Electromembrane Extraction of Posaconazole for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
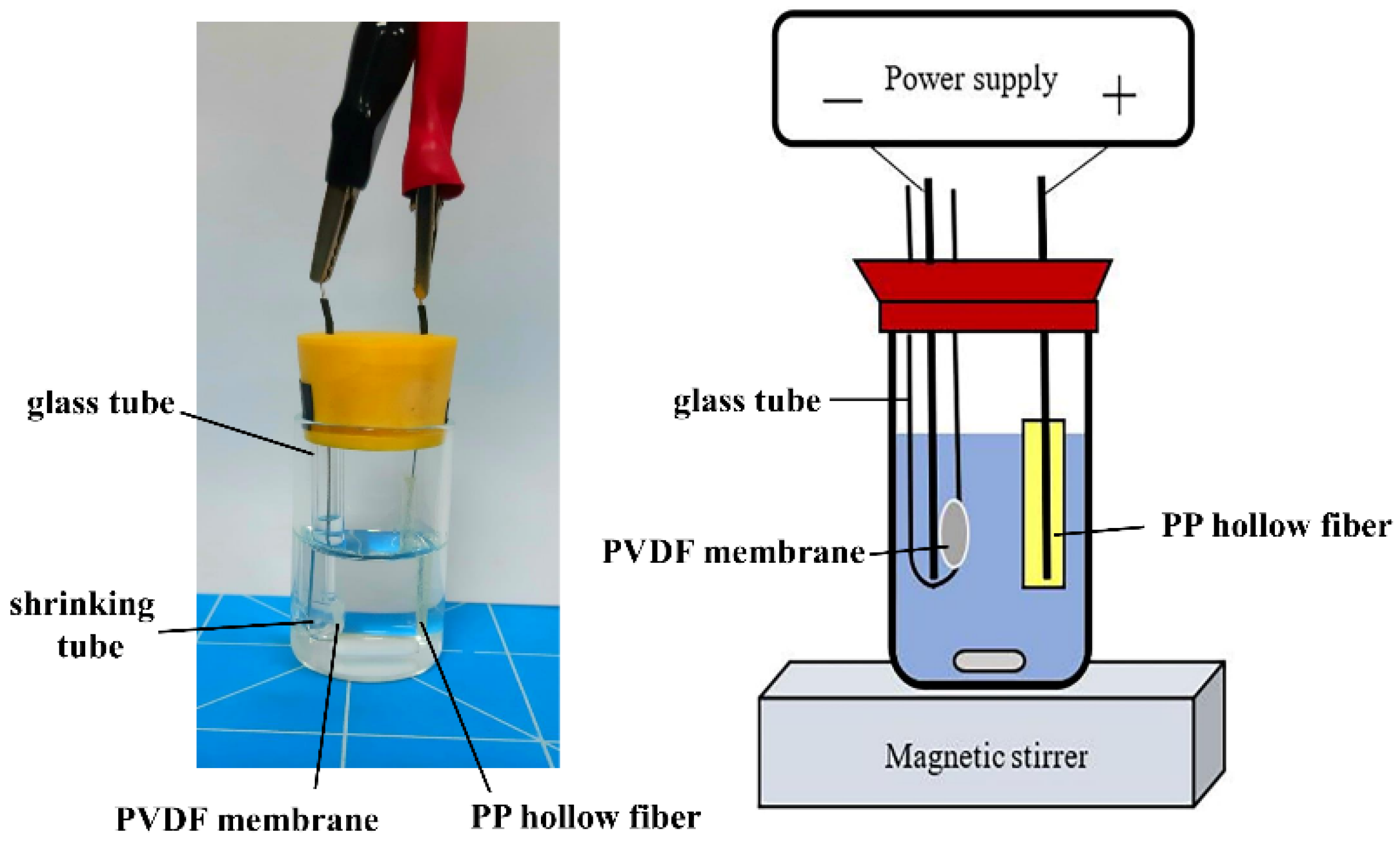
2.2. EME Procedure
2.3. MALDI/MS Measurements
2.4. Preparation of Human Serum Sample
3. Results and Discussions
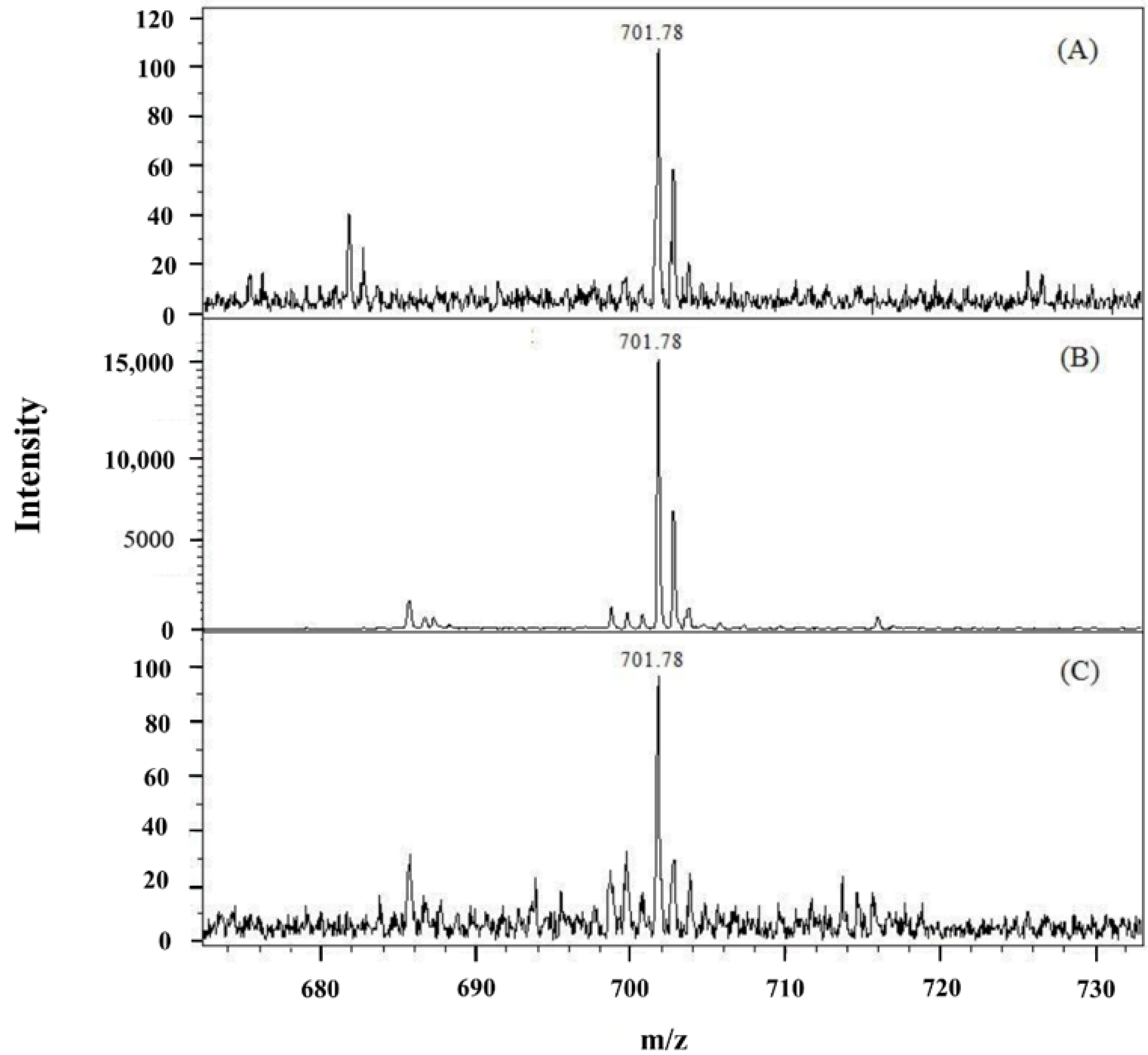
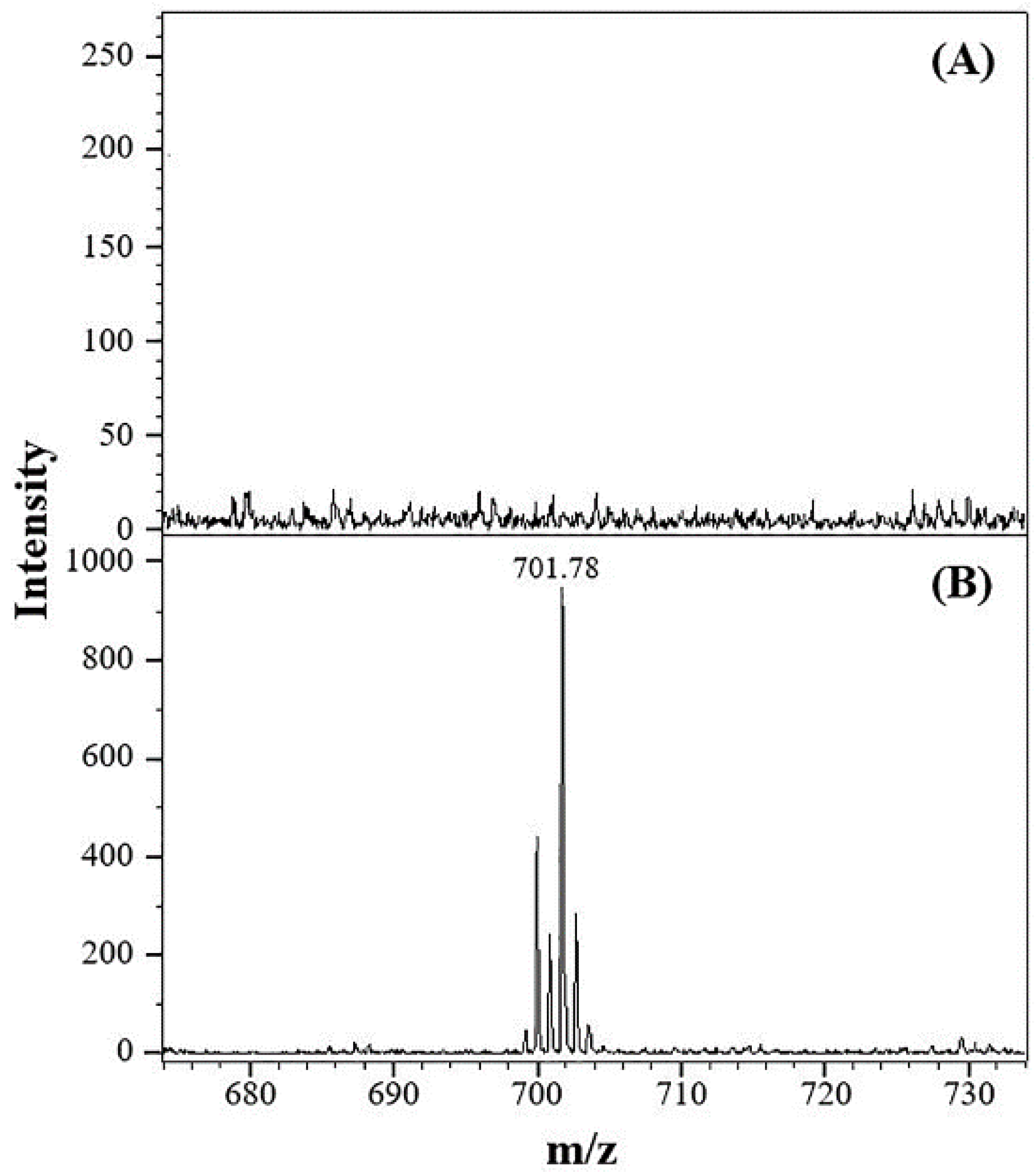
3.1. Detection of Posaconazole on Membrane by MALDI/MS
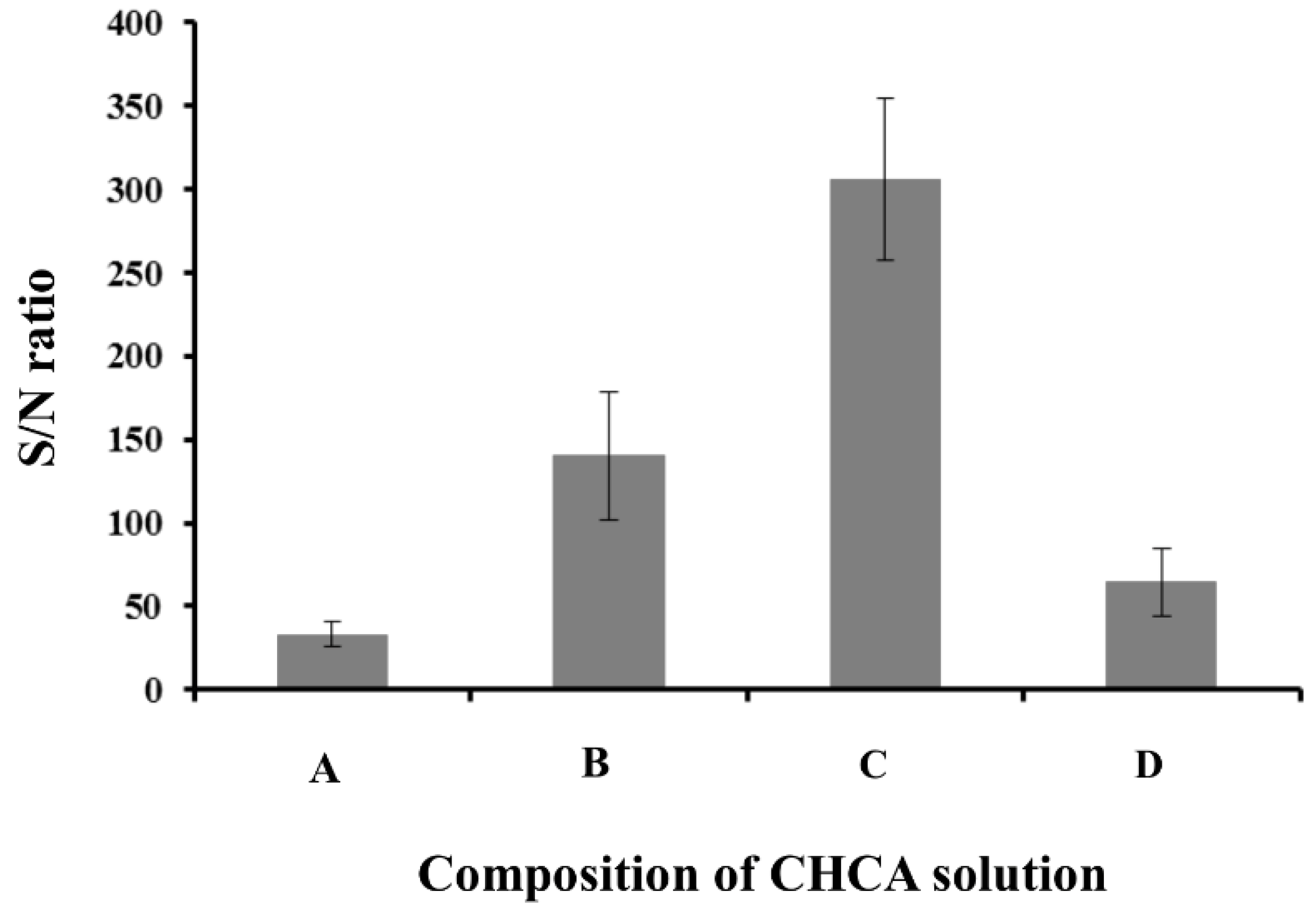
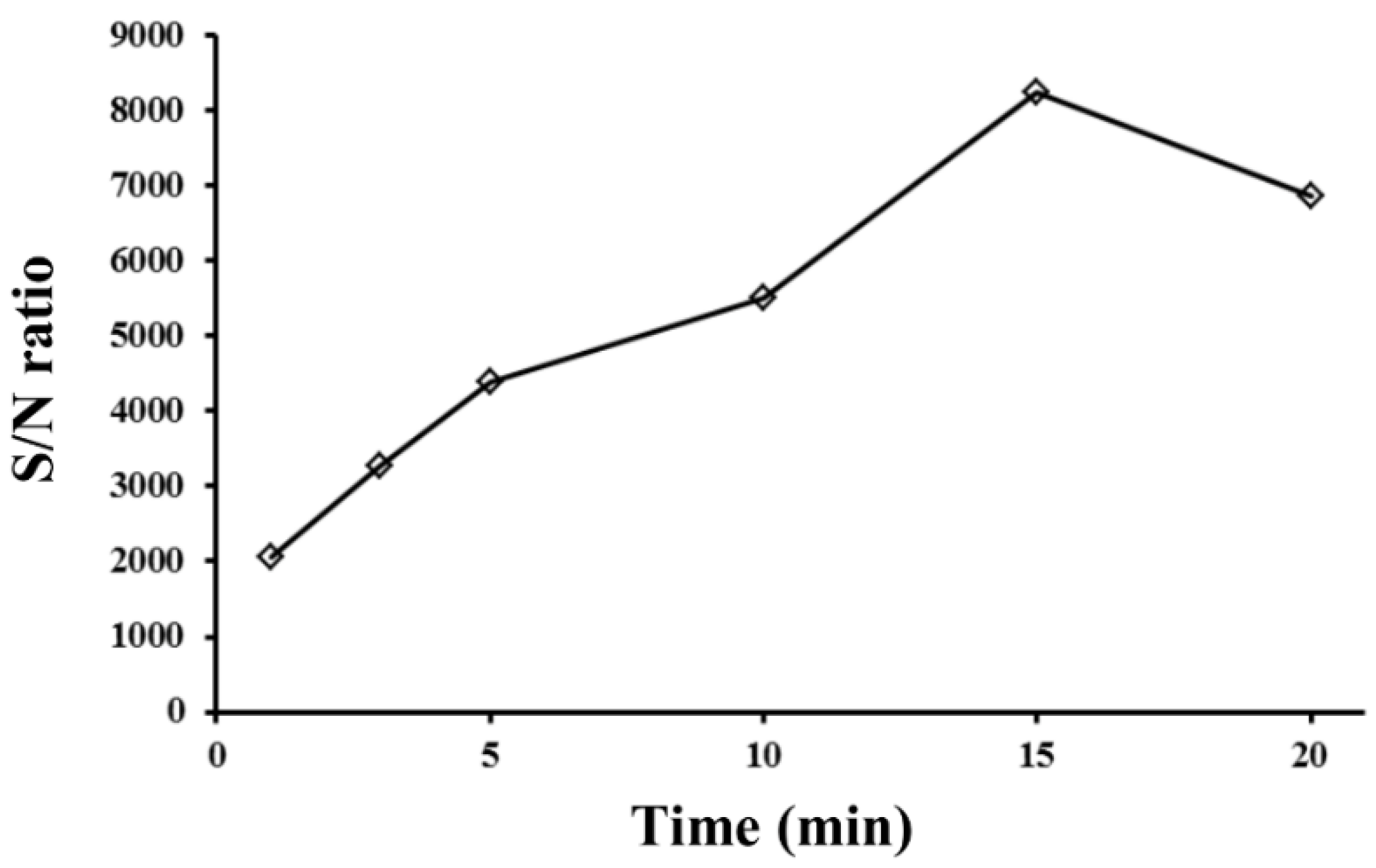
3.2. Optimization of EME Conditions
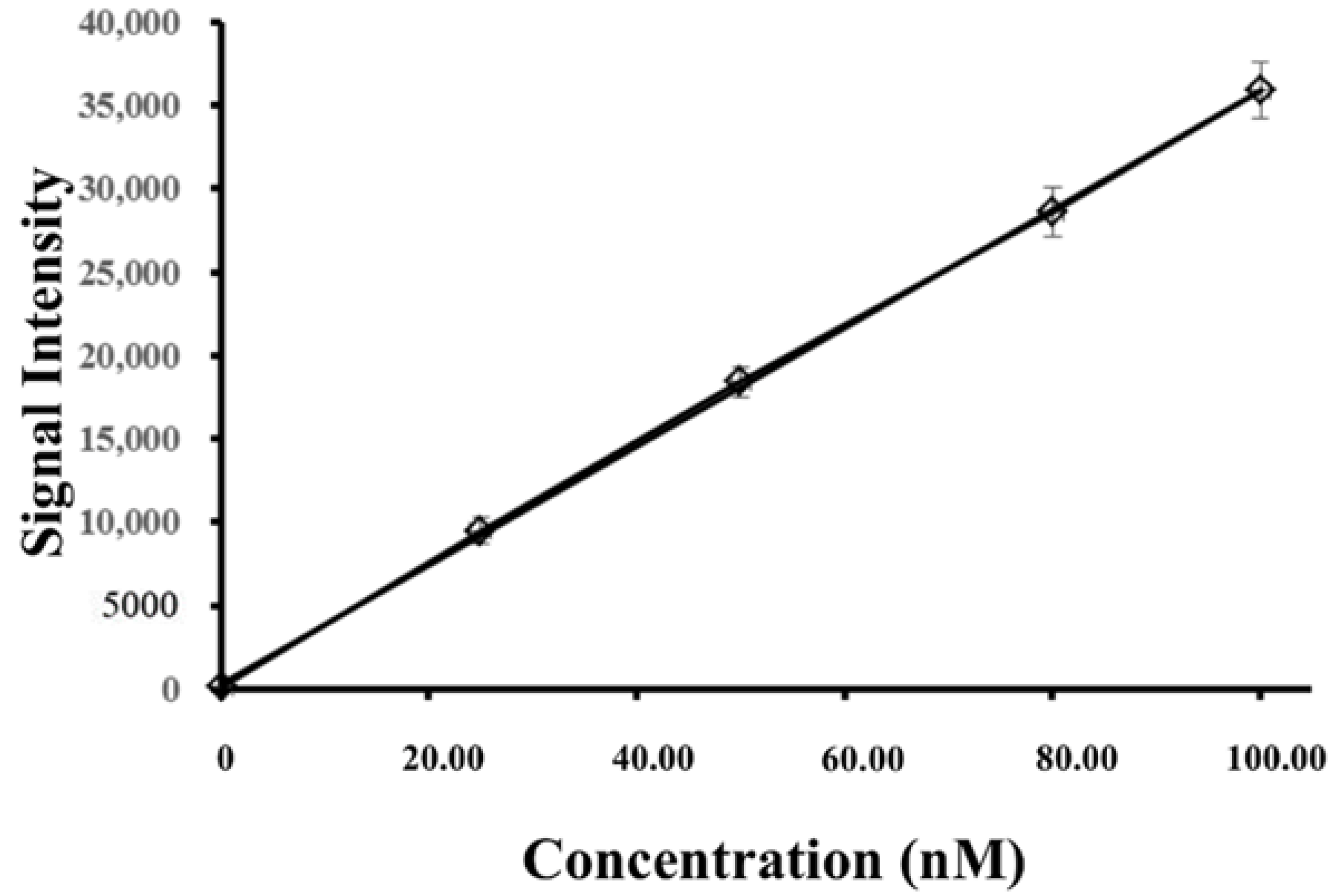
3.3. Analytical Characteristics
3.4. Analysis of Serum Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drouin, N.; Kubáň, P.; Rudaz, S.; Pedersen-Bjergaard, S. Elecromembrane extraction: Overview of the last decade. Trends Anal. Chem. 2019, 113, 357–363. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Rasmussen, K.E. Electrokinetic migration across artificial liquid membranes new concept for rapid sample preparation of biological fluids. J. Chromatogr. A 2006, 1109, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pourahadi, A.; Nojavan, S.; Davarani, S.S.H. Gel-electromembrane extraction of peptides: Determination of five hypothalamic agents in human plasma samples. Talanta 2020, 217, 121025. [Google Scholar] [CrossRef] [PubMed]
