Evidence for a Physiological Role of T-Type Ca Channels in Ventricular Cardiomyocytes of Adult Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Mice
2.3. Isolation of Ventricular Cardiomyocytes
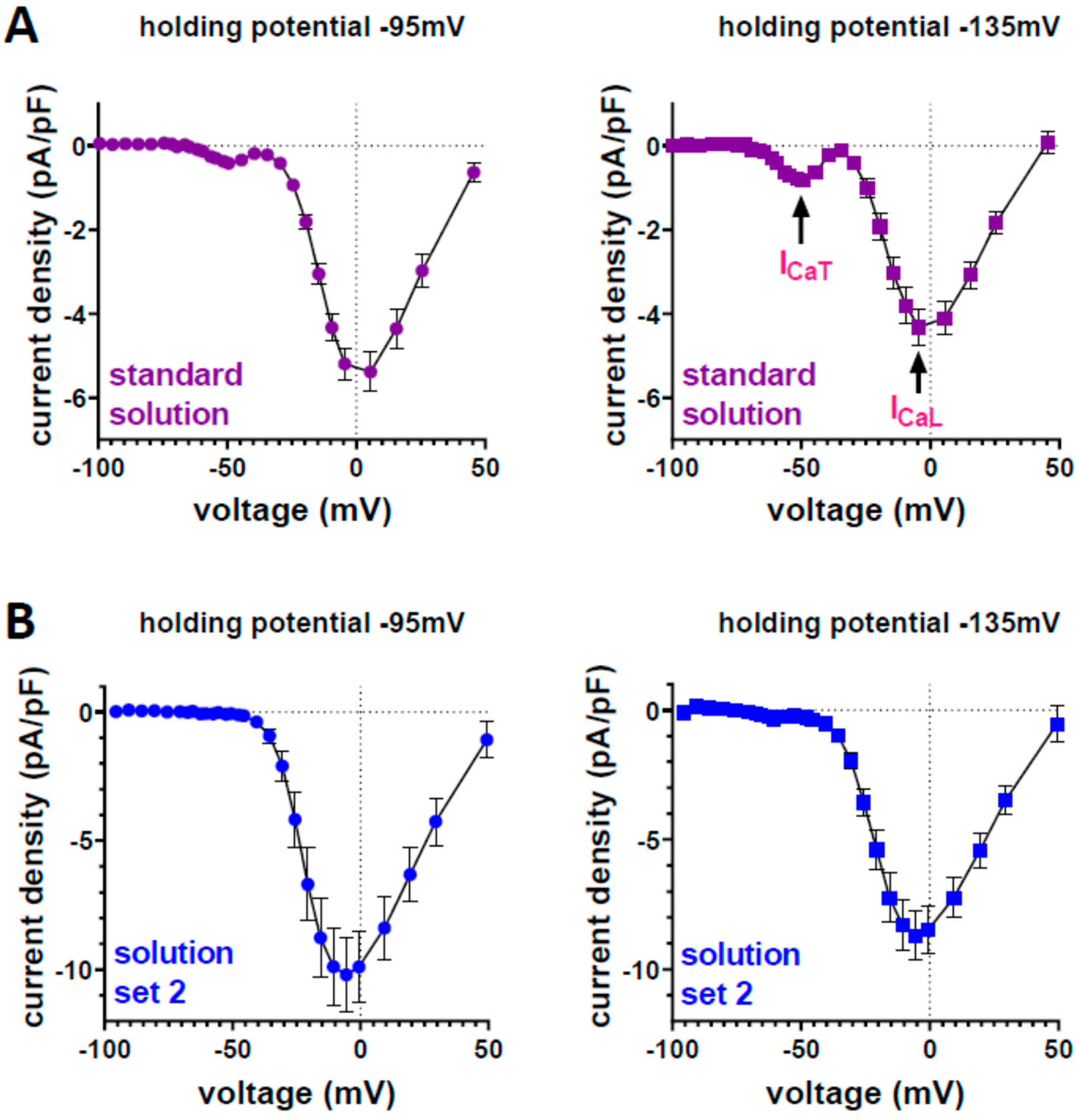
2.4. Detection of Ca Currents
2.5. Immunostaining
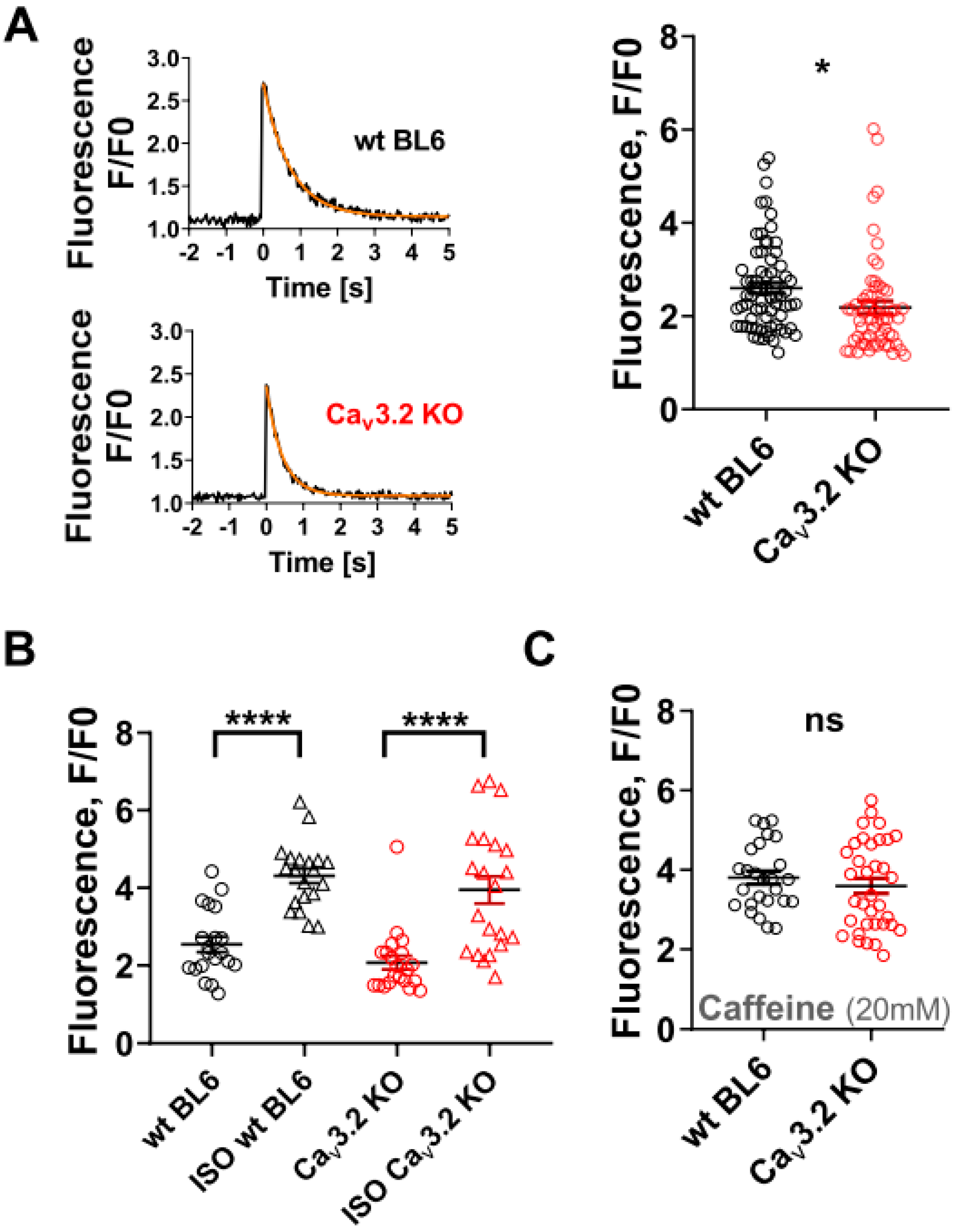
2.6. Intracellular Ca Measurements
2.7. Statistical Analyses
3. Results
4. Discussion
4.1. Physiological Role of T-Type Ca Channels in Ventricular Cardiomyocytes of Healthy Adult Mice
4.2. Potential Factors Responsible for Overlooking Actually Present ICaT in the Ventricular Cardiomyocytes of Adult Mammals
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiwara, N.; Irisawa, H.; Kameyama, M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 1988, 395, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, L.L. T-type calcium channel expression and function in the diseased heart. Channels 2010, 4, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Qin, D.; Deng, L.; Boutjdir, M.; El-Sherif, N. Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Cardiovasc. Res. 2000, 46, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Vassort, G.; Talavera, K.; Alvarez, J.L. Role of T-type Ca2+ channels in the heart. Cell Calcium 2006, 40, 205–220. [Google Scholar] [CrossRef]
- Ono, K.; Iijima, T. Cardiac T-type Ca(2+) channels in the heart. J. Mol. Cell. Cardiol. 2010, 48, 65–70. [Google Scholar] [CrossRef]
- Rubi, L.; Gawali, V.S.; Kubista, H.; Todt, H.; Hilber, K.; Koenig, X. Proper Voltage-Dependent Ion Channel Function in Dysferlin-Deficient Cardiomyocytes. Cell. Physiol. Biochem. 2015, 36, 1049–1058. [Google Scholar] [CrossRef]
- Ebner, J.; Cagalinec, M.; Kubista, H.; Todt, H.; Szabo, P.L.; Kiss, A.; Podesser, B.K.; Cserne Szappanos, H.; Hool, L.C.; Hilber, K.; et al. Neuronal nitric oxide synthase regulation of calcium cycling in ventricular cardiomyocytes is independent of Ca v 1.2 channel modulation under basal conditions. Pflug. Arch. 2020, 472, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Sen, L.; Smith, T.W. T-type Ca2+ channels are abnormal in genetically determined cardiomyopathic hamster hearts. Circ. Res. 1994, 75, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Mitra, R.; Morad, M. Two types of calcium channels in guinea pig ventricular myocytes. Proc. Natl. Acad. Sci. USA 1986, 83, 5340–5344. [Google Scholar] [CrossRef] [Green Version]
- Sipido, K.R.; Carmeliet, E.; van de Werf, F. T-type Ca2+ current as a trigger for Ca2+ release from the sarcoplasmic reticulum in guinea-pig ventricular myocytes. J. Physiol. 1998, 508, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Masumiya, H.; Agata, N.; Tanaka, H.; Shigenobu, K. Developmental changes in action potential and membrane currents in fetal, neonatal and adult guinea-pig ventricular myocytes. J. Mol. Cell. Cardiol. 1996, 28, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; O’Connell, R.P.; Deo, M.; Milstein, M.L.; Furspan, P.; Herron, T.J.; Pandit, S.V.; Musa, H.; Berenfeld, O.; Jalife, J.; et al. The ionic bases of the action potential in isolated mouse cardiac Purkinje cell. Heart Rhythm 2013, 10, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamayo, M.; Manzanares, E.; Bas, M.; Martín-Nunes, L.; Val-Blasco, A.; Larriba, M.J.; Fernández-Velasco, M.; Delgado, C. Calcitriol (1,25-dihydroxyvitamin D 3) increases L-type calcium current via protein kinase A signaling and modulates calcium cycling and contractility in isolated mouse ventricular myocytes. Heart Rhythm 2017, 14, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Szabó, P.L.; Ebner, J.; Koenig, X.; Hamza, O.; Watzinger, S.; Trojanek, S.; Abraham, D.; Todt, H.; Kubista, H.; Schicker, K.; et al. Cardiovascular phenotype of the Dmd mdx rat—A suitable animal model for Duchenne muscular dystrophy. Dis. Model. Mech. 2021, 14, dmm047704. [Google Scholar] [CrossRef]
