Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Membrane (Alfa Laval-NF)
2.2. Methods
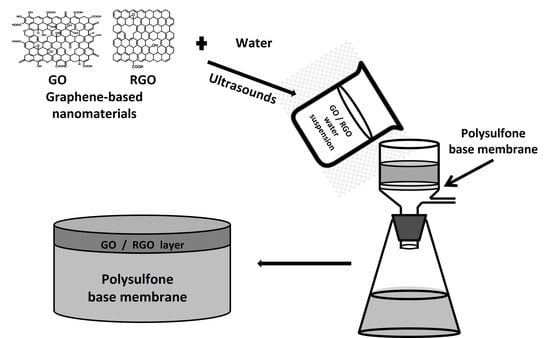
2.2.1. Membranes Modification
2.2.2. Morphological Characterization of the Membranes
2.2.3. Physico-Chemical Characterization of the Membranes
2.2.4. Analytical Methods
3. Results and Discussion
3.1. Morphological Characterization of the Membranes
3.2. Physico-Chemical Characterization of the Membranes
3.2.1. Solvent Permeability
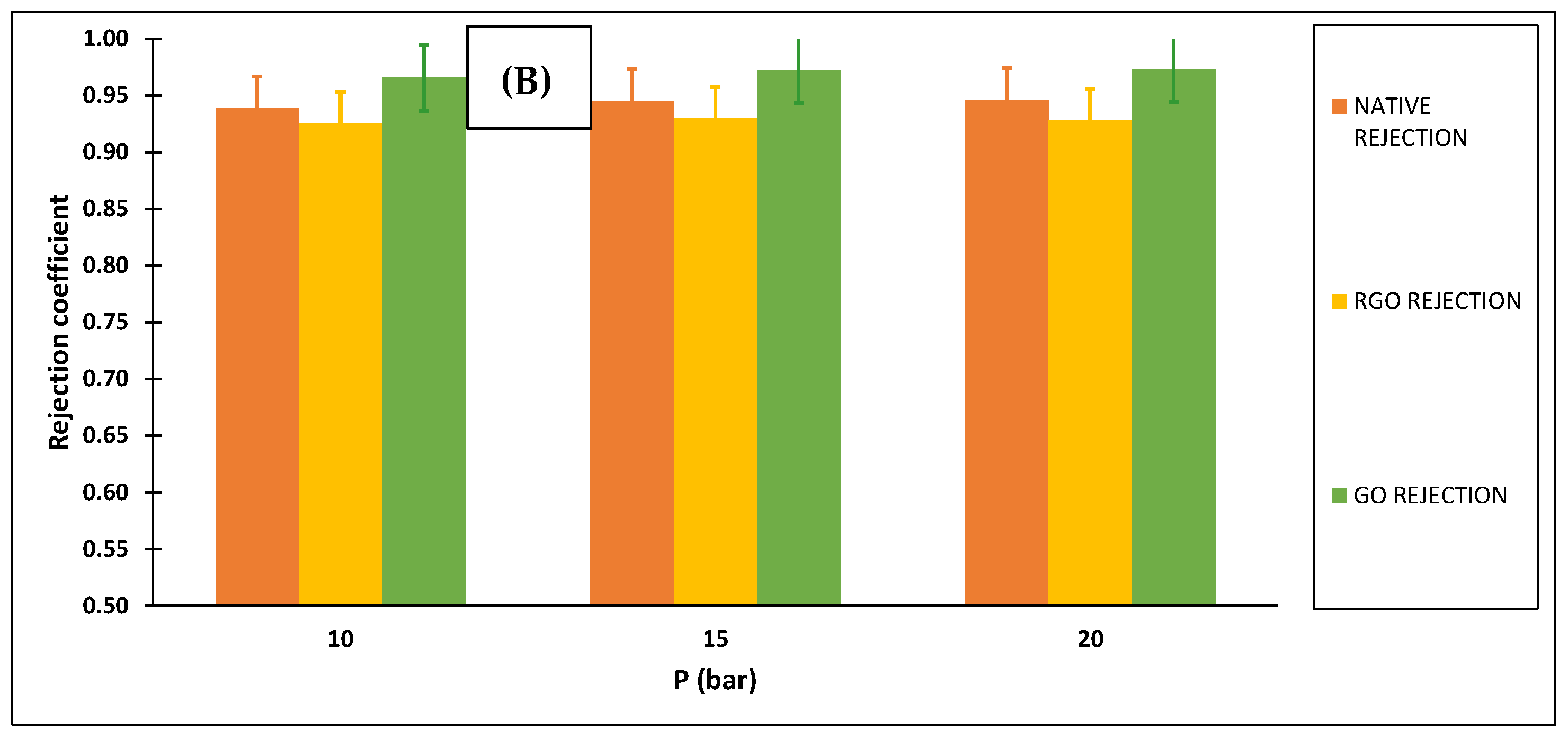
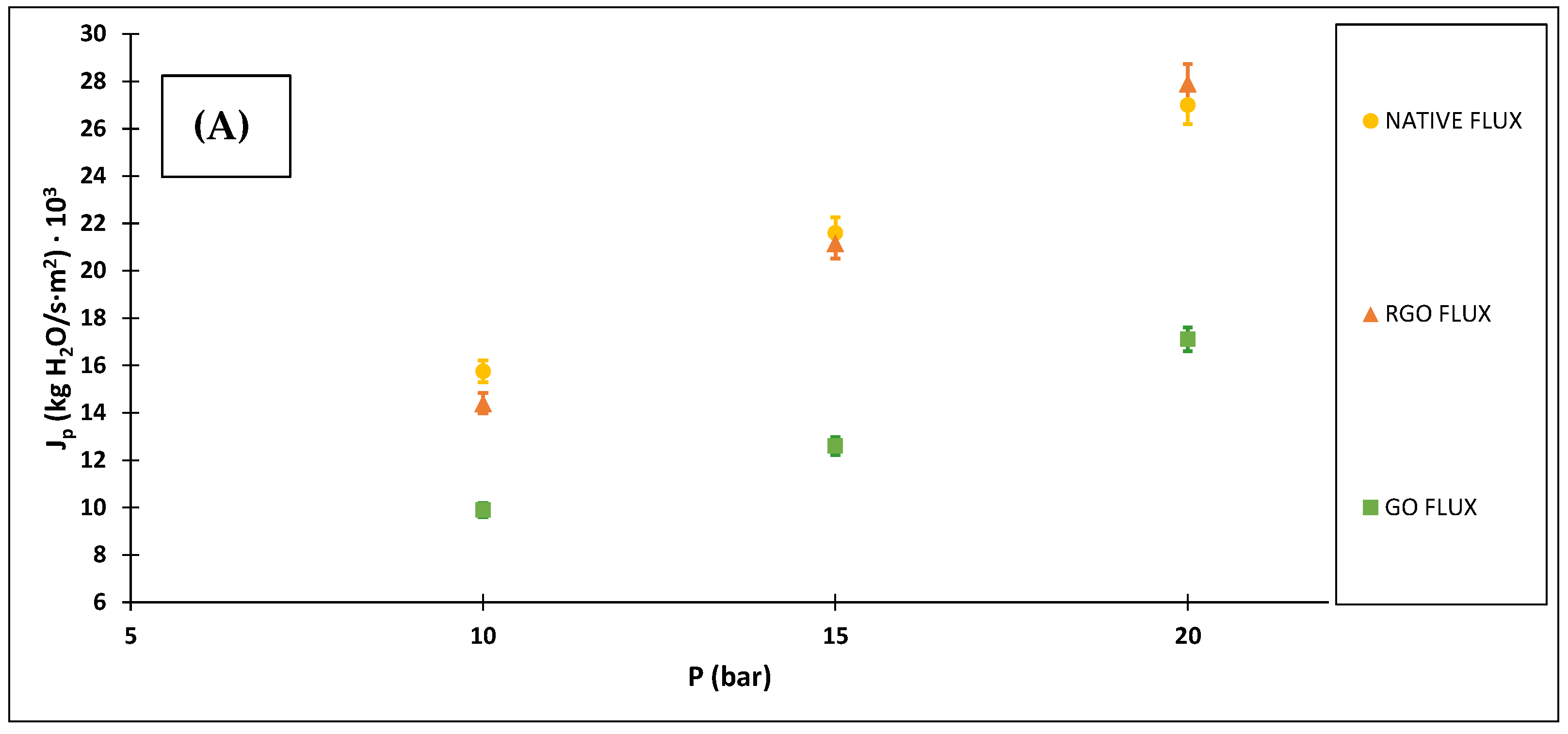
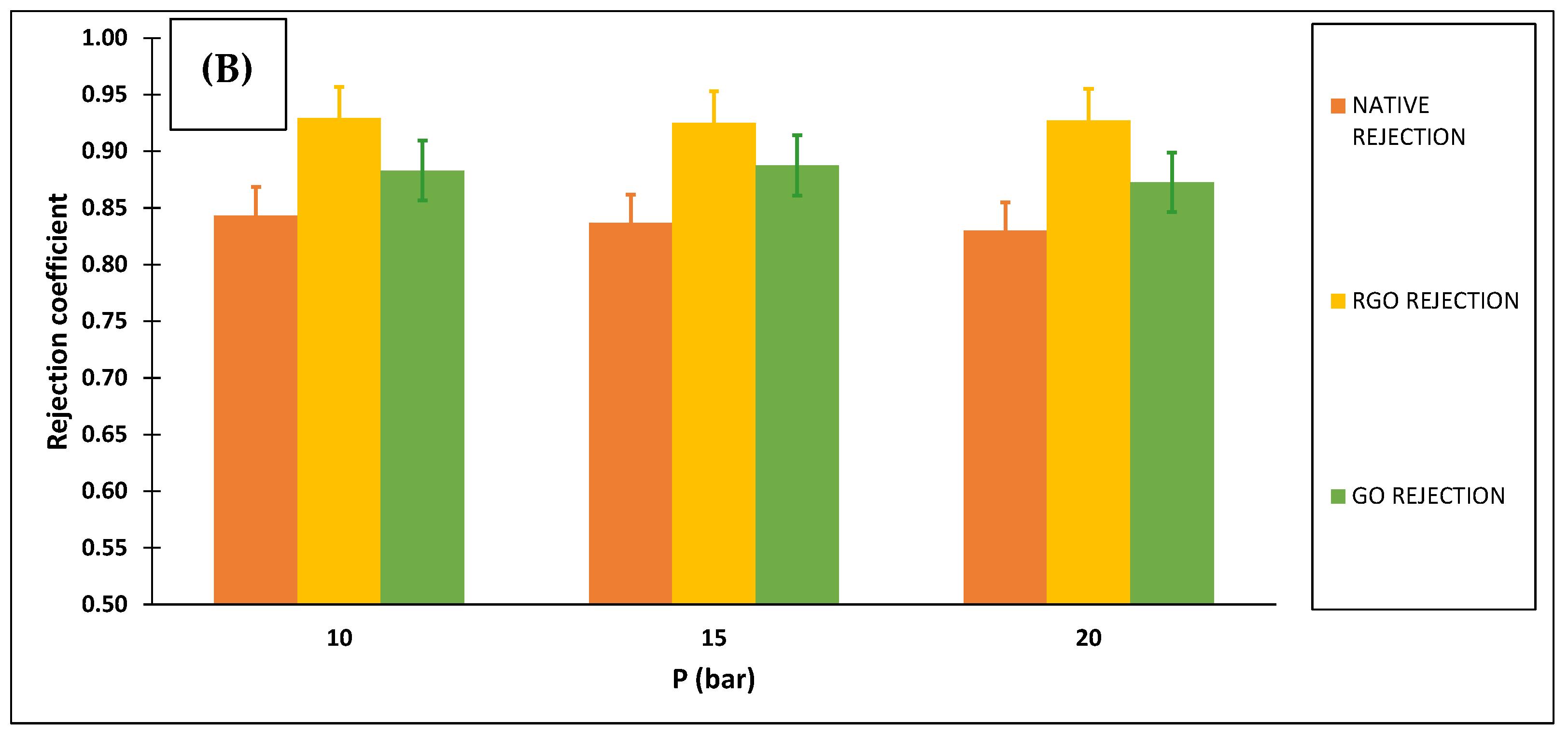
3.2.2. Membranes Selectivity
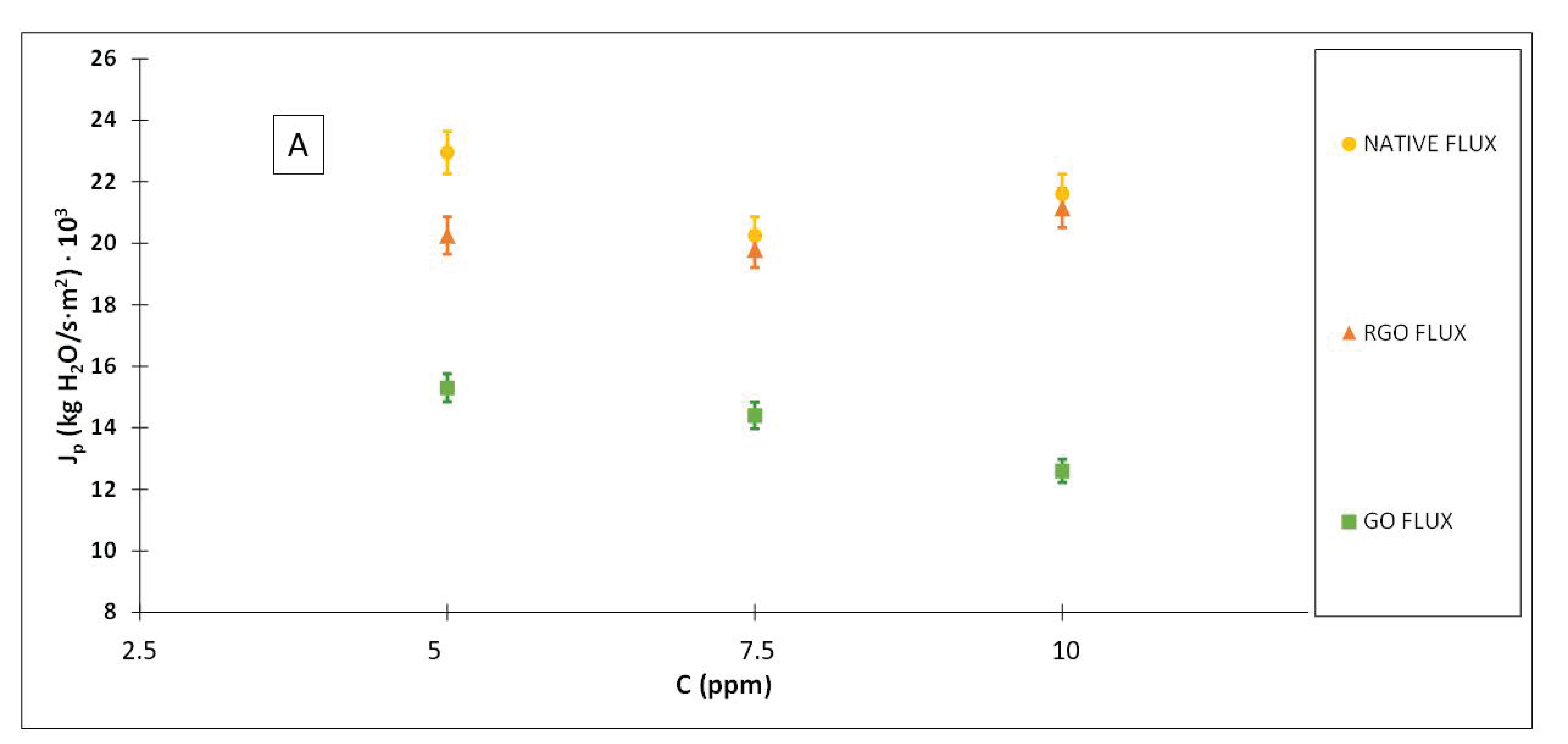
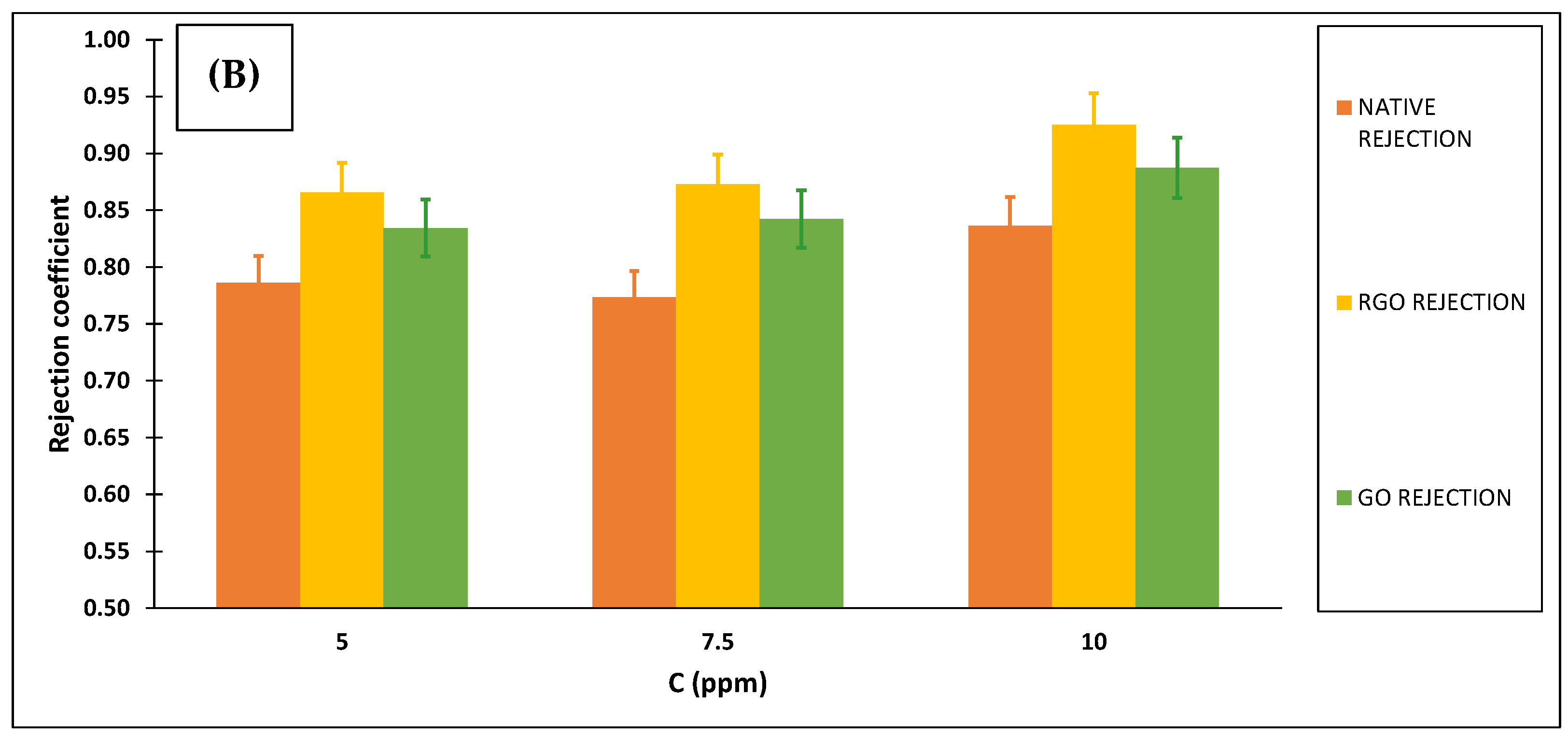
3.2.3. Ibuprofen Removal
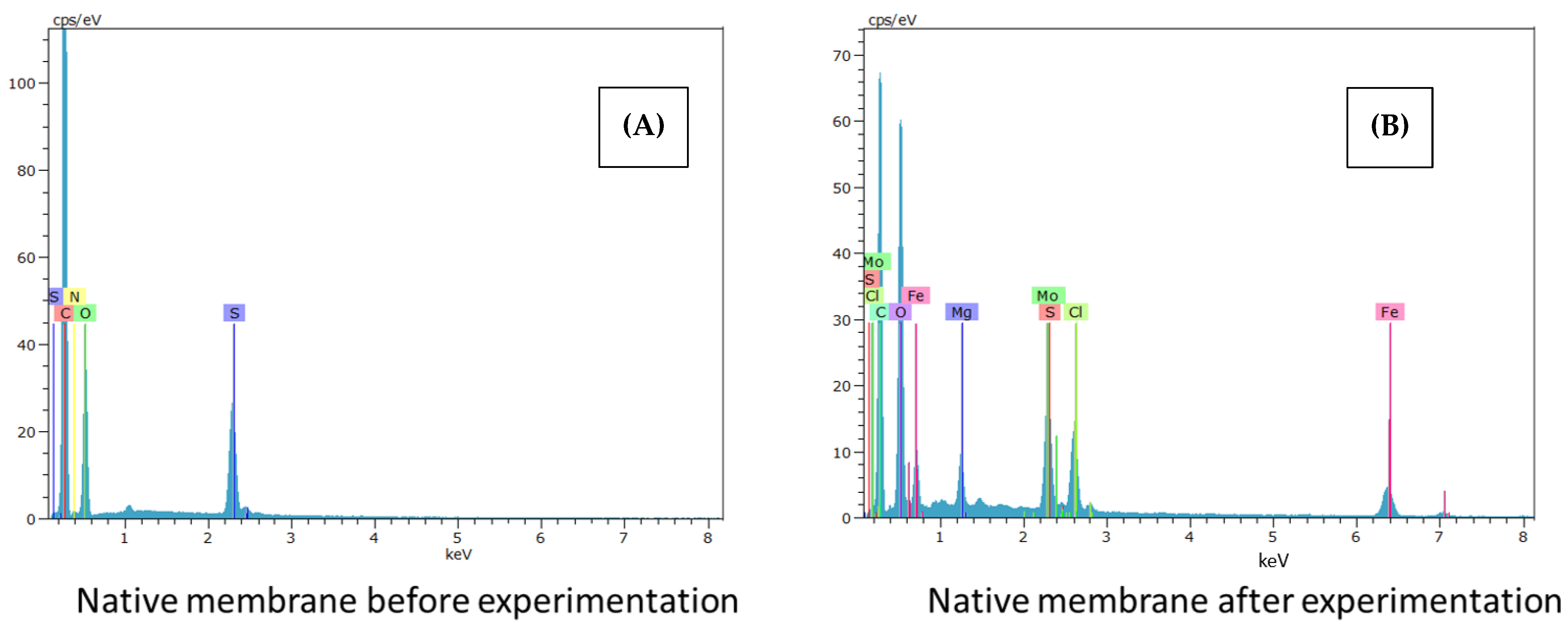
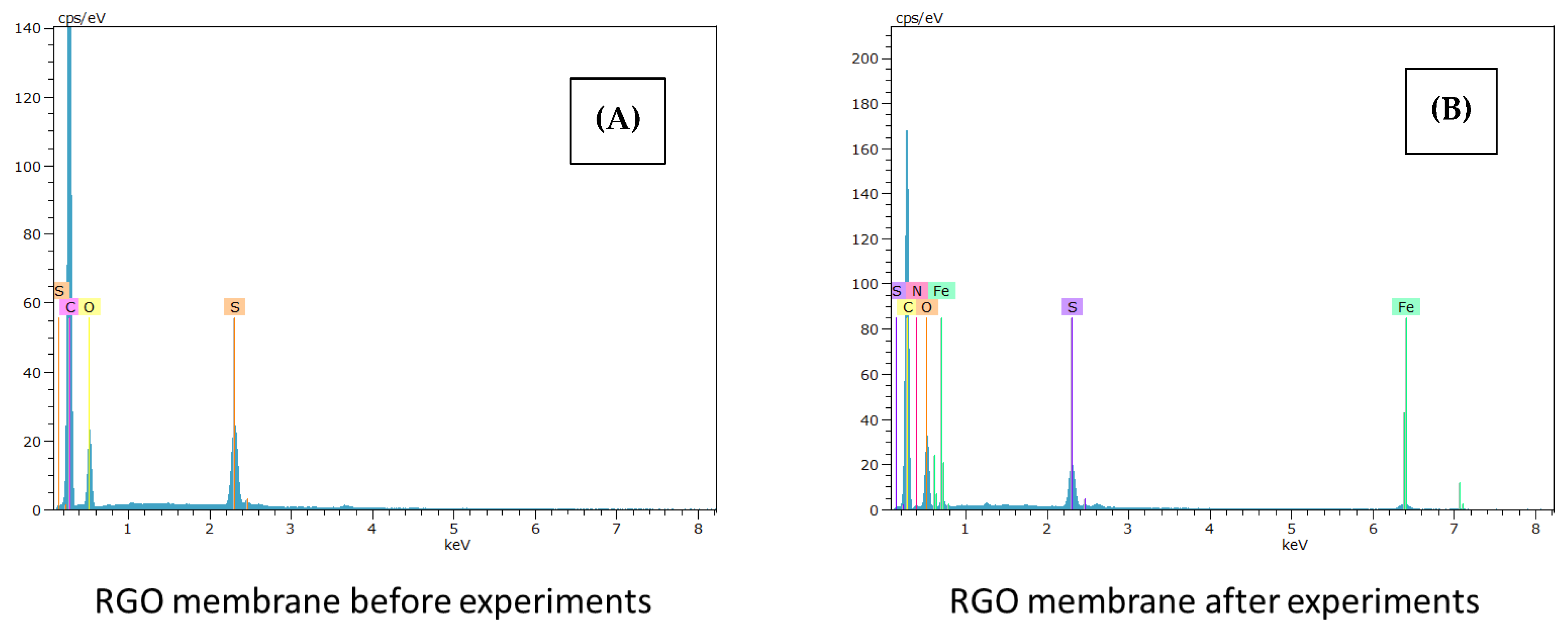
3.2.4. Fouling Study and Membrane Deterioration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuhorka, J.; Wallace, E.; Mikulášek, P. Removal of micropollutants from water by commercially available nanofiltration membranes. Sci. Total Environ. 2020, 720, 137474–137484. [Google Scholar] [CrossRef] [PubMed]
- Visanji, Z.; Sadr, S.M.K.; Johns, M.B.; Savic, D.; Memon, F.A. Optimising wastewater treatment solutions for the removal of contaminants of emerging concern (CECs): A case study for application in India. J. Hydroinform. 2019, 22, 93–110. [Google Scholar] [CrossRef]
- Zhou, S.; Di Paolo, C.; Wu, X.; Shao, Y.; Seiler, T.B.; Hollert, H. Optimization of screening-level risk assessment and priority selection of emerging pollutants—The case of pharmaceuticals in European surface waters. Environ. Int. 2019, 128, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, J.; Posthuma, L.; Cousins, I.T.; Lindim, C.; De Zwart, D.; Bunke, D.; Kutsarova, S.; Müller, C.; Munthe, J.; Slobodnik, J.; et al. The European Collaborative Project SOLUTIONS developed models to provide diagnostic and prognostic capacity and fill data gaps for chemicals of emerging concern. Environ. Sci. Eur. 2019, 31, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A critical evaluation of advanced oxidation processes for emerging contaminants removal. Environ. Process. 