- Cristina, R.-H.; Jesús, M.-V.M.; Rut, F.-T.; Ángel, B.-L.M. Use of polymer inclusion membranes (PIMs) as support for electromembrane extraction of non-steroidal anti-inflammatory drugs and highly polar acidic drugs. Talanta 2018, 179, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.A.; Kubáň, P.; Øiestad, E.L.; Pedersen-Bjergaard, S. Electromembrane extraction of highly polar bases from biological samples-Deeper insight into bis(2-ethylhexyl) phosphate as ionic carrier. Anal. Chim. Acta 2020, 1115, 23–32. [Google Scholar] [CrossRef]
- Balchen, M.; Lund, H.; Reubsaet, L.; Pedersen-Bjergaard, S. Fast, selective, and sensitive analysis of low-abundance peptides in human plasma by electromembrane extraction. Anal. Chim. Acta 2012, 716, 16–23. [Google Scholar] [CrossRef]
- Hasheminasab, K.S.; Fakhari, A.R. Application of nonionic surfactant as a new method for the enhancement of electromembrane extraction performance for determination of basic drugs in biological samples. J. Chromatogr. A 2015, 1378, 1–7. [Google Scholar] [CrossRef]
- Šlampová, A.; Kubáň, P.; Boček, P. Quantitative aspects of electrolysis in electromembrane extractions of acidic and basic analytes. Anal. Chim. Acta 2015, 887, 92–100. [Google Scholar] [CrossRef]
- Sedehi, S.; Tabani, H.; Nojavan, S. Electro-driven extraction of polar compounds using agarose gel as a new membrane: Determination of amino acids in fruit juice and human plasma samples. Talanta 2018, 179, 318–325. [Google Scholar] [CrossRef]
- Mamat, N.A.; See, H.H. Simultaneous electromembrane extraction of cationic and anionic herbicides across hollow polymer inclusion membranes with a bubbleless electrode. J. Chromatogr. A 2017, 1504, 9–16. [Google Scholar] [CrossRef]
