-66-NH2/MOF). Sep. Purif. Technol. 2020, 251, 117010. [Google Scholar] [CrossRef]
- Lee, W.; Ng, Z.; Hubadillah, S.; Goh, P.; Lau, W.; Othman, M.; Ismail, A.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 11433. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and wetting in the membrane distillation driven wastewater reclamation process—A review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauke, N.M.; Moutloali, R.M.; Ramontja, J. Influence of the Zeolite ZSM-22 Precursor on a UF-PES Selective Substrate Layer for Salts Rejection. Membranes 2022, 12, 553. https://doi.org/10.3390/membranes12060553
Chauke NM, Moutloali RM, Ramontja J. Influence of the Zeolite ZSM-22 Precursor on a UF-PES Selective Substrate Layer for Salts Rejection. Membranes. 2022; 12(6):553. https://doi.org/10.3390/membranes12060553
Chicago/Turabian StyleChauke, Nyiko M., Richard M. Moutloali, and James Ramontja. 2022. "Influence of the Zeolite ZSM-22 Precursor on a UF-PES Selective Substrate Layer for Salts Rejection" Membranes 12, no. 6: 553. https://doi.org/10.3390/membranes12060553
APA StyleChauke, N. M., Moutloali, R. M., & Ramontja, J. (2022). Influence of the Zeolite ZSM-22 Precursor on a UF-PES Selective Substrate Layer for Salts Rejection. Membranes, 12(6), 553. https://doi.org/10.3390/membranes12060553