Development of 3D ZnO-CNT Support Structures Impregnated with Inorganic Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis of ZnO-CNT Composite Nanopowder
2.2. Preparation of ZnO-CNT Nanopowders-Based Pastes for 3D Printing
2.3. Additive Manufacturing of 3D Structures Based on ZnO-CNT Pastes
2.4. Post-Printing Treatment of ZnO-CNT 3D Support Structures
2.5. Impregnation of ZnO-CNT 3D Structures with Inorganic Salts (PCMs)
2.6. Characterization Methods
2.6.1. Morpho-Structural Characterization
- D—the average crystallite size, in nm
- β—the line broadening at half the maximum intensity, in radians,
- λ—the X-ray wavelength, in Å;
- k—constant; k = 0.9 according to Bragg or 0.70 < k < 1.70 according to Klug and Alexander
- θ—diffraction (Bragg) angle
2.6.2. Thermal Characterization
3. Results and Discussion
3.1. Morphostructural Characterization of ZnO-CNT Composite-Based 3D Support Structures
3.2. Morphostructural Characterization of PCMs-Impregnated 3D Structures
3.3. Thermal Stability of the as-Fabricated 3D Support Structures
3.4. Thermal Properties and Stability of 3D Structures Impregnated with PCM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Winkless, L. Are we a step closer to 3D printed carbon nanotube composites? Mater. Today 2017, 20, 228. [Google Scholar] [CrossRef]
- Gonzalez, G.; Chiappone, A.; Roppolo, I.; Fantino, E.; Bertana, V.; Perrucci, F.; Scaltrito, L.; Pirri, F.; Sangermano, M. Development of 3D printable formulations containing CNT with enhanced electrical properties. Polymer 2017, 109, 246–253. [Google Scholar] [CrossRef]
- Pervaiz, S.; Qureshi, T.A.; Kashwani, G.; Kannan, S. 3D Printing of Fiber-Reinforced Plastic Composites Using Fused Deposi tion Modeling: A Status Review. Materials 2021, 14, 4520. [Google Scholar] [CrossRef] [PubMed]
- Velu, R.; Raspall, F.; Singamneni, S. 3D printing technologies and composite materials for structural applications. In Green Composites for Automotive Applications; Koronis, G., Silva, A., Eds.; Woodhead Publishing Series in Composites Science and Engineering: Sawston, UK, 2019; Chapter 8; pp. 171–196. [Google Scholar]
- Agrawaal, H.; Thompson, J.E. Additive manufacturing (3D printing) for analytical chemistry. Talanta Open 2021, 3, 100036. [Google Scholar] [CrossRef]