- Balchen, M.; Halvorsen, T.G.; Reubsaet, L.; Pedersen-Bjergaard, S. Rapid isolation of angiotensin peptides from plasma by electromembrane extraction. J. Chromatogr. A 2009, 1216, 6900–6905. [Google Scholar] [CrossRef] [PubMed]
- Balchen, M.; Reubsaet, L.; Pedersen-Bjergaard, S. Electromembrane extraction of peptides. J. Chromatogr. A 2008, 1194, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Mollahosseini, A.; Elyasi, Y.; Rastegari, M. An improvement of electrospun membrane reusability via titanium dioxide nanoparticles and silane compounds for the electromembrane extraction. Anal. Chim. Acta 2019, 1088, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, M.; Yamini, Y.; Seidi, S.; Ebrahimpour, B. Electromembrane surrounded solid phase microextraction: A novel approach for efficient extraction from complicated matrices. J. Chromatogr. A 2013, 1280, 16–22. [Google Scholar] [CrossRef]
- Tabani, H.; Shokri, A.; Tizro, S.; Nojavan, S.; Varanusupakul, P.; Alexovič, M. Evaluation of dispersive liquid-liquid microextraction by coupling with green-based agarose gel-electromembrane extraction: An efficient method to the tandem extraction of basic drugs from biological fluids. Talanta 2019, 199, 329–335. [Google Scholar] [CrossRef]