2017, 4, 283–302. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen—Toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. 2018, 25, 21498–21524. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Bhattachary, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, M.J.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.E.; Hayball, J.D. Activated carbon, carbon nanotubes and graphene: Materials and composites for advanced water purification. J. Carbon Res. 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Kárászová, M.; Bourassi, M.; Gaálová, J. Membrane removal of emerging contaminants from water: Which kind of membranes should we use? Membranes 2020, 10, 305. [Google Scholar] [CrossRef]
- Kabbani, H.M.; Al-Hindi, M.; Ayoub, G.M.; Ahmad, M. The effects of salt concentration on the rejection of pharmaceutically active compounds by nanofiltration membranes. J. Sustain. Dev. Energy Water Environ. Syst. 2019, 9, 1080356–1080372. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Gequinto, L.R.D.O.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.; Her, N.; Park, C.M.; Yu, M.; Yoon, Y. Removal of contaminants of emerging concern by FO, RO, and UF membranes in water and wastewater. In Contaminants of Emerging Concern in Water and Wastewater; Hernández-Maldonado, A.J., Blaney, L., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; Chapter 5; pp. 139–176. [Google Scholar]
- Shad, M.F. Evaluating occurrence of contaminants of emerging concerns in microfiltration and reverse osmosis treatment of primary effluent in a novel water recycling process. Masters Thesis, California State Polytechnic University, Pomona, CA, USA, 2017. [Google Scholar]