- Low, Z.X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-Y.; Tan, W.S.; An, J.; Chua, C.K.; Tang, C.Y.; Fane, A.G.; Chong, T.H. The potential to enhance membrane module design with 3D printing technology. J. Membr. Sci. 2016, 499, 480–490. [Google Scholar] [CrossRef]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination, and water treatment. Appl. Mater. Today 2019, 18, 100486. [Google Scholar] [CrossRef]
- Dommati, H.; Ray, S.S.; Wang, J.-C.; Chen, S.-S. A comprehensive review of recent developments in 3D printing technique for ceramic membrane fabrication for water purification. RSC Adv. 2019, 9, 16869–16883. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Ostwal, M.; Asatekin, A.; Geise, G.M.; Smith, Z.P.; Phillip, W.A.; Lively, R.P.; McCutcheon, J.R. A critical review and commentary on recent progress of additive manufacturing and its impact on membrane technology. J. Membr. Sci. 2022, 645, 120041. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Miliozzi, A.; Crescenzi, T.; Torre, L.; Kenny, J.M. A New Phase Change Material Based on Potassium Nitrate with Silica and Alumina Nanoparticles for Thermal Energy Storage. Nanoscale Res. Lett. 2015, 10, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energ. Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Sharma, S.D.; Sagara, K. Latent Heat Storage Materials and Systems: A Review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C. Preparation, and thermal energy storage properties of poly(n-butyl methacrylate)/fatty acids composites as form-stable phase change materials. Polym. Compos. 2012, 33, 92–98. [Google Scholar] [CrossRef]
- Tamme, R.; Bauer, T.; Buschle, J.; Laing, D.; Müller-Steinhagen, H.; Steinmann, W.-D. Latent heat storage above 120 °C for applications in the industrial process heat sector and solar power generation. Int. J. Energy Res. 2008, 32, 264–271. [Google Scholar] [CrossRef]
- Jiang, Z.; Palacios, A.; Zou, B.; Zhao, Y.; Deng, W.; Zhang, X.; Ding, Y. A review on the fabrication methods for structurally stabilised composite phase change materials and their impacts on the properties of materials. Renew. Sustain. Energy Rev. 2022, 159, 112134. [Google Scholar] [CrossRef]