- Behpour, M.; Nojavan, S.; Asadi, S.; Shokri, A. Combination of gel-electromembrane extraction with switchable hydrophilicity solvent-based homogeneous liquid-liquid microextraction followed by gas chromatography for the extraction and determination of antidepressants in human serum, breast milk and wastewater. J. Chromatogr. A 2020, 1621, 461041. [Google Scholar] [CrossRef]
- Kamankesh, M.; Mohammadi, A.; Mollahosseini, A.; Seidi, S. Application of a novel electromembrane extraction and microextraction method followed by gas chromatography-mass spectrometry to determine biogenic amines in canned fish. Anal. Methods 2019, 11, 1898–1907. [Google Scholar] [CrossRef]
- Tahmasebi, Z.; Davarani, S.S.H.; Asgharinezhad, A.A. Highly efficient electrochemical determination of proplthiouracil in urine samples after selective electromembrane extraction by copper nanoparticles-decorated hollow fibers. Biosens. Bioelectron. 2018, 114, 66–71. [Google Scholar] [CrossRef]
- Mofidi, Z.; Norouzi, P.; Seidi, S.; Ganjali, M.R. Determination of diclofenac using electromembrane extraction coupled with stripping FFT continuous cyclic voltammetry. Anal. Chim. Acta 2017, 972, 38–45. [Google Scholar] [CrossRef]
- Davarani, S.S.H.; Shekhi, N.; Nojavan, S.; Ansari, R.; Mansori, S. Electromembrane extraction of heavy metal cations from aqueous media based on flat membrane: Method transfer from hollow fiber to flat membrane. Anal. Methods 2015, 7, 2680–2686. [Google Scholar] [CrossRef]
