Biorefinery of Tomato Leaves by Integrated Extraction and Membrane Processes to Obtain Fractions That Enhance Induced Resistance against Pseudomonas syringae Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Biomolecules and Total Solids Quantification
2.2.2. Production of Aqueous Extract from Lyophilized Tomato Leaf
2.2.3. Biopesticide Activity of the Collected Fractions
2.2.4. Membrane Process Equipment
3. Results
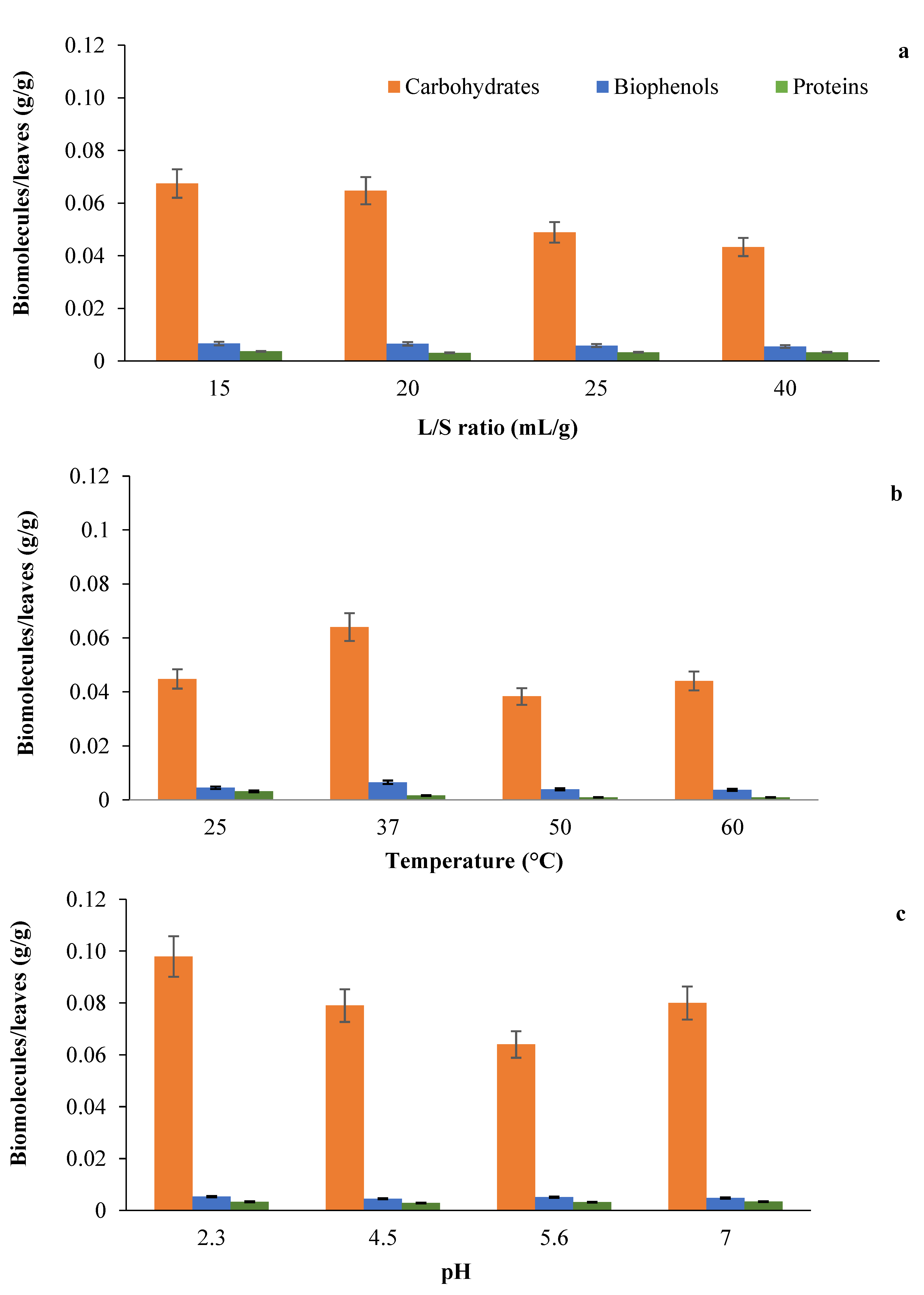
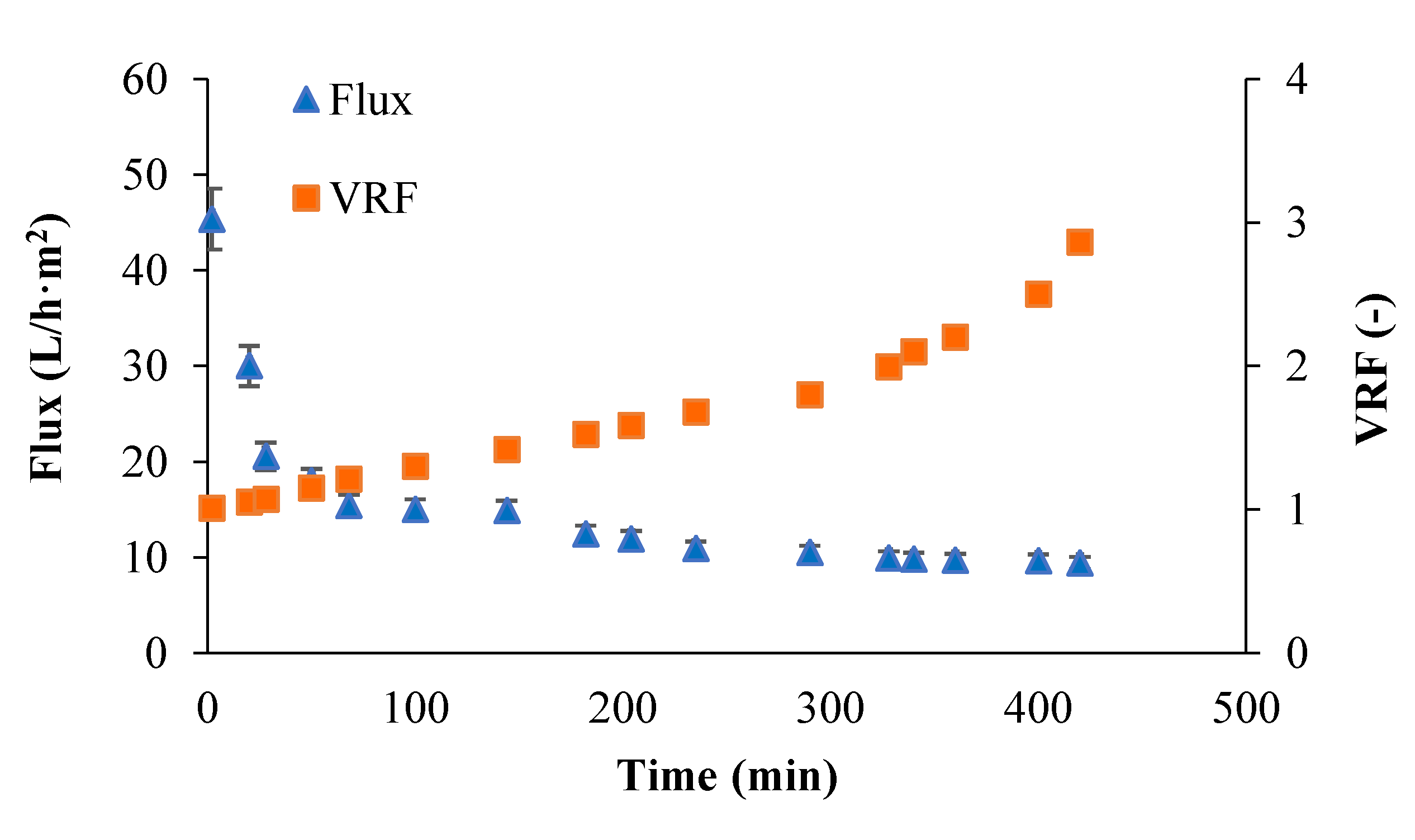
3.1. Production of Tomato Leaves Extract
3.2. Ultrafiltration (UF) of Aqueous Extract
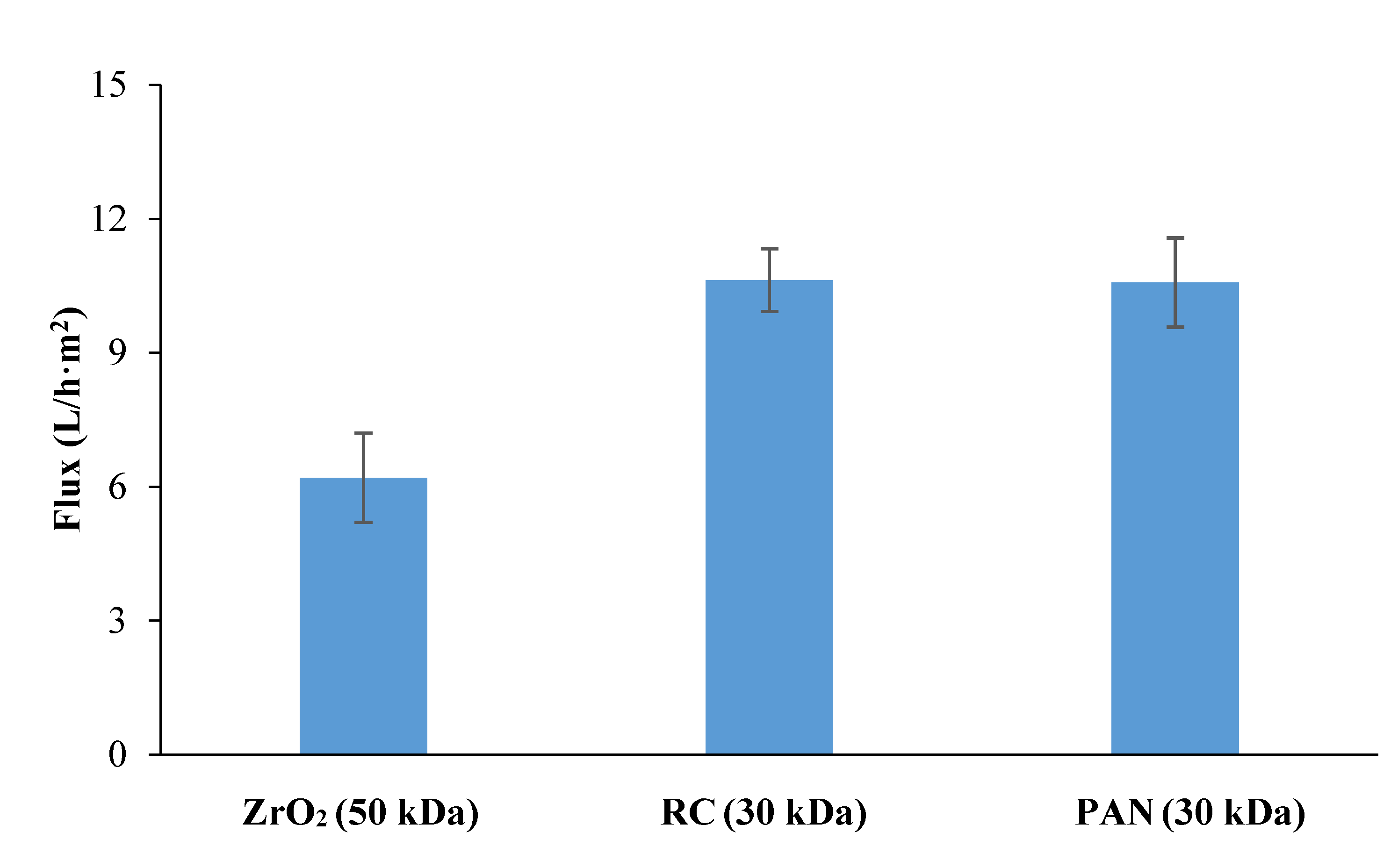
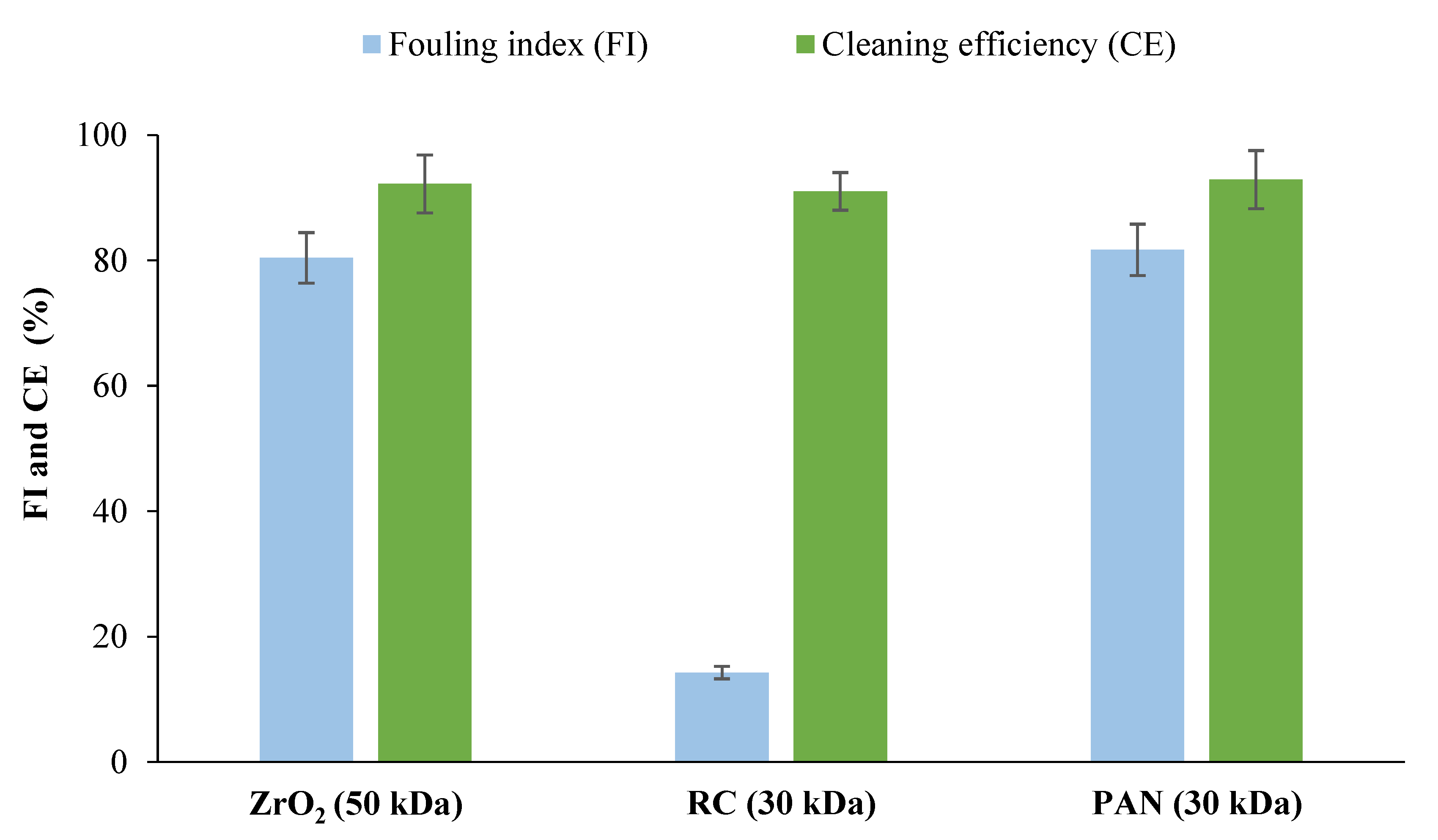
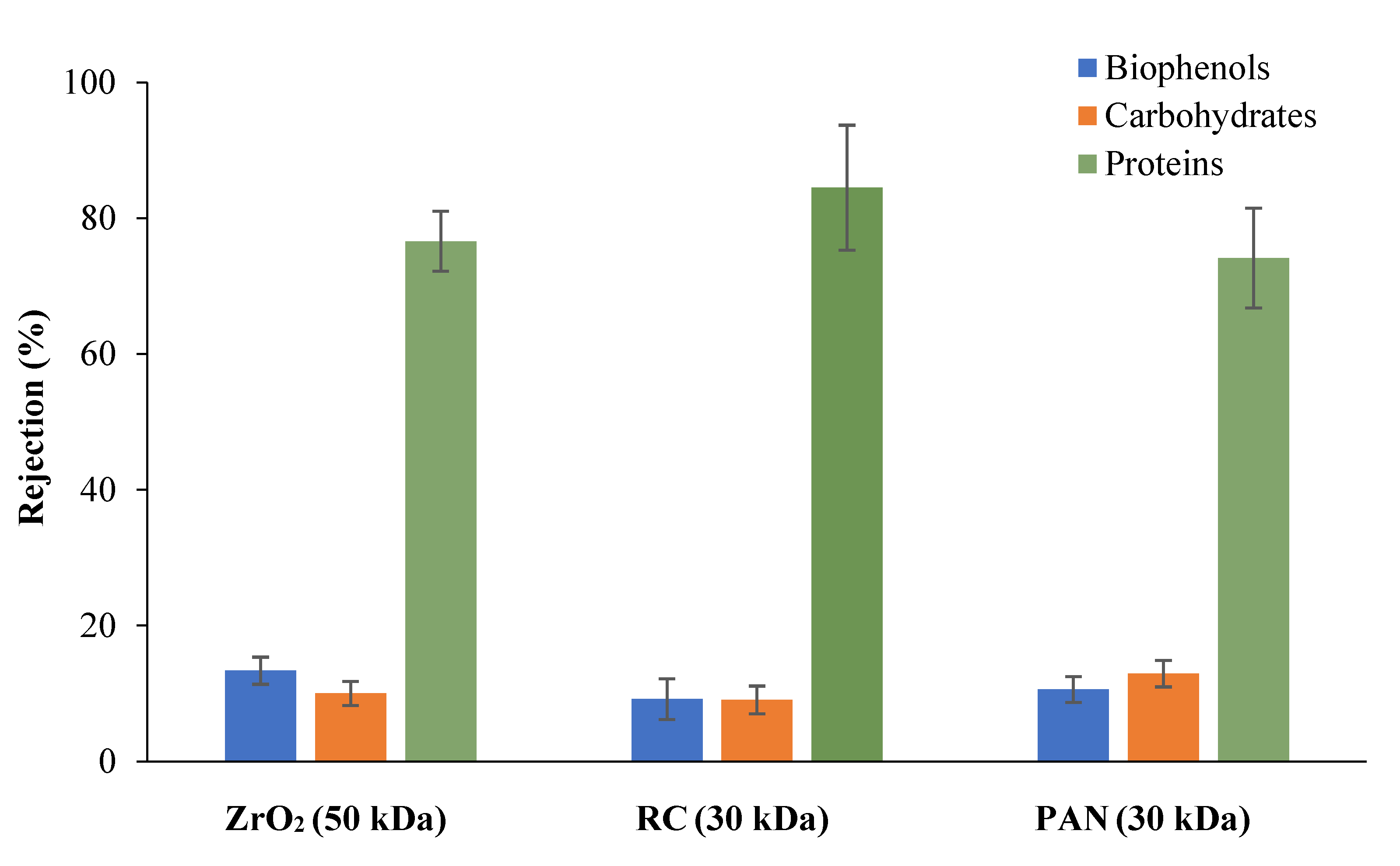
3.2.1. Selection of Membrane Material for Protein Removal from Tomato Leaves Extract
3.2.2. Selection of Membrane Material for Carbohydrates Fractionation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Mazzei, R.; Piacentini, E.; Nardi, M.; Poerio, T.; Bazzarelli, F.; Procopio, A.; Di Gioia, M.L.; Ceraldi, R.; Morelli, C.; Giorno, L.; et al. Production of plant-derived oleuropein aglycone by a combined membrane process and evaluation of its breast anticancer properties. Front. Bioeng. Biotechnol. 2020, 908. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Mazzei, R.; Poerio, T.; Bazzarelli, F.; Wu, Z.; Li, K.; Giorno, L. Biorefinery of olive leaves to produce dry oleuropein aglycone: Use of homemade ceramic capillary biocatalytic membranes in a multiphase system. Chem. Eng. Sci. 2018, 185, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Junker-Frohn, L.V.; Lück, M.; Schmittgen, S.; Wensing, J.; Carraresi, L.; Thiele, B.; Groher, T.; Reimer, J.J.; Bröring, S.; Noga, G.; et al. Tomato’s green gold: Bioeconomy potential of residual tomato leaf biomass as a novel source for the secondary metabolite rutin. ACS Omega 2019, 4, 19071–19080. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.A.; Fraser, P.D. Solanesol: Added value from solanaceous waste. Phytochemistry 2011, 72, 1323−1327. [Google Scholar] [CrossRef]
- Campbell, R.; Freitag, S.; Bryan, G.J.; Stewart, D.; Taylor, M.A. Environmental and genetic factors associated with solanesol accumulation in potato leaves. Front. Plant Sci. 2016, 7, 1263. [Google Scholar] [CrossRef] [Green Version]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-Food Industry Waste as Resource of Chemicals: The Role of Membrane Technology in Their Sustainable Recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Mazzei, R.; Gebreyohannes, A.Y.; Papaioannou, E.; Nunes, S.P.; Vankelecom, I.F.; Giorno, L. Enzyme catalysis coupled with artificial membranes towards process intensification in biorefinery-a review. Bioresour. Technol. 2021, 335, 125248. [Google Scholar] [CrossRef] [PubMed]