- Dhivya, S.; Hussain, S.I.; Jeyasheela, S.; Kalaiselvam, S. Experimental study on microcapsules of Ag doped ZnO nanomaterials enhanced Oleic-Myristic acid eutectic PCM for thermal energy storage. Thermochim. Acta 2019, 671, 70–82. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent Thermal Energy Storage Technologies, and Applications: A Review. Int. J. Thermofluid 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.M.; Vaka, M.; Walvekar, R.; Khalid, M.; Abdullah, E.C.; Nizamuddin, S.; Karri, R.R. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano-Struct. Nano-Objects 2019, 20, 100399. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Al Omari, S.A.B.; Elnajjar, E.; Mahmoud, F.; Al-Ketan, O.; Al-Rub, R.A. Thermal characterization of 3D-Printed lattices based on triply periodic minimal surfaces embedded with organic phase change material. Case Stud. Therm. Eng. 2021, 27, 101315. [Google Scholar] [CrossRef]
- Righetti, G.; Savio, G.; Meneghello, R.; Doretti, L.; Mancin, S. Experimental study of phase change material (PCM) embedded in 3D periodic structures realized via additive manufacturing. Int. J. Therm. Sci. 2020, 153, 106376. [Google Scholar] [CrossRef]
- Elias, C.N.; Stathopoulos, V.N. A comprehensive review of recent advances in materials aspects of phase change materials in thermal energy storage. Energy Procedia 2019, 161, 385–394. [Google Scholar] [CrossRef]
- Junaid, M.F.; ur Rehman, Z.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic phase change materials in thermal energy storage: A review on perspectives and technological advances in building applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, J.; Kim, H.; Seo, Y.; Linga, P. Hydrates for cold energy storage and transport: A Review. Adv. Appl. Energy 2021, 2, 100022. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, J.; Liu, L.; Xu, T.; Wu, H.; Zhou, X. Study on properties of phase change foam concrete block mixed with paraffin/fumed silica composite phase change material. Renew. Energy 2020, 150, 1127–1135. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kumar, P.; Szulejko, J.E.; Adelodun, A.A.; Junaid, M.F.; Uchimiya, M.; Chambers, S. Toward a better understanding of the impact of mass transit air pollutants on human health. Chemosphere 2017, 174, 268–279. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement effects on thermal properties of nanoporous shape-stabilized composite PCMs: A review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Tian, Y.; Zhou, D. Comprehensive evaluation of Paraffin-HDPE shape stabilized PCM with hybrid carbon nano-additives. Appl. Therm. Eng. 2019, 163, 114404. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, G.; Hao, J.; Ren, Z.; Deng, Z.; Xu, G.; Yang, C.; Chang, L. Fabrication and characterization of the novel shape-stabilized composite PCMs of Na2CO3-K2CO3/MgO/glass. Sol. Energy 2019, 189, 228–234. [Google Scholar] [CrossRef]