- Lawler, J. Graphene-based nanosheet functionalized membranes-industrial applications. Nanotechnol. Res. J. 2016, 9, 263–291. [Google Scholar]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Chen, J.T.; Yu, M. Printing ultrathin graphene oxide nanofiltration membranes for water purification. J. Mater. Chem. A 2017, 5, 20860–20866. [Google Scholar] [CrossRef]
- Sun, M.; Li, J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today 2018, 20, 121–137. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Novel polysulfone ultrafiltration membranes incorporating polydopamine functionalized graphene oxide with enhanced flux and fouling resistance. J. Membr. Sci. 2021, 620, 118900–118914. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of dyes using graphene oxide (GO) mixed matrix membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef]
- De Melo, L.; Pereira, F.; Grosseli, G.M.; Sakamoto, I.K.; Fadini, P.S.; Silva, E.L.; Amâncio, M.B. Influence of ethanol and nitrate on ibuprofen removal in batch reactors under denitrifying conditions. Process Saf. Environ. Protect. 2022, 160, 297–309. [Google Scholar]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef]
- Wang, J.; He, B.; Yan, D.; Hu, X. Implementing ecopharmacovigilance (EPV) from a pharmacy perspective: A focus on non-steroidal anti-inflammatory drugs. Sci. Total Environ. 2017, 603–604, 772–784. [Google Scholar] [CrossRef]
- Bialk-Bielinska, A.; Kumirska, J.; Borecka, M.; Caban, M.; Paszkiewicz, M.; Pazdro, K.; Stepnowski, P. Selected analytical challenges in the determination of pharmaceuticals in drinking/marine waters and soil/sediment samples. J. Pharmaceut. Biomed. Anal. 2016, 121, 271–296. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; León, G.; Gómez, M.; Murcia, M.D.; Gómez, E.; Macario, J.A. Removal of different dye solutions: A comparison study using polyamide NF membrane. Membranes 2020, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.M.; El-Zahhar, A.A. Cellulose acetate butyrate graphene oxide nanocomposite membrane: Fabrication, characterization and performance. Chem. Ind. Chem. Eng. 2021, 27, 35–44. [Google Scholar] [CrossRef]