- Tahmasebi, Z.; Davarani, S.S.H. Selective and sensitive speciation analysis of Cr(VI) and Cr(III), at sub-μg L-1 levels in water samples by electrothermal atomic absorption spectrometry after electromembrane extraction. Talanta 2016, 161, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Davarani, S.S.H.; Moazami, H.R.; Keshtkar, A.R.; Banitaba, M.H.; Nojavan, S. A selective electromembrane extraction of uranium(VI) prior to its fluorometric determination in water. Anal. Chim. Acta 2013, 83, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-S.; Cheng, P.-S.; Chang, S.Y. Solvent-free electromembrane extraction: A new concept in electro-driven extraction. Talanta 2019, 199, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, T.; Rahimi, A.; Nojavan, S. Study on electrical current variations in electromembrane extraction process: Relation between extraction recovery and magnitude of electrical current. Anal. Chim. Acta 2016, 903, 81–90. [Google Scholar] [CrossRef]
- Munayyer, H.K.; Mann, P.A.; Chau, A.S.; Yarosh-Tomaine, T.; Greene, J.R.; Hare, R.S.; Heimark, L.; Palermo, R.E.; Loebenberg, D.; McNicholas, P.M. Posaconazole is potent inhibitor of sterol 14α-demethylation in yeasts and molds. Antimicrob. Agents Chemother. 2004, 48, 3690–3696. [Google Scholar] [CrossRef] [Green Version]
- Pitisuttithum, P.; Negroni, R.; Graybill, J.R.; Bustamante, B.; Pappas, P.; Chapman, S.; Hare, R.S.; Hardalo, C.J. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 2005, 56, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.M.; Schoeppler, K.E.; Jaeger, J.; Mueller, S.W.; MacLaren, R.; Fish, D.N.; Kiser, T.H. Voriconazole and posaconazole therapeutic drug monitoring: A retrospective study. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 60. [Google Scholar] [CrossRef]