- Cardenas-Ramirez, C.; Jaramillo, F.; Gomez, M. Systematic review of encapsulation and shape-stabilization of phase change materials. J. Energy Storage 2020, 30, 101495. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Huang, Y. Morphology and thermal properties of electrospun fatty acids/polyethylene terephthalate composite fibers as novel form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2008, 92, 1382–1387. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S. Kumar. Improvement in thermal properties of PCM/Expanded vermiculite/expanded graphite shape stabilized composite PCM for building energy applications. Renew. Energy 2021, 176, 295–304. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sari, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Sari, A. Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials. Energy Convers. Manag. 2016, 117, 132–141. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, T.; Wang, K.; Xu, C.; Ye, F. Development and characterization of NaCl-KCl/Kaolin composites for thermal energy storage. Sol. Energy 2021, 227, 468–476. [Google Scholar] [CrossRef]
- Jafaripour, M.; Sadrameli, S.M.; Pahlavanzadeh, H.; Mousavi, S.A.H. Seyed. Fabrication and optimization of kaolin/stearic acid composite as a form-stable phase change material for application in the thermal energy storage systems. J. Energy Storage 2021, 33, 102155. [Google Scholar] [CrossRef]
- Ren, M.; Zhao, H.; Gao, X. Effect of modified diatomite based shape-stabilized phase change materials on multiphysics characteristics of thermal storage mortar. Energy 2022, 241, 122823. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Sadrameli, S.M.; Mousavi, S.A.H.S.; Jafaripour, M. Preparation and characterization of high temperature shape stable NaNO3/diatomite phase change materials with nanoparticles for solar energy storage applications. J. Energy Storage 2022, 45, 103735. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Lohrasbi, S.; Nsofor, E.C. Hybrid heat transfer enhancement for latent-heat thermal energy storage systems: A review. Int. J. Heat Mass Transf. 2019, 137, 630–649. [Google Scholar] [CrossRef]