- Koenig, X.; Dysek, S.; Kimbacher, S.; Mike, A.K.; Cervenka, R.; Lukacs, P.; Nagl, K.; Dang, X.B.; Todt, H.; Bittner, R.E.; et al. Voltage-gated ion channel dysfunction precedes cardiomyopathy development in the dystrophic heart. PLoS ONE 2011, 6, e20300. [Google Scholar] [CrossRef] [Green Version]
- Talavera, K.; Janssens, A.; Klugbauer, N.; Droogmans, G.; Nilius, B. Extracellular Ca2+ modulates the effects of protons on gating and conduction properties of the T-type Ca2+ channel alpha1G (CaV3.1). J. Gen. Physiol. 2003, 121, 511–528. [Google Scholar] [CrossRef] [Green Version]
- Rubi, L.; Todt, H.; Kubista, H.; Koenig, X.; Hilber, K. Calcium current properties in dystrophin-deficient ventricular cardiomyocytes from aged mdx mice. Physiol. Rep. 2018, 6, e13567. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, F.; Zhang, X.; Qi, Z.; Tang, M.; Szeto, C.; Li, Y.; Zhang, H.; Chen, X. β-Adrenergic stimulation increases Cav3.1 activity in cardiac myocytes through protein kinase A. PLoS ONE 2012, 7, e39965. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kuwahara, K.; Takano, M.; Arai, Y.; Kuwabara, Y.; Yasuno, S.; Nakagawa, Y.; Nakanishi, M.; Harada, M.; Fujiwara, M.; et al. T-type Ca2+ channel blockade prevents sudden death in mice with heart failure. Circulation 2009, 120, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Williams, I.A.; Allen, D.G. Intracellular calcium handling in ventricular myocytes from mdx mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H846–H855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangoni, M.E.; Traboulsie, A.; Leoni, A.-L.; Couette, B.; Marger, L.; Le Quang, K.; Kupfer, E.; Cohen-Solal, A.; Vilar, J.; Shin, H.-S.; et al. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ. Res. 2006, 98, 1422–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, P.B.L. Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: News from the world of knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R227–R237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.-S.; Huang, C.-H.; Chieng, H.; Chang, Y.-T.; Chang, D.; Chen, J.-J.; Chen, Y.-C.; Chen, Y.-H.; Shin, H.