- Davalos Monteiro, R.; van de Wetering, J.; Krawczyk, B.; Engelberg, D.L. Corrosion behaviour of type 316L stainless steel in hot caustic aqueous environments. Met. Mater. Int. 2019, 26, 630–640. [Google Scholar] [CrossRef] [Green Version]
- Tahaikt, M.; El-Ghzizel, S.; Essafi, N.; Hafsi, M.; Taky, M.; Elmidaoui, A. Technical-economic comparison of nanofiltration and reverse osmosis in the reduction of fluoride ions from groundwater: Experimental, modeling, and cost estimate. Desalin. Water Treat. 2021, 216, 83–95. [Google Scholar] [CrossRef]
- Chu, K.H.; Fathizadeh, M.; Yu, M.; Flora, J.R.; Jang, A.; Jang, M.; Park, C.M.; Yoo, S.S.; Her, N.; Yoon, Y. Evaluation of removal mechanisms in a graphene oxide-coated ceramic ultrafiltration membrane for retention of natural organic matter, pharmaceuticals, and inorganic salts. Appl. Mater. Interfaces 2017, 9, 40369–40377. [Google Scholar] [CrossRef]
- Peng, H.; Tang, Q.; Tang, S.; Gong, J.; Zhao, Q. Surface modified polyamide nanofiltration membranes with high permeability and stability. J. Membr. Sci. 2019, 592, 117386–117393. [Google Scholar] [CrossRef]
- Park, M.J.; Wang, C.; Seo, D.H.; Gonzales, R.R.; Matsuyama, H.; Shon, H.K. Inkjet printed single walled carbon nanotube as an interlayer for high performance thin film composite nanofiltration membrane. J. Membr. Sci. 2021, 620, 118901–118912. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Biswas, P.; Fortner, J.D. Graphene oxides as nanofillers in polysulfone ultrafiltration membranes: Shape Matters. J. Membr. Sci. 2019, 581, 453–461. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, B.; Liu, J.; Zhang, H.; Song, C. Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 2013, 447, 452–462. [Google Scholar] [CrossRef]