- Nofal, M.; Pan, Y.; Al-Hallaj, S. Selective Laser Sintering of Phase Change Materials for Thermal Energy Storage Applications. Procedia Manuf. 2017, 10, 851–865. [Google Scholar] [CrossRef]
- Lachheb, M.; Karkri, M.; Albouchi, F.; Mzali, F.; Nasrallah, S.B. Thermophysical properties estimation of paraffin/graphite composite phase change material using an inverse method. Energy Convers. Manag. 2014, 82, 229–237. [Google Scholar] [CrossRef]
- Liu, X.; Yang, F.; Li, M.; Sun, C.; Wu, Y. Development of cost-effective PCM-carbon foam composites for thermal energy storage. Energy Rep. 2022, 8, 1696–1703. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef]
- Mishra, R.K.; Verma, K.; Mishra, V.; Chaudhary, B. A review on carbon-based phase change materials for thermal energy storage. J. Energy Storage 2022, 50, 104166. [Google Scholar] [CrossRef]
- Mayilvelnathan, V.; Arasu, A.V. Characterization and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar]
- Du, Y.; Zhou, T.; Zhao, C.; Ding, Y. Molecular dynamics simulation on thermal enhancement for carbon nano tubes (CNTs) based phase change materials (PCMs). Int. J. Heat Mass Transf. 2022, 182, 122017. [Google Scholar] [CrossRef]
- Yu, Z.; Feng, D.; Feng, Y.; Zhang, X. Thermal conductivity and energy storage capacity enhancement and bottleneck of shape-stabilized phase change composites with graphene foam and carbon nanotubes. Compos. A Appl. Sci. Manuf. 2022, 152, 106703. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Dittrich, B.; Schartel, B.; Roso, M.; Modesti, M. Expandable graphite in polyurethane foams: The effect of expansion volume and intercalants on flame retardancy. J. Appl. Polym. Sci. 2017, 134, 45173. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Liu, P.; Gao, H.; Chang, Y.; Wang, G. Smart Utilization of Multifunctional Metal Oxides in Phase Change Materials. Matter 2020, 3, 708–741. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.; Zhao, Y.M.; Li, G.D.; Pan, W.G. Preparation and thermal properties of palmitic acid ZnO/Expanded graphite composite phase change material for heat storage. Energy 2022, 242, 122972. [Google Scholar] [CrossRef]
- Orozco, M.A.; Acurio, K.; Vásquez-Aza, F.; Martínez-Gómez, J.; Chico-Proano, A. Thermal Storage of Nitrate Salts as Phase Change Materials (PCMs). Materials 2021, 14, 7223. [Google Scholar] [CrossRef]
