Effect of Membrane Surface Area on Solute Removal Performance of Dialyzers with Fouling
Abstract
:1. Introduction
2. Experimental
2.1. Materials
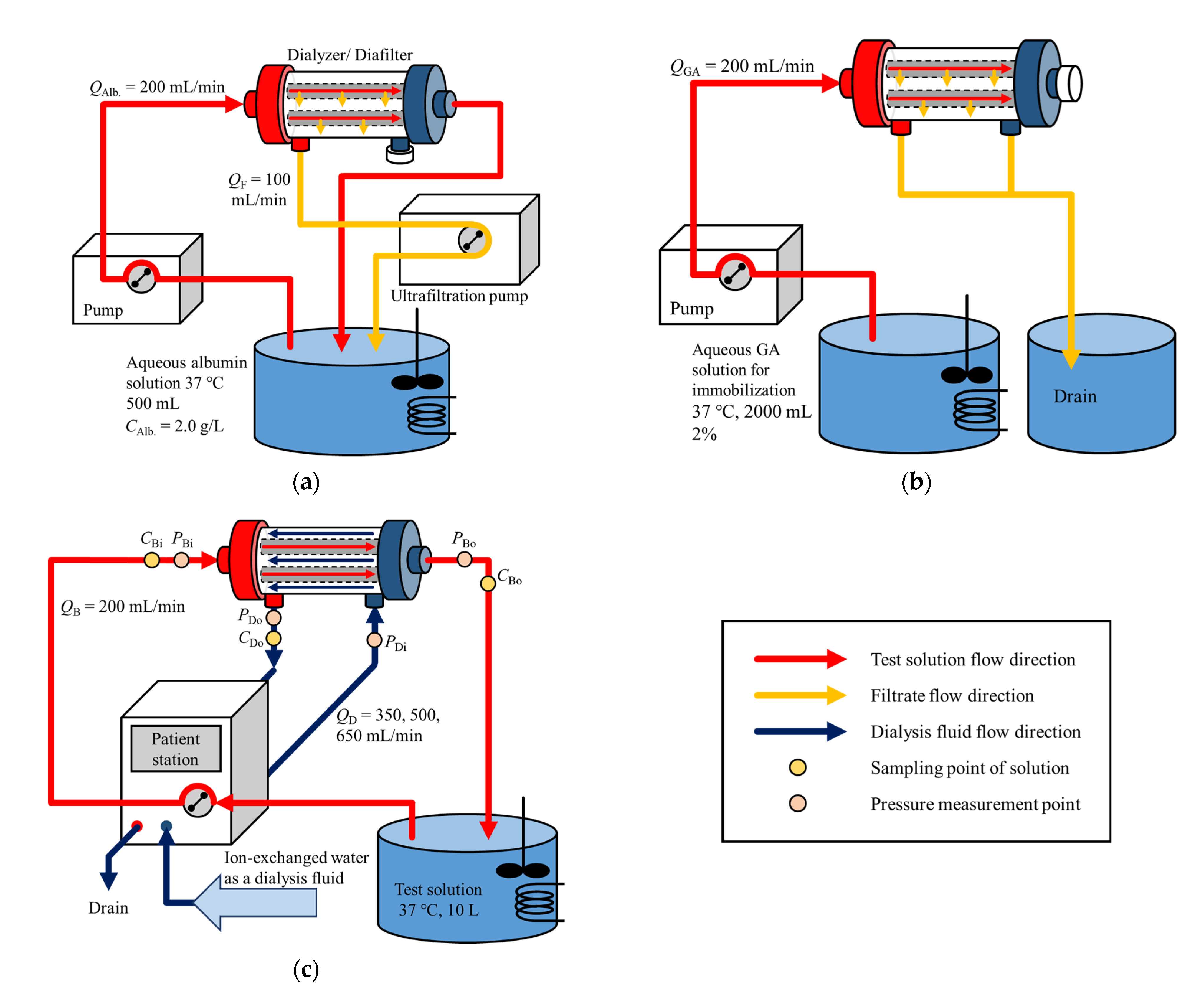
2.2. Albumin Immobilization
2.3. Dialysis Experiment
2.4. Evaluation of Mass Transfer in Dialysis Experiments
2.5. Computation of Pressure Distribution in a Dialyzer
3. Results and Discussion
3.1. Albumin Adsorption to the Test Module for Replication of Fouling
3.2. Evaluation of Solute Removal Performance in Two Types of Dialyzers
3.3. Computation of Pressure Distribution in Test Models
3.4. Effect of Solute Molecular Weight on the Removal Performance of Dialyzer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, Y.; Mineshima, M.; Ishimori, I.; Kaneko, I.; Akiba, T.; Teraoka, S. Effect of hollow fiber length on solute removal and quantification of internal filtration rate by Doppler ultrasound. Int. J. Artif. Organs 2003, 26, 129–134. [Google Scholar] [CrossRef]
- Ronco, C.; Brendolan, A.; Lupi, A.; Metry, G.; Levin, N.W. Effects of a reduced inner diameter of hollow fibers in hemodialyzers. Kidney Int. 2000, 58, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, A.C.; Fujita, R.; Tomisawa, N.; Jinbo, Y.; Yamamura, M. Effect of packing density of hollow fibers on solute removal performances of dialyzers. Hemodial. Int. 2009, 13, S2–S7. [Google Scholar] [CrossRef]
- Donato, D.; Storr, M.; Krause, B. Design optimization of hollow fiber dialyzers to enhance internal filtration based on a mathematical model. J. Membr. Sci. 2020, 598, 117690. [Google Scholar] [CrossRef]
- Hirano, A.; Kida, S.; Yamamoto, K.; Sakai, K. Experimental evaluation of flow and dialysis performance of hollow-fiber dialyzers with different packing densities. J. Artif. Organs 2012, 15, 168–175. [Google Scholar] [CrossRef]
- Mandolfo, S.; Malberti, F.; Imbasciati, E.; Cogliati, P.; Gauly, A. Impact of blood and dialysate flow and surface on performance of new polysulfone hemodialysis dialyzers. Int. J. Artif. Organs 2003, 26, 113–120. [Google Scholar] [CrossRef]