- Tarazona, I.; Chisvert, A.; Salvador, A. Determination of benzophenone-3 and its main metabolites in human serum by dispersive liquid-liquid microextraction followed by liquid chromatography tandem mass spectrometry. Talanta 2013, 116, 388–395. [Google Scholar] [CrossRef]
- Leung, S.; Poulakos, M.N.; Machin, J. Posaconazole: An update of its clinical use. Pharmacy 2015, 3, 210–268. [Google Scholar] [CrossRef] [Green Version]
- Courtney, R.; Wexler, D.; Radwanski, E.; Lim, J.; Laughlin, M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharm. 2004, 57, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Ara, K.M.; Raofie, F. Low-voltage electrochemically stimulated stir membrane liquid-liquid microextraction as a novel technique for the determination of methadone. Talanta 2017, 168, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Raterink, R.J.; Lindenburg, P.W.; Vreeken, R.J.; Hankemeier, T. Three-phase electroextraction: A new sample purification and enrichmenr method for bioanalysis. Anal. Chem. 2013, 85, 7762–7768. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Andes, D. Therapeutic drug monitoring of antifungals: Pharmacokinetic and pharmacodynamic considerations. Ther. Drug Monit. 2008, 30, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Marchillo, K.; Conklin, R.; Krishna, G.; Ezzet, F.; Cacciapuoti, A.; Loebenberg, D. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 2004, 48, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.Y.A.; Sultan, M.A.; El-Alamin, M.M.A.; Atia, M.A.; Aboul-Enein, H.Y. A disposable carbon nanotubes-screen printed Electrode (CNTs-SPE) for determination of the antifungal agent posaconazole in biological samples. Electroanalysis 2017, 29, 843–849. [Google Scholar] [CrossRef]
- Lin, S.-C.; Lin, H.-Y.; Lin, S.-W.; Yao, M.; Wu, U.-I.; Kuo, H.-P.; Kuo, C.-H. Simultaneous determination of triazole antifungal drugs in human plasma by sweeping-micellar electrokinetic chromatography. Anal. Bioanal. Chem. 2012, 404, 217–228. [Google Scholar] [CrossRef]
- Liao, H.-W.; Lin, S.-W.; Wu, U.-I.; Kuo, C.-H. Rapid and sensitive determination of posaconazole in patient plasma by capillary electrophoresis with field-amplified sample stacking. J. Chromatogr. A 2012, 1226, 48–54. [Google Scholar] [CrossRef]
- Tang, P.H. Determination of posaconazole in plasma/serum by high-performance liquid chromatography with fluorescence detection. Separations 2017, 4, 16. [Google Scholar] [CrossRef]
- Beste, K.Y.; Burkhardt, O.; Kaever, V. Rapid HPLC-MS/MS method for simultaneous quantitation of four routinely administered triazole antifungals in human plasma. Clin. Chim. Acta 2012, 413, 240–245. [Google Scholar] [CrossRef]