- Sun, H.; Tang, B.; Wu, P. Development of hybrid ultrafiltration membranes with improved water separation properties using modified superhydrophilic metal-organic framework nanoparticles. Appl. Mater. Interfaces 2017, 9, 21473–21484. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.-J. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Lou, Y.; Tan, F.J.; Zeng, R.; Wang, M.; Li, P.; Xia, S. Preparation of cross-linked graphene oxide on polyethersulfone membrane for pharmaceuticals and personal care products removal. Polymers 2020, 12, 1921. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lee, C.H. Elucidating the rejection mechanisms of PPCPs by nanofiltration and reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 6798–6806. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Oluwasola, E.I.; Ismail, S.; Shoparwe, N.F. Graphene oxide-doped polymer inclusion membrane for remediation of pharmaceutical contaminant of emerging concerns: Ibuprofen. Membranes 2022, 12, 24. [Google Scholar] [CrossRef]
- Bareera, M.; Buscio, V.; Odabasi, S.U.; Buyukgungor, H. A study on behavior, interaction and rejection of paracetamol, diclofenac and ibuprofen (PhACs) from wastewater by nanofiltration membranes. Environ. Technol. Innov. 2020, 18, 100641–100679. [Google Scholar]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E. Viability of a low-pressure nanofilter in treating recycled water for water reuse applications: A pilot-scale study. Water Res. 2007, 41, 3948–3958. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Hawkes, S. Effects of membrane fouling on the nanofiltration of pharmaceutically active compounds (PhACs): Mechanisms and role of membrane pore size. Sep. Purif. Technol. 2007, 57, 176–184. [Google Scholar] [CrossRef]
- Verliefde, A.R.; Cornelissen, E.R.; Heijman, S.G.J.; Petrinic, I.; Luxbacher, T.; Amy, G.L.; Van Dijk, J.C. Influence of membrane fouling by (pretreated) surface water on rejection of pharmaceutically active compounds (PhACs) by nanofiltration membranes. J. Membr. Sci. 2009, 330, 90–103. [Google Scholar] [CrossRef]
- Alturki, A.A.; Tadkaew, N.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 2010, 365, 206–215. [Google Scholar] [CrossRef]