- Giorno, F.; Mazzei, R.; Giorno, L. Purification of triacylglycerols for biodiesel production from Nannochloropsis microalgae by membrane technology. Bioresour. Technol. 2013, 140, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Djamai, W.; Mazzei, R.; Bazzarelli, F.; Dahmani, B.; Giorno, L. Membrane-assisted biorefinery of microalgae to obtain enriched fractions of bioderived molecules. Biofuels Bioprod. Biorefining 2019, 13, 878–888. [Google Scholar] [CrossRef]
- Acosta-Fernández, R.; Poerio, T.; Nabarlatz, D.; Giorno, L.; Mazzei, R. Enzymatic hydrolysis of xylan from coffee parchment in membrane bioreactors. Ind. Eng. Chem. Res. 2020, 59, 7346–7354. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Quantick, P.; Petropakis, H. A study of the direct osmotic concentration of tomato juice in tubular membrane–module configuration. I. The effect of certain basic process parameters on the process performance. J. Membr. Sci. 1998, 150, 99–110. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Turchini, A.; Della Valle, P.; Trevisan, M. Integrated membrane processes for the concentration of tomato juice. Desalination 2002, 148, 73–77. [Google Scholar] [CrossRef]
- Razi, B.; Aroujalian, A.; Raisi, A.; Fathizadeh, M. Clarification of tomato juice by cross-flow microfiltration. J. Food Sci. Technol. 2011, 46, 138–145. [Google Scholar] [CrossRef]
- Bahçeci, K.S.; Akıllıoğlu, H.G.; Gökmen, V. Osmotic and membrane distillation for the concentration of tomato juice: Effects on quality and safety characteristics. Innov. Food Sci. Emerg. Technol. 2015, 31, 131–138. [Google Scholar] [CrossRef]
- Yodjun, P.; Soontarapa, K.; Eamchotchawalit, C. Separation of lycopene/solvent mixture by chitosan membranes. J. Met. Mater. Miner. 2011, 21, 107–113. [Google Scholar]
- de Souza, A.L.R.; Gomes, F.D.S.; Tonon, R.V.; da Silva, L.F.M.; Cabral, L.M.C. Coupling membrane processes to obtain a lycopene-rich extract. J. Food Process. Preserv. 2019, 43, 14164. [Google Scholar] [CrossRef]
- Filho, R.L.; De Souza, R.M.; Ferreira, A.; Quecine, M.C.; Alves, E.; De Azevedo, J.L. Biocontrol activity of Bacillus against a GFP-marked Pseudomonas syringae pv. tomato on tomato phylloplane. Australas. Plant Pathol. 2013, 42, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Singh, A.K.; Kumar, A. Disease management of tomato through PGPB: Current trends and future perspective. 3 Biotech 2017, 7, 255. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M. The Use of Flat Ceramic Membranes for Purification of the Liquid Fraction of the Digestate from Municipal Waste Biogas Plants. Energies 2021, 14, 3947. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Giorno, L. Enzyme-loaded membrane reactor to degrade a pesticide in vegetative waters. J. Membr. Sci. 2021, 635, 119438. [Google Scholar] [CrossRef]
- Luján-Facundo, M.J.; Mendoza-Roca, J.A.; Cuartas-Uribe, B.; Álvarez-Blanco, S. Evaluation of cleaning efficiency of ultrafiltration membranes fouled by BSA using FTIR–ATR as a tool. J. Food Eng. 2015, 163, 1–8. [Google Scholar] [CrossRef]
- Esmaeili, M.; Virtanen, T.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Vanillin as an antifouling and hydro-philicity promoter agent in surface modification of polyethersulfone membrane. Membranes 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Guo, S.; Rao, L.; Qin, J.; Xu, X.; Liang, Y. Optimization of the technology of extracting water-soluble polysaccharides from Morus alba L. leaves. Afr. J. Biotechnol. 2011, 10, 12684–12690. [Google Scholar]
- Shang, H.; Zhou, H.; Duan, M.; Li, R.; Wu, H.; Lou, Y. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from comfrey (Symphytum officinale L.) root. Int. J. Biol. Macromol. 2018, 112, 889–899. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, Y.; Wang, C.; Yang, Q.; Jiang, X.; Zhu, C. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis. Mar. Drugs 2020, 18, 539. [Google Scholar] [CrossRef]
- Tan, S.S.; Xu, Q.; Ziping, L.; Zilin, L.; Shiyu, T.; Qiliang, X.; Haiyan, Y.; Lingli, Y. Inquiry of water-soluble polysaccharide extraction conditions from grapefruit skin. Engineering 2011, 3, 8659. [Google Scholar]
- Bazzarelli, F.; Poerio, T.; Mazzei, R.; D’Agostino, N.; Giorno, L. Study of OMWWs suspended solids destabilization to improve membrane processes performance. Sep. Purif. Technol. 2015, 149, 183–189. [Google Scholar] [CrossRef]
- Abd-Razak, N.H.; Zairossani, M.N.; Chew, Y.M.; Bird, M.R. Fouling analysis and the recovery of phytosterols from orange juice using regenerated cellulose ultrafiltration membranes. Food Bioprocess Technol. 2020, 13, 2012–2028. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; Lorenzo, G.D. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Biologically active compounds from goji (Lycium barbarum L.) leaves aqueous extracts: Purification and concentration by membrane processes. Biomolecules 2020, 10, 935. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef]