- Abe, M.; Masakane, I.; Wada, A.; Nakai, S.; Nitta, K.; Nakamoto, H. Dialyzer surface area is a significant predictor of mortality in patients on hemodialysis: A 3-year nationwide cohort study. Sci. Rep. 2021, 11, 20616. [Google Scholar] [CrossRef]
- Ronco, C.; Marchionna, N.; Brendolan, A.; Neri, M.; Lorenzin, A.; Martínez Rueda, A.J. Expanded haemodialysis: From operational mechanism to clinical results. Nephrol. Dial. Transplant. 2018, 33, iii41–iii47. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama, R.; Ishimori, I.; Akiba, T.; Minesima, M. Effect of blood flow rate on internal filtration in a high-flux dialyzer with polysulfone membrane. J. Artif. Organs 2012, 15, 266–271. [Google Scholar] [CrossRef]
- Fukuda, M.; Miyazaki, M.; Uezumi, S.; Yoshida, M. Design and assessment of the new APS dialyzer (APS-SA series). J. Artif. Organs 2006, 9, 192–198. [Google Scholar] [CrossRef]
- Nakashima, A.; Ogata, S.; Doi, S.; Yamahira, M.; Naraki, S.; Takasugi, N.; Ohmoto, T.; Ito, T.; Masaki, T.; Yorioka, N. Performance of polysulfone membrane dialyzers and dialysate flow pattern. Clin. Exp. Nephrol. 2006, 10, 210–215. [Google Scholar] [CrossRef]
- Said, N.; Lau, W.J.; Ho, Y.-C.; Lim, S.K.; Zainol Abidin, M.N.; Ismail, A.F. A review of commercial developments and recent laboratory research of dialyzers and membranes for hemodialysis application. Membranes 2021, 11, 767. [Google Scholar] [CrossRef]
- Yamashita, A.C.; Tomisawa, N.; Takezawa, A.; Sakurai, K.; Sakai, T. Blood compatibility and filtration characteristics of a newly developed polyester polymer alloy membrane. Hemodial. Int. 2004, 8, 368–371. [Google Scholar] [CrossRef]
- Tomisawa, N.; Yamashita, A.C. Amount of adsorbed albumin loss by dialysis membranes with protein adsorption. J. Artif. Organs 2009, 12, 194–199. [Google Scholar] [CrossRef]
- Yamashita, A.C.; Fujita, R.; Hosoi, N. Effect of sterilization on solute transport performances of super high-flux dialyzers. Hemodial. Int. 2012, 16, S10–S14. [Google Scholar] [CrossRef]
- Yamashita, A.C.; Ono, T.; Tomisawa, N. Verification of physicochemical structures of dialysis membrane using reversal dialysis technique. Hemodial. Int. 2017, 21, S3–S9. [Google Scholar] [CrossRef