- Yangali Quintanilla, V. Rejection of emerging organic contaminants by nanofiltration and reverse osmosis membranes: Effects of fouling, modelling and water reuse. Doctoral Thesis, Delft University of Technology, Delft, The Netherlands, 2010. [Google Scholar]
- Garcia-Ivars, J.; Martella, L.; Massella, M.; Carbonell-Alcaina, C.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I. Nanofiltration as tertiary treatment method for removing trace pharmaceutically active compounds in wastewater from wastewater treatment plants. Water Res. 2017, 125, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.J.; Duranceau, S.J. Removal of enantiomeric ibuprofen in a nanofiltration membrane process. Membranes 2020, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Kudlek, E.; Bohdziewicz, J.; Dudziak, M. Influence of water matrix on the retention of pharmaceuticals by high-pressure membrane filtration. Ecol. Chem. Eng. A 2015, 22, 469–479. [Google Scholar]
- Marszałek, A.; Puszczało, E. Effect of photooxidation on nanofiltration membrane fouling during wastewater treatment from the confectionery industry. Water 2020, 12, 793. [Google Scholar] [CrossRef] [Green Version]



| Manufacturer | Alfa Laval (Denmark) |
|---|---|
| Product denomination | NF |
| Composition | Polysulfone |
| Pore size (Da) | 300 |
| Maximum pressure (N m−2) | 55 × 105 |
| Operating pressure range (N m−2) | 15–42 × 105 |
| Temperature range (°C) | 5–50 |
| Cl free concentration (ppm) | <0.1 |
| pH range (Treference = 25 °C) | 3–10 |
| Coefficient of Permeability to Solvent 10−8 (s/m) | ||
|---|---|---|
| Native | RGO | GO |
| 17.15 | 16.11 | 8.33 |
| Membrane | Material | Experimental Conditions | Permeate Flux (L/m2h) | Rejection (%) | Reference |
|---|---|---|---|---|---|
| NF | Aromatic polyamide | pH = 7.5 P = 445–504 kPa | - | 45 | (Yoon et al., 2007) [38] |
| NF 4040 | Polypiperazine-amid thin-film composite | pH = 6.3 C = 250 ng/L P = 60–70 psi | 20.4 | 100 | (Bellona et al., 2007) [39] |
| NF90 NF270 TFC-SR2 | Polyamide thin-film with a microporous polysulfone | pH = 4–9.8 pH = 6.3–9.8 pH = 9.8 | - | 100 99 84 | (Nghiem et al., 2007) [40] |