-S.; Campbell, K.P.; et al. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ. Res. 2009, 104, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Jaleel, N.; Nakayama, H.; Chen, X.; Kubo, H.; MacDonnell, S.; Zhang, H.; Berretta, R.; Robbins, J.; Cribbs, L.; Molkentin, J.D.; et al. Ca2+ influx through T- and L-type Ca2+ channels have different effects on myocyte contractility and induce unique cardiac phenotypes. Circ. Res. 2008, 103, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Asfaw, T.N.; Tyan, L.; Glukhov, A.V.; Bondarenko, V.E. A compartmentalized mathematical model of mouse atrial myocytes. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H485–H507. [Google Scholar] [CrossRef]
- Fan, G.; Kaßmann, M.; Cui, Y.; Matthaeus, C.; Kunz, S.; Zhong, C.; Zhu, S.; Xie, Y.; Tsvetkov, D.; Daumke, O.; et al. Age attenuates the T-type Ca V 3.2-RyR axis in vascular smooth muscle. Aging Cell 2020, 19, e13134. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Kaßmann, M.; Hashad, A.M.; Welsh, D.G.; Gollasch, M. Differential targeting and signalling of voltage-gated T-type Ca v 3.2 and L-type Ca v 1.2 channels to ryanodine receptors in mesenteric arteries. J. Physiol. 2018, 596, 4863–4877. [Google Scholar] [CrossRef] [Green Version]
- Markandeya, Y.S.; Fahey, J.M.; Pluteanu, F.; Cribbs, L.L.; Balijepalli, R.C. Caveolin-3 regulates protein kinase A modulation of the Ca(V)3.2 (alpha1H) T-type Ca2+ channels. J. Biol. Chem. 2011, 286, 2433–2444. [Google Scholar] [CrossRef] [Green Version]
- Markandeya, Y.S.; Phelan, L.J.; Woon, M.T.; Keefe, A.M.; Reynolds, C.R.; August, B.K.; Hacker, T.A.; Roth, D.M.; Patel, H.H.; Balijepalli, R.C. Caveolin-3 Overexpression Attenuates Cardiac Hypertrophy via Inhibition of T-type Ca2+ Current Modulated by Protein Kinase Cα in Cardiomyocytes. J. Biol. Chem. 2015, 290, 22085–22100. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.R.; Perez-Reyes, E.; Jones, S.W. State-dependent inactivation of the alpha1G T-type calcium channel. J. Gen. Physiol. 1999, 114, 185–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hering, J.; Feltz, A.; Lambert, R.C. Slow inactivation of the Ca(V)3.1 isotype of T-type calcium channels. J. Physiol. 2004, 555, 331–344. [Google Scholar] [CrossRef] [PubMed]