- Motoc, A.M.; Tudor, A.I.; Petriceanu, M.; Badilita, V.; Palomo del Barrio, E.; Jana, P.; Fierro, V.; Celzard, A.; Piticescu, R.R. In-situ synthesis and attachment of colloidal ZnO nanoparticles inside porous carbon structures. Mater. Chem. Phys. 2015, 161, 219–227. [Google Scholar]
- Pant, B.; Park, M.; Prasad Ojha, G.; Park, J.; Kuk, Y.-S.; Lee, E.-J.; Kim, H.-Y.; Park, S.-J. Carbon Nanofibers Wrapped with Zinc Oxide Nano-flakes as Promising Electrode Material for Supercapacitors. J. Colloid Interface Sci. 2018, 522, 40–47. [Google Scholar] [CrossRef]
- Prasad Ojha, G.; Pant, B.; Acharya, J.; Park, M. Prussian Red Anions Immobilized Freestanding Three-Dimensional Porous Carbonaceous Networks: A New Avenue to Attain Capacitor- and Faradic-Type Electrodes in a Single Frame for 2.0 V Hybrid Supercapacitors. ACS Sustain. Chem. Eng. 2022, 10, 2994–3006. [Google Scholar] [CrossRef]
- Cursaru, L.-M.; Valsan, S.N.; Puscasu, M.-E.; Tudor, I.A.; Zarnescu-Ivan, N.; Vasile, B.S.; Piticescu, R.M. Study of ZnO-CNT Nanocomposites in High Pressure Conditions. Materials 2021, 14, 5330. [Google Scholar] [CrossRef]
- Al Abdullah, K.; Awad, S.; Zaraket, J.; Salame, C. Synthesis of ZnO Nanopowders by Using Sol-Gel and Studying Their Structural and Electrical Properties at Different Temperature. Energy Procedia 2017, 119, 565–570. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. ISRN Nanotechnol. 2012, 2012, 372505. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.C.; Ericksen, G.E. An X-ray Diffraction Method for Semiquantitative Mineralogical Analysis of Chilean Nitrate Ore. U.S. GEOLOGICAL SURVEY. Open-File Report 94-240. Rev. Geol. Chile 1997, 24, 45–53. [Google Scholar]
- Córdova Reyes, I.; Salmones, J.; Zeifert, B.; Contreras, J.L.; Rojas, F. Transesterification of canola oil catalized by calcined Mg–Al hydrotalcite doped with nitratine. Chem. Eng. Sci. 2014, 119, 174–181. [Google Scholar] [CrossRef]
- Ataev, M.B.; Gafurov, M.M.; Emirov, R.M.; Rabadanov, K.S.; Amirov, A.M. Phase composition and the structure of (1–x) KNO3+xAl2O3 nanocomposites by X-ray diffraction. Phys. Solid State 2016, 58, 2423–2426. [Google Scholar] [CrossRef]
- D’Agostino, D.; Macchia, A.; Cataldo, R.; Campanella, L.; Campbell, A. Microclimate and Salt Crystallization in the Crypt of Lecce’s Duomo. Int. J. Archit. Herit. 2015, 9, 290–299. [Google Scholar] [CrossRef]