| TS80 DESAL HL | Cross-linked aromatic polyamide top layer | pH = 6.5–7.5 C = 2 µg/L P = 5 bar | - | 99 98 | (Verliefde et al., 2009) [41] |
| NF270 NF90 | Thin aromatic or semiaromatic polyamide | pH = 7.4–7.6 C = 2 µg/L P = 12 bar | 41.0 | 99 99 | (Alturki et al., 2010) [42] |
| NF200 NF90 | Aromatic polyamide | pH = 6–7 C =2 µg/L P = 12 bar | - | 89 96 | (Yangali Quintanilla et al., 2010) [43] |
| MPS-34 TFC-SR2 NF270 | Polysulfone composite Polysulfone composite Polyamide thin-film composite | pH = 8 P = 5 bar | - | 99 58 95 | (García-Ivars et al., 2017) [44] |
| Ceramic membrane Ceramic GO | Ceramic membrane Ceramic GO | pH = 7 C = 10 µM P = 3 bar | 25.1 14.4 | 70 92 | (Chu et al., 2017) [27] |
| NF50 NF10 | Sulfonated polyethersulfone | pH = 6–7 | - | 80.54 12 | (Bareera et al., 2020) [37] |
| NF270 TS40 | Polyamide thin-film composite Polypiperazine amide | pH = 4 C = 400 µg/L | 42.4 | 20–30 37–42 | (Higgins and Duranceau, 2020) [45] |
| G1 G2 G3 | Polymer inclusion membrane G1 = 0.15% GO G2 = 0.45% GO G3 = 0.75% GO | pH = 2 C = 10 mg/L P = 100 psi | - | 70 75 77 | (Ahmad et al., 2021) [36] |
| AFC30 AFC40 AFC80 | Polyamide | pH = 7 C = 1 mg/L P = 2 MPa | 34.2 | 98 98 90 | (Kudlek et al., 2015) [46] |
| NF270 | Polyamide thin-film composite | pH = 7 C = 10 mg/L P = 130 psi | - | 85 | (Kabbani et al., 2021) [10] |
| NF GO RGO | Polysulfone GO 0.15% RGO 0.15% | pH = 7 C = 7.5 mg/L P = 15 bar | 75.6 52.2 72.0 | 77 85 88 | This work |
| Native Membrane | ||||
|---|---|---|---|---|
| P (bar) | Permeability | |||
| Initial (Jw (kg H2O/s m2)103) | Final (Jw (kg H2O/s m2)103) | F | ||
| 10 | 19.156 | 16.111 | 0.159 | |
| 15 | 28.053 | 24.444 | 0.129 | |
| 20 | 36.304 | 32.222 | 0.112 | |
| P (bar) | Selectivity | |||
| Initial | Final | |||
| Jp (kg H2O/s m2)103 | Rejection coefficient | Jp (kg H2O/s m2)103 | Rejection coefficient | |
| 10 | 18.826 | 0.939 | 16.111 | 0.976 |
| 15 | 25.471 | 0.945 | 24.444 | 0.978 |