- Fatiguso, G.; Favata, F.; Zedda, I.; Nicolò, A.D.; Cusato, J.; Avataneo, V.; Perri, G.D.; D’Avolio, A. A simple high performance liquid chromatography-mass spectrometry method for therapeutic drug monitoring of isavuconazole and four other antifungal drugs in human plasma samples. J. Pharm. Biomed. Anal. 2017, 145, 718–724. [Google Scholar] [CrossRef]

| Analyte | Concentration (μM) | Intraday | Interday | ||
|---|---|---|---|---|---|
| Precision a (%) | Accuracy b (%) | Precision a (%) | Accuracy b (%) | ||
| Posaconazole | 0.5 | 11.3 | 4.7 | 11.6 | 3.9 |
| 5 | 10.9 | 9.1 | 12.5 | 8.9 | |
| 15 | 9.3 | −4.0 | 7.4 | 1.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-S.; Chen, W.-C.; Chang, S.Y. Electromembrane Extraction of Posaconazole for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Detection. Membranes 2022, 12, 620. https://doi.org/10.3390/membranes12060620
Chen C-S, Chen W-C, Chang SY. Electromembrane Extraction of Posaconazole for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Detection. Membranes. 2022; 12(6):620. https://doi.org/10.3390/membranes12060620
Chicago/Turabian StyleChen, Chi-Sheng, Wen-Chi Chen, and Sarah Y. Chang. 2022. "Electromembrane Extraction of Posaconazole for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Detection" Membranes 12, no. 6: 620. https://doi.org/10.3390/membranes12060620
APA StyleChen, C.-S., Chen, W.-C., & Chang, S. Y. (2022). Electromembrane Extraction of Posaconazole for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Detection. Membranes, 12(6), 620. https://doi.org/10.3390/membranes12060620