- Vernhet, A.; Bellon-Fontaine, M.N.; Brillouet, J.M.; Roesink, E.; Moutounet, M. Wetting properties of microfiltration membrane: Determination by means of the capillary rise technique and incidence on the adsorption of wine polysaccharide and tannins. J. Membr. Sci. 1997, 128, 163–174. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Coupling ultrafiltration-based processes to concentrate phenolic compounds from aqueous goji berry extracts. Molecules 2020, 25, 3761. [Google Scholar] [CrossRef]


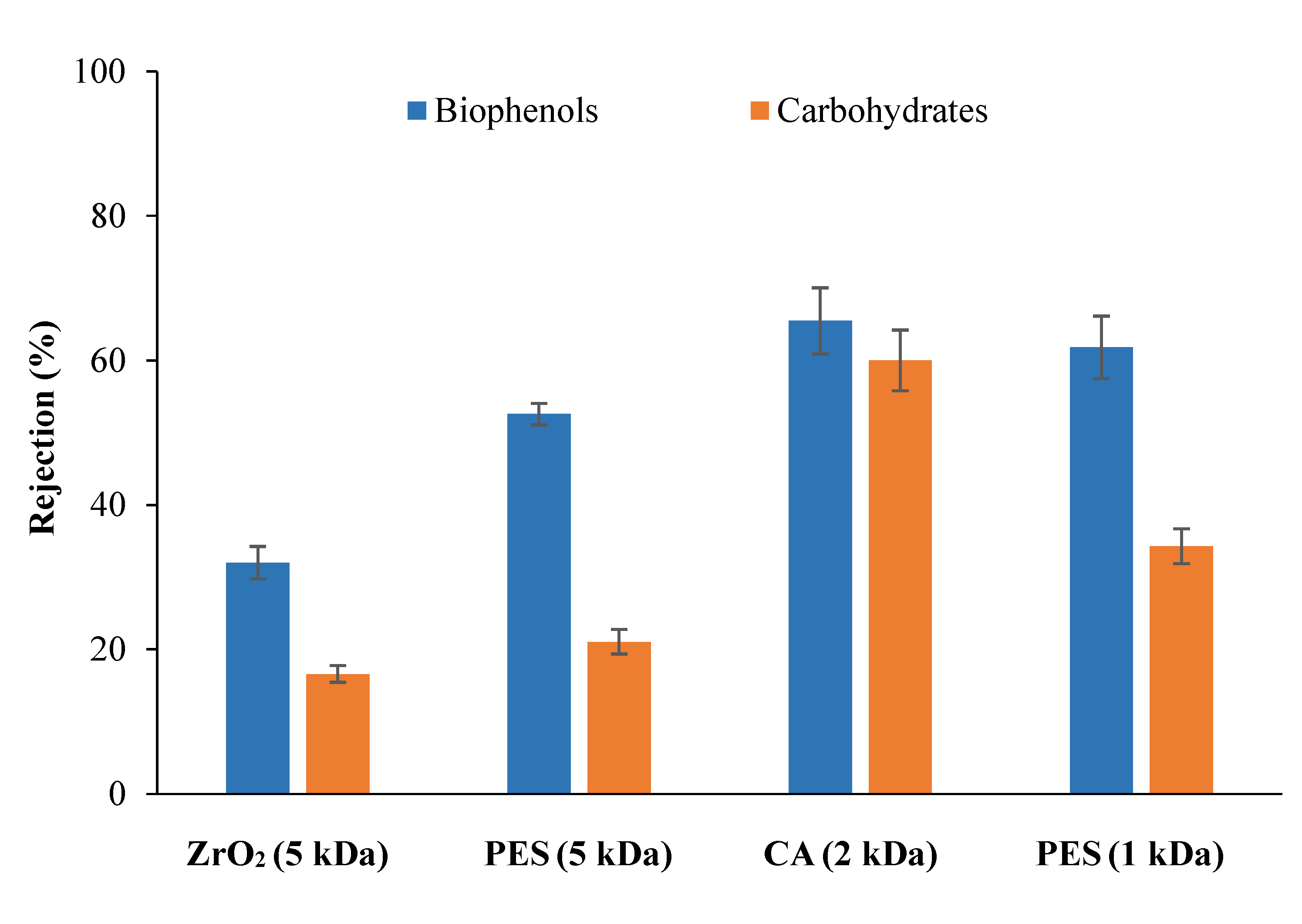
| Membrane Type | ZrO2 | RC | PAN | PES | ZrO2 | CA | PES |
|---|---|---|---|---|---|---|---|
| Manufacturer | Tami | Merck Millipore | GE Osmonics | Microdyn- Nadir | Tami | Microdyn-Nadir | Microdyn-Nadir |
| Configuration | tubular | flat | flat | flat | tubular | flat | flat |
| NMWCO (kDa) | 50 | 30 | 30 | 5 | 5 | 2 | 1 |
| Membrane surface area (cm2) | 42 | 12.6 | 12.6 | 12.6 | 42 | 12.6 | 12.6 |
| Water contact angle (°) | 42.0 a | 19.0 b | 34.5 c | 54.3 d | 58.0 a | 71.8 c | 72.0 e |
| Samples | P. syringae Leaf Population (CFU/mL) |
|---|---|
| Control | >7 × 105 |
| Feed | 3.9 ± 2.1 × 104 |
| Permeate | 1.9 ± 0.1 × 104 |