| 20 | 37.099 | 0.946 | 31.667 | 0.976 |
| RGO Membrane | ||||
|---|---|---|---|---|
| P (bar) | Permeability | |||
| Initial (Jw (kg H2O/s m2)103) | Final (Jw (kg H2O/s m2)103) | F | ||
| 10 | 15.556 | 16.111 | −0.036 | |
| 15 | 24.444 | 23.333 | 0.045 | |
| 20 | 31.667 | 28.889 | 0.088 | |
| P (bar) | Selectivity | |||
| Initial | Final | |||
| Jp (kg H2O/s m2)103 | Rejection coefficient | Jp (kg H2O/s m2)103 | Rejection coefficient | |
| 10 | 16.111 | 0.925 | 14.444 | 0.846 |
| 15 | 23.333 | 0.930 | 23.889 | 0.825 |
| 20 | 31.111 | 0.928 | 30.000 | 0.835 |
| GO Membrane | ||||
|---|---|---|---|---|
| P (bar) | Permeability | |||
| Initial (Jw (kg H2O/s m2)103) | Final (Jw (kg H2O/s m2)103) | F | ||
| 10 | 11.667 | 11.111 | 0.048 | |
| 15 | 15.000 | 17.222 | −0.148 | |
| 20 | 20.000 | 22.778 | −0.139 | |
| P (bar) | Selectivity | |||
| Initial | Final | |||
| Jp (kg H2O/s m2)103 | Rejection coefficient | Jp (kg H2O/s m2)103 | Rejection coefficient | |
| 10 | 8.889 | 0.966 | 8.333 | 0.790 |
| 15 | 14.444 | 0.972 | 13.333 | 0.831 |
| 20 | 18.889 | 0.973 | 18.333 | 0.858 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; León, G.; Miguel, B.; Gago, I.; Martínez, P.M. Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes. Membranes 2022, 12, 562. https://doi.org/10.3390/membranes12060562
Hidalgo AM, Gómez M, Murcia MD, León G, Miguel B, Gago I, Martínez PM. Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes. Membranes. 2022; 12(6):562. https://doi.org/10.3390/membranes12060562
Chicago/Turabian StyleHidalgo, Asunción M., María Gómez, María D. Murcia, Gerardo León, Beatriz Miguel, Israel Gago, and Pilar M. Martínez. 2022. "Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes" Membranes 12, no. 6: 562. https://doi.org/10.3390/membranes12060562
APA StyleHidalgo, A. M., Gómez, M., Murcia, M. D., León, G., Miguel, B., Gago, I., & Martínez, P. M. (2022). Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes. Membranes, 12(6), 562. https://doi.org/10.3390/membranes12060562