- Castro-Quijada, M.; Faundez, D.; Rojas, R.; Videla, A. Improving the working fluid based on a NaNO3-KNO3-NaCl-KCl molten salt mixture for concentrating solar power energy storage. Sol. Energy 2022, 231, 464–472. [Google Scholar] [CrossRef]
- D’Aguanno, B.; Karthik, M.; Grace, A.N.; Floris, A. Thermostatic properties of nitrate molten salts and their solar and eutectic mixtures. Sci. Rep. 2018, 8, 10485. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Lincu, D.; Buhǎlţeanu, L.; Berger, D.; Matei, C. Shape-stabilized phase change materials using molten NaNO3–KNO3 eutectic and mesoporous silica matrices. Sol. Energy Mater Sol. Cells 2020, 215, 110644. [Google Scholar] [CrossRef]

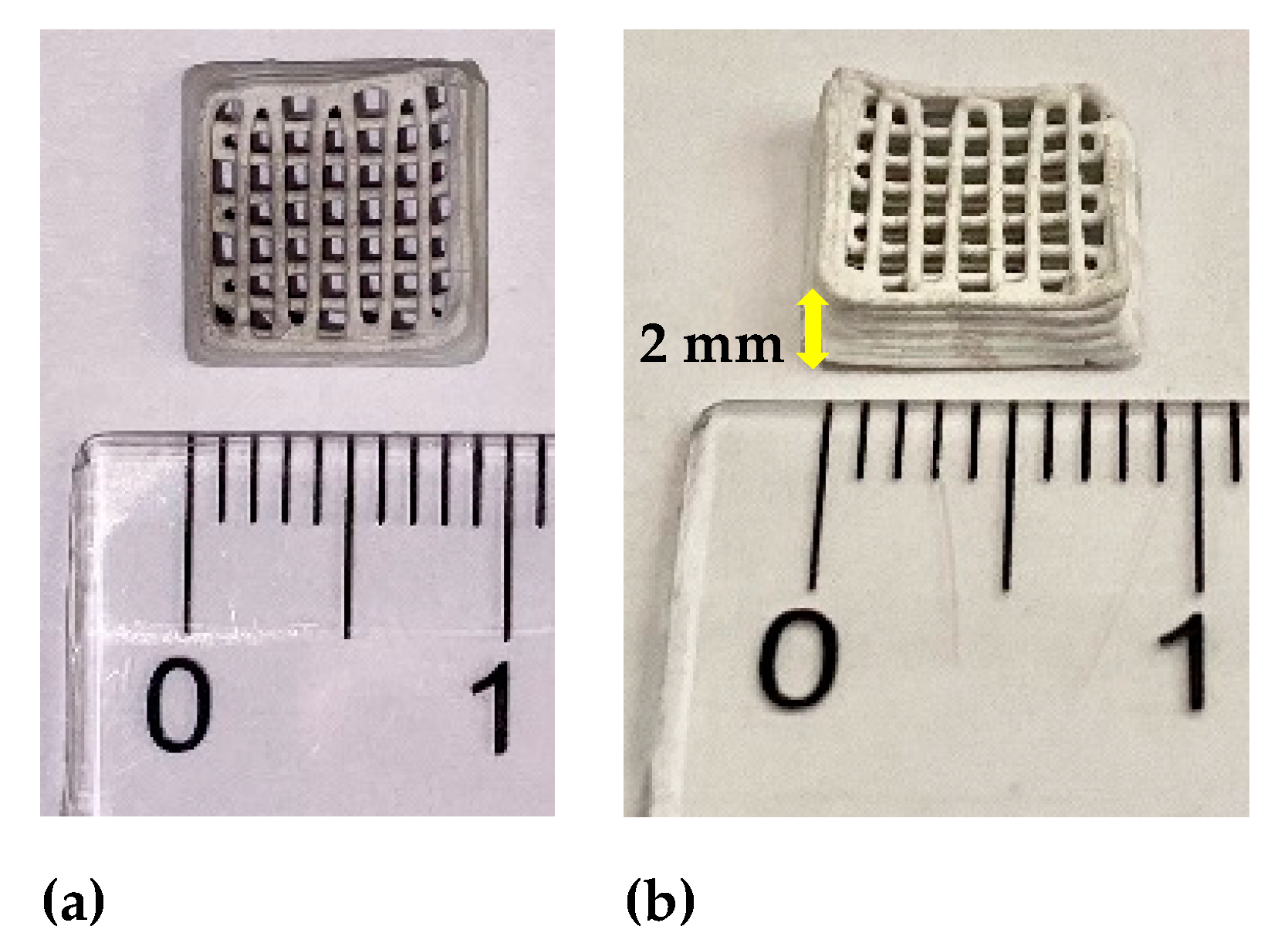
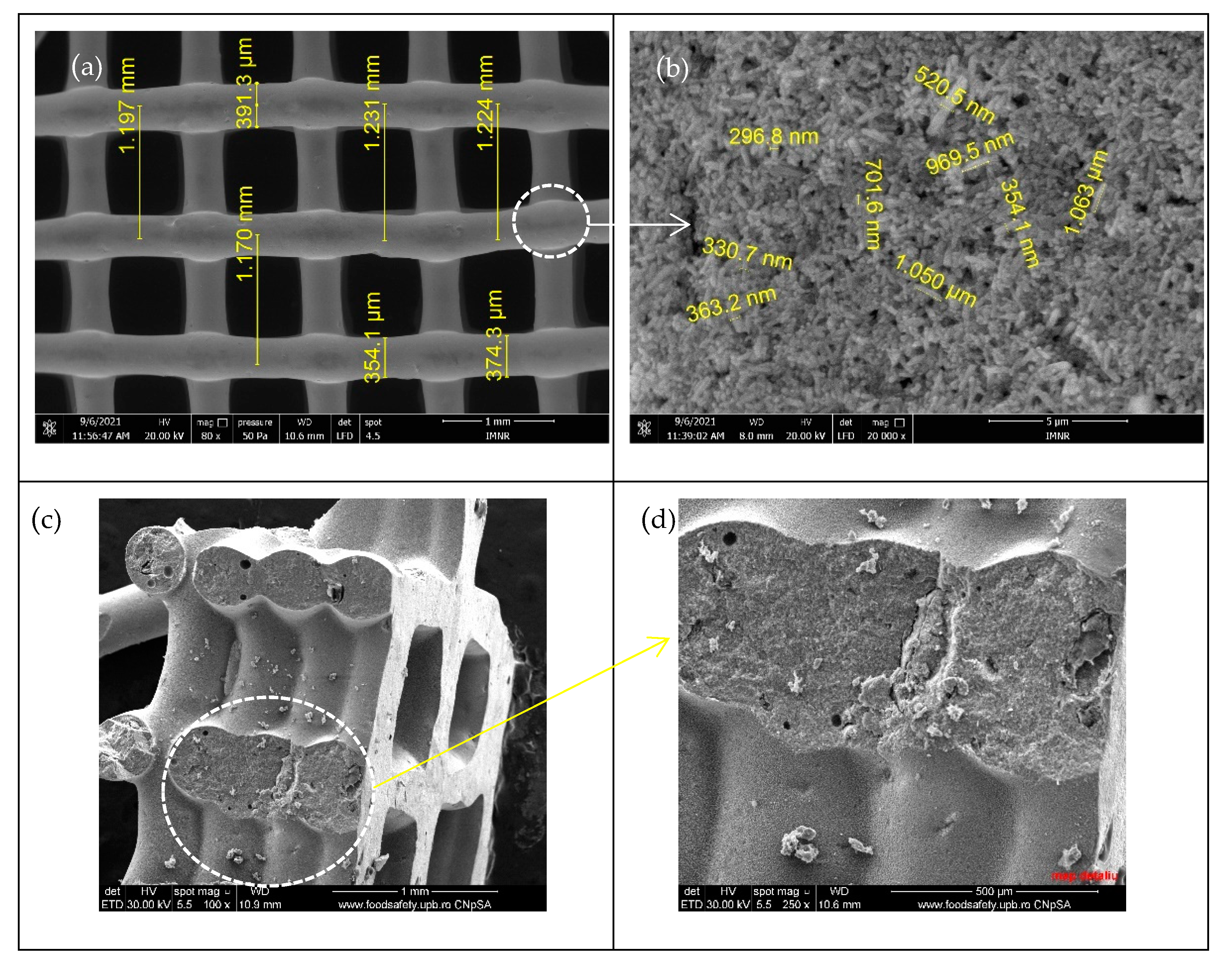
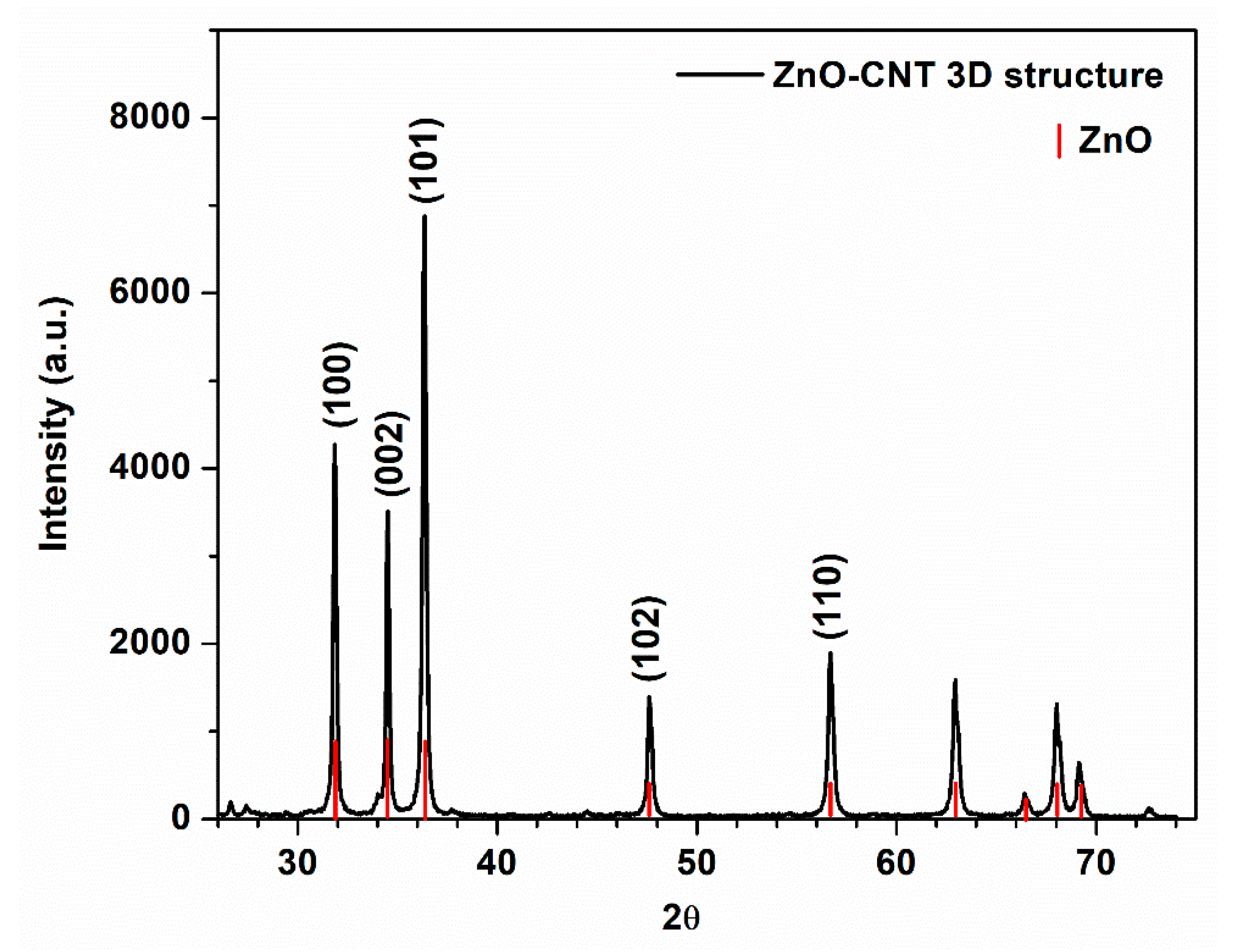


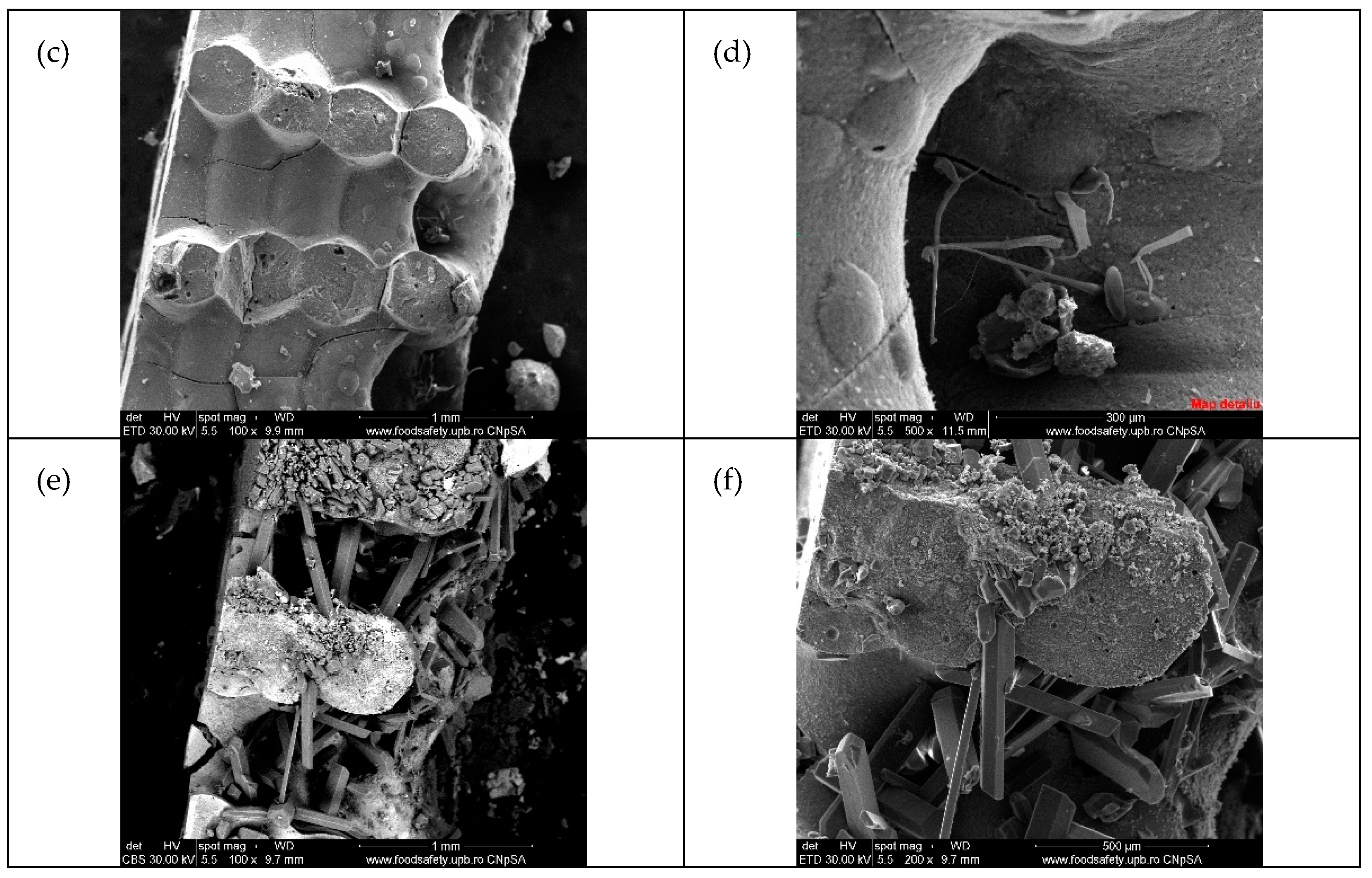
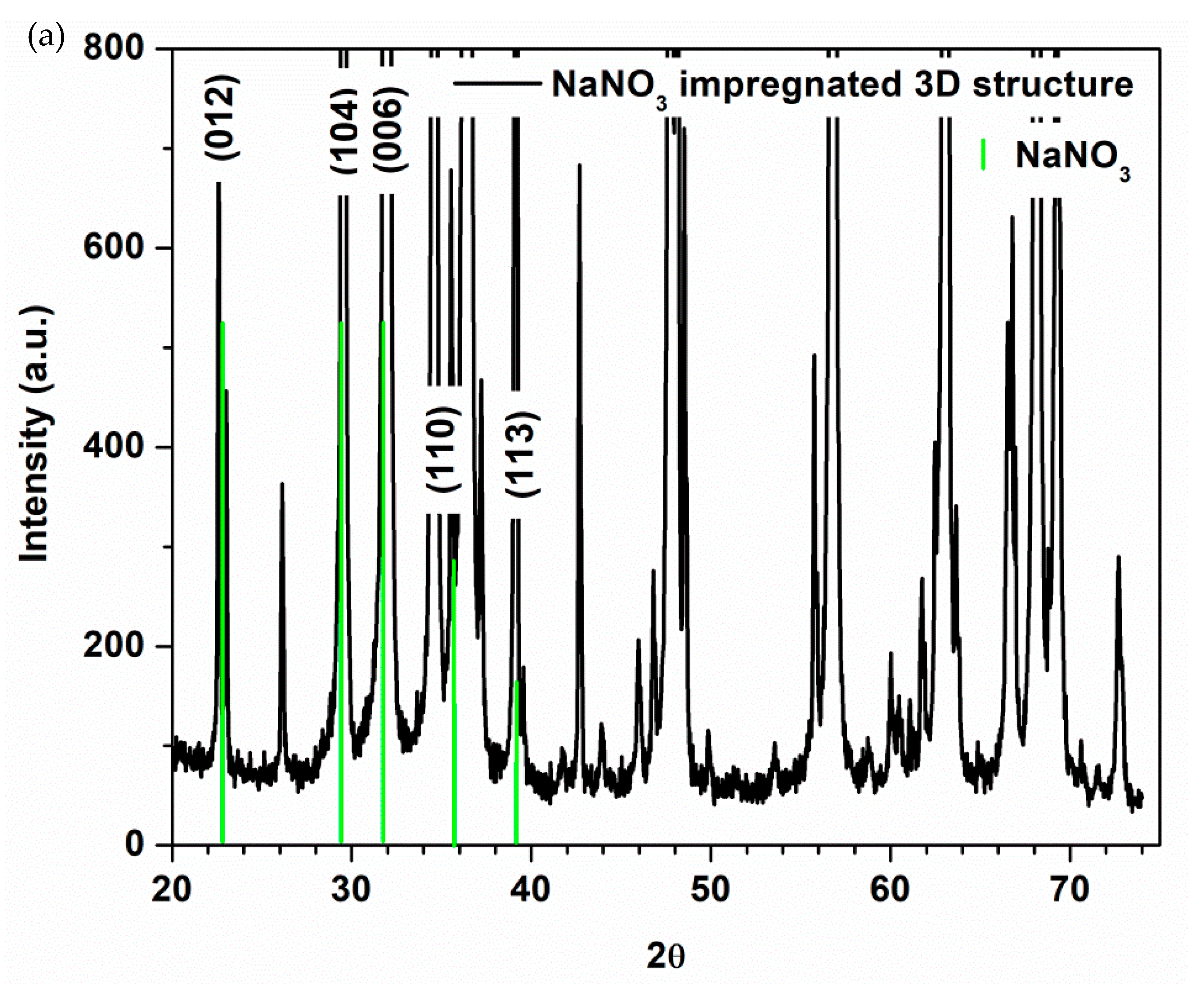
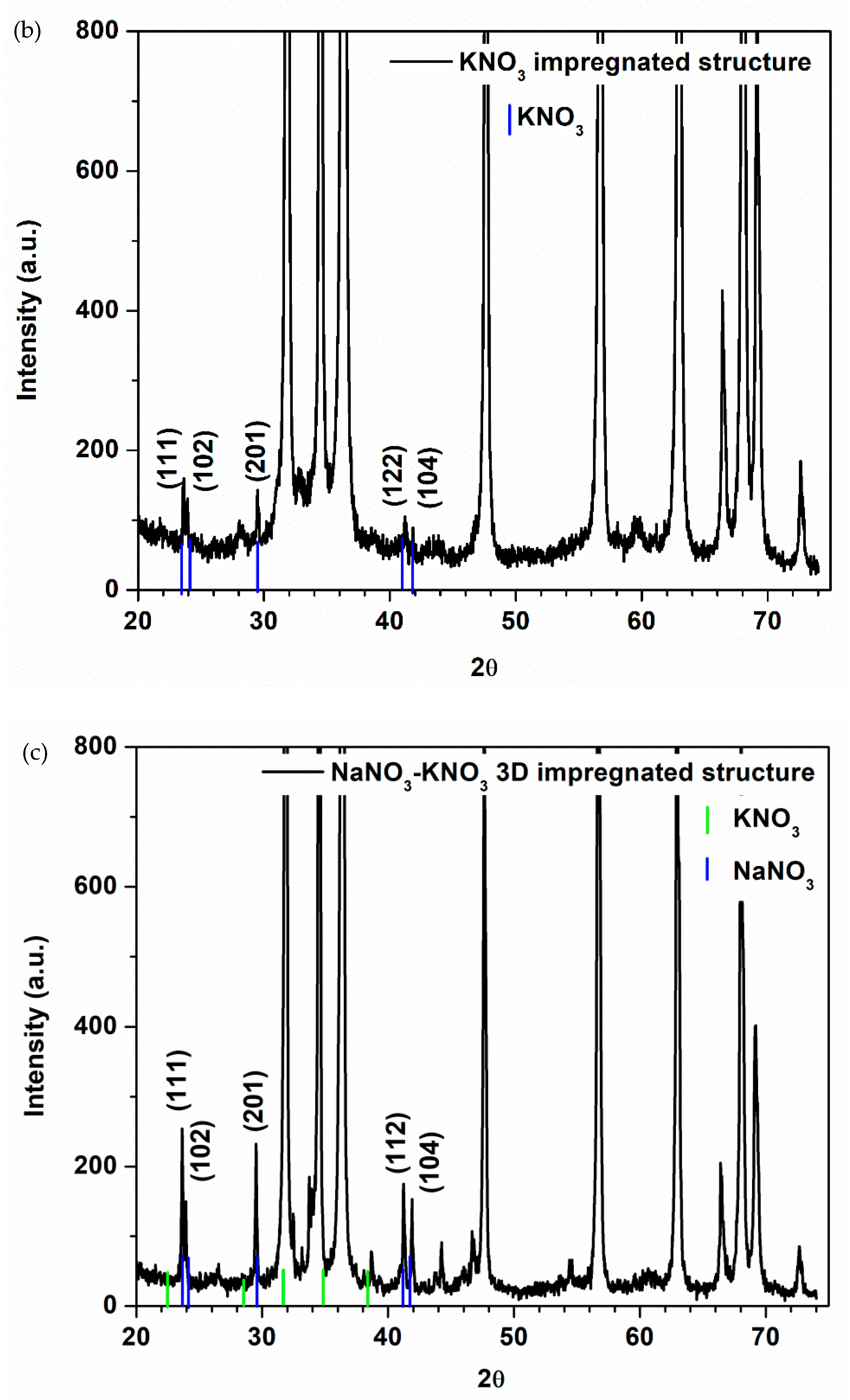
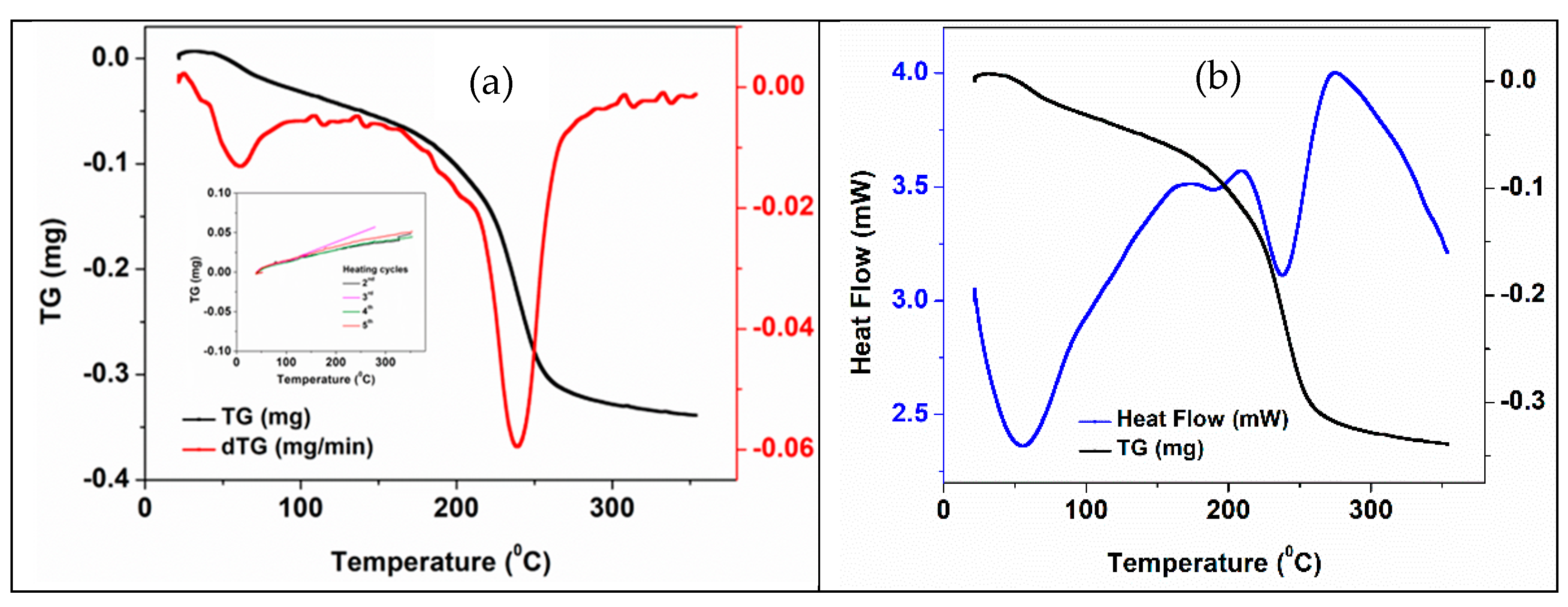


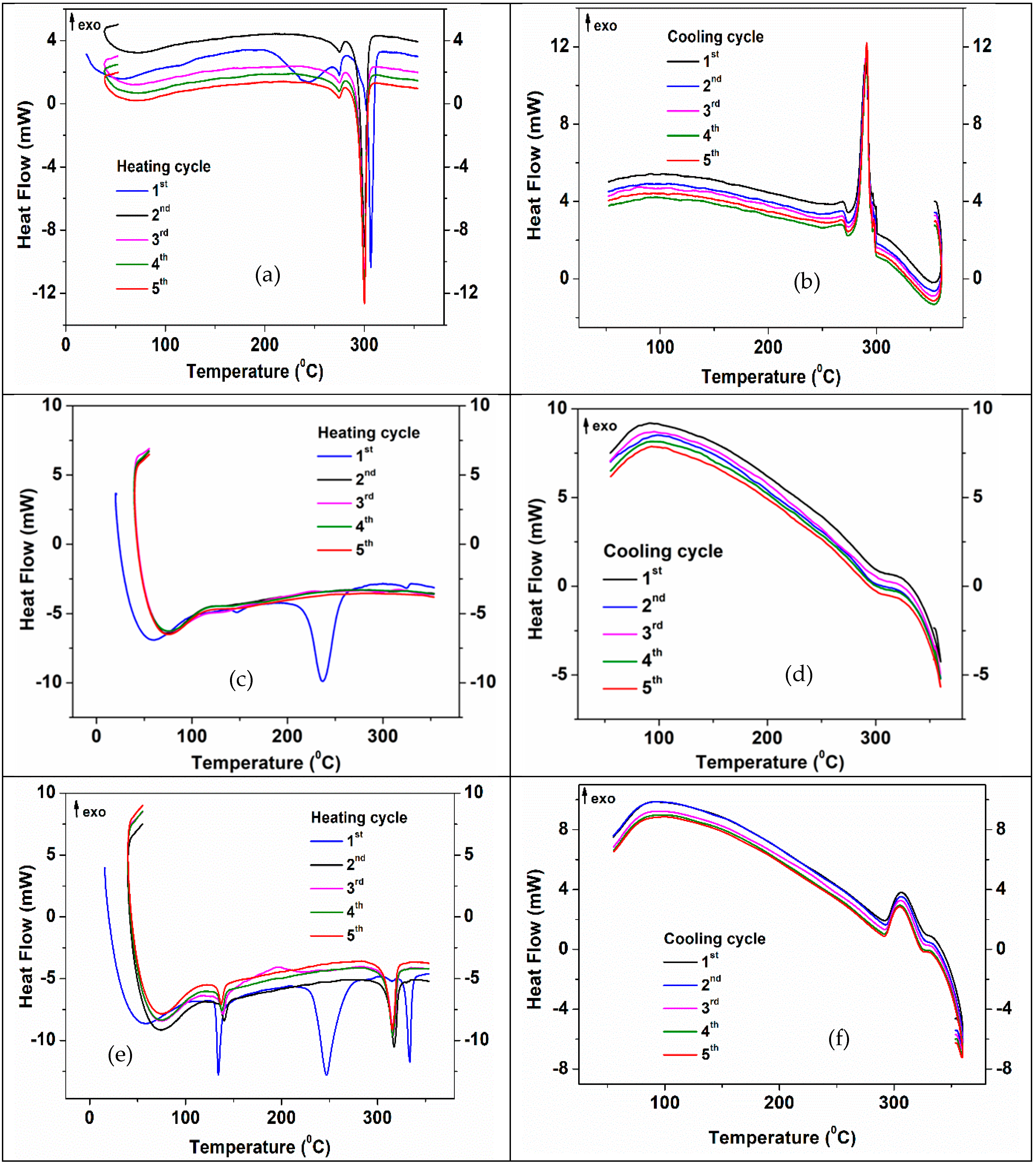
| Type of 3D Structure | Lattice Constants of ZnO | Crystalite Size of ZnO for (101) Diffraction Peak, nm | Percentage Composition, % | ||
|---|---|---|---|---|---|
| a = b, nm | c, nm | ZnO | SALT | ||
| Non-impregnated sample | 0.32505 | 0.5207 | 96.98 | 100 | - |
| NaNO3 impregnated sample | 0.3251 | 0.5208 | 101.19 | 44.94 | 55.06 |
| KNO3 impregnated sample | 0.3249 | 0.5206 | 69.95 | 95.55 | 4.45 |
| NaNO3:KNO3 impregnated sample | 0.32501 | 0.5206 | 55.64 | 84.45 | 15.55 |
| Type of 3D Structure | Impregnated PCM Phase | Lattice Constants of Salt | Average Crystalite Size, nm | ||
|---|---|---|---|---|---|
| a, nm | b, nm | c, nm | |||
| NaNO3 impregnated sample | NaNO3 | 0.507 | 0.507 | 1.683 | µm—tens of µm |
| KNO3 impregnated sample | KNO3 | 0.642 | 0.541 | 0.917 | 38.00 |
| NaNO3:KNO3 impregnated sample | NaNO3:KNO3 | 0.642 * | 0.541 * | 0.917 * | 88.56 * |
| Type of 3D Structure | Cycle No. | Heating Cycles | Cooling Cycles |
|---|---|---|---|
| Total Mass Loss/Gain, % | Total Mass Loss, % | ||
| Non-impregnated sample | 1 | −1.376 | −0.245 |
| 2 | 0.147 | −0.229 | |
| 3 | 0.169 | −0.243 | |
| 4 | 0.162 | −0.241 | |
| 5 | 0.182 | −0.241 |
| Type of 3D Structure | Cycle No. | Heating Cycles | Cooling Cycles |
|---|---|---|---|