| Retentate | 1.5 ± 0.3 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzarelli, F.; Mazzei, R.; Papaioannou, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Biorefinery of Tomato Leaves by Integrated Extraction and Membrane Processes to Obtain Fractions That Enhance Induced Resistance against Pseudomonas syringae Infection. Membranes 2022, 12, 585. https://doi.org/10.3390/membranes12060585
Bazzarelli F, Mazzei R, Papaioannou E, Giannakopoulos V, Roberts MR, Giorno L. Biorefinery of Tomato Leaves by Integrated Extraction and Membrane Processes to Obtain Fractions That Enhance Induced Resistance against Pseudomonas syringae Infection. Membranes. 2022; 12(6):585. https://doi.org/10.3390/membranes12060585
Chicago/Turabian StyleBazzarelli, Fabio, Rosalinda Mazzei, Emmanouil Papaioannou, Vasileios Giannakopoulos, Michael R. Roberts, and Lidietta Giorno. 2022. "Biorefinery of Tomato Leaves by Integrated Extraction and Membrane Processes to Obtain Fractions That Enhance Induced Resistance against Pseudomonas syringae Infection" Membranes 12, no. 6: 585. https://doi.org/10.3390/membranes12060585
APA StyleBazzarelli, F., Mazzei, R., Papaioannou, E., Giannakopoulos, V., Roberts, M. R., & Giorno, L. (2022). Biorefinery of Tomato Leaves by Integrated Extraction and Membrane Processes to Obtain Fractions That Enhance Induced Resistance against Pseudomonas syringae Infection. Membranes, 12(6), 585. https://doi.org/10.3390/membranes12060585