| Standard Solution | Solution Set 2 | ||
|---|---|---|---|
| Bath solution (mM) | Pipette solution (mM) | Bath solution (mM) | Pipette solution (mM) |
| TEA-Cl (145) | Cs-aspartate (145) | NMDG (150) | CsCl (102) |
| HEPES (10) | HEPES (10) | HEPES (15) | HEPES (10) |
| CaCl2 (2) | MgCl2 (2) | Glucose (5) | EGTA (10) |
| TEA-OH to pH 7.4 | Mg-ATP (2) | CsCl (5) | TEA-Cl (10) |
| Cs-EGTA (0.1) | CaCl2 (2) | MgCl2 (5) | |
| CsOH to pH 7.4 | HCl to pH 7.4 | Na2ATP (5) | |
| pH 7.4 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marksteiner, J.; Ebner, J.; Salzer, I.; Lilliu, E.; Hackl, B.; Todt, H.; Kubista, H.; Hallström, S.; Koenig, X.; Hilber, K. Evidence for a Physiological Role of T-Type Ca Channels in Ventricular Cardiomyocytes of Adult Mice. Membranes 2022, 12, 566. https://doi.org/10.3390/membranes12060566
Marksteiner J, Ebner J, Salzer I, Lilliu E, Hackl B, Todt H, Kubista H, Hallström S, Koenig X, Hilber K. Evidence for a Physiological Role of T-Type Ca Channels in Ventricular Cardiomyocytes of Adult Mice. Membranes. 2022; 12(6):566. https://doi.org/10.3390/membranes12060566
Chicago/Turabian StyleMarksteiner, Jessica, Janine Ebner, Isabella Salzer, Elena Lilliu, Benjamin Hackl, Hannes Todt, Helmut Kubista, Seth Hallström, Xaver Koenig, and Karlheinz Hilber. 2022. "Evidence for a Physiological Role of T-Type Ca Channels in Ventricular Cardiomyocytes of Adult Mice" Membranes 12, no. 6: 566. https://doi.org/10.3390/membranes12060566
APA StyleMarksteiner, J., Ebner, J., Salzer, I., Lilliu, E., Hackl, B., Todt, H., Kubista, H., Hallström, S., Koenig, X., & Hilber, K. (2022). Evidence for a Physiological Role of T-Type Ca Channels in Ventricular Cardiomyocytes of Adult Mice. Membranes, 12(6), 566. https://doi.org/10.3390/membranes12060566