| Mass Loss/Gain, % | Mass Loss, % | ||
| NaNO3 impregnated sample | 1 | −1.6 | −0.128 |
| 2 | 0.104 | −0.123 | |
| 3 | 0.121 | −0.124 | |
| 4 | 0.116 | −0.117 | |
| 5 | 0.126 | −0.124 | |
| KNO3 impregnated sample | 1 | −3.078 | −0.171 |
| 2 | 0.11 | −0.179 | |
| 3 | 0.135 | −0.146 | |
| 4 | 0.118 | −0.190 | |
| 5 | 0.148 | −0.185 | |
| NaNO3:KNO3 impregnated sample | 1 | −2.098 | −0.126 |
| 2 | 0.061 | −0.119 | |
| 3 | 0.145 | −0.108 | |
| 4 | 0.086 | −0.121 | |
| 5 | 0.082 | −0.118 |
| Type of PCM Impregnated in 3D Structure | Cycle No. | Heating Cycles | Cooling Cycles | |||
|---|---|---|---|---|---|---|
| Peak1 endo, °C | Peak2 endo, °C | Peak3 endo, °C | Peak 1 exo, °C | Peak 2 exo, °C | ||
| NaNO3 | 1 | 277.5 | 310.3 | 295.3 | ||
| 2 | 276.5 | 306.6 | 295.2 | |||
| 3 | 276.3 | 304.6 | 295.3 | |||
| 4 | 276.4 | 304.5 | 295.6 | |||
| 5 | 276.3 | 304.5 | 295.6 | |||
| KNO3 | 1 | 138.4 | 238.8 | 327.6 | 292.6 | |
| 2 | 142.2 | 320.4 | 293 | |||
| 3 | 141.9 | 319.9 | 293.1 | |||
| 4 | 141.8 | 320.3 | 292.6 | |||
| 5 | 141.5 | 320.2 | 293 | |||
| NaNO3:KNO3/1:1 | 1 | 137.5 | 249 | 335.8 | 330 | 306.9 |
| 2 | 144.1 | 321.4 | 335.1 | 330.6 | 307.6 | |
| 3 | 143.7 | 321.2 | 335 | 330.7 | 307.8 | |
| 4 | 143.2 | 321 | 334.9 | 330.5 | 307.8 | |
| 5 | 142.9 | 320.8 | 334.8 | 330 | 307.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, S.; Puscasu, M.-E.; Tudor, I.A.; Matei, A.C.; Cursaru, L.M.; Piticescu, R.R. Development of 3D ZnO-CNT Support Structures Impregnated with Inorganic Salts. Membranes 2022, 12, 588. https://doi.org/10.3390/membranes12060588
Chiriac S, Puscasu M-E, Tudor IA, Matei AC, Cursaru LM, Piticescu RR. Development of 3D ZnO-CNT Support Structures Impregnated with Inorganic Salts. Membranes. 2022; 12(6):588. https://doi.org/10.3390/membranes12060588
Chicago/Turabian StyleChiriac, Stefania, Maria-Eliza Puscasu, Ioan Albert Tudor, Alexandru Cristian Matei, Laura Madalina Cursaru, and Radu Robert Piticescu. 2022. "Development of 3D ZnO-CNT Support Structures Impregnated with Inorganic Salts" Membranes 12, no. 6: 588. https://doi.org/10.3390/membranes12060588
APA StyleChiriac, S., Puscasu, M.-E., Tudor, I. A., Matei, A. C., Cursaru, L. M., & Piticescu, R. R. (2022). Development of 3D ZnO-CNT Support Structures Impregnated with Inorganic Salts. Membranes, 12(6), 588. https://doi.org/10.3390/membranes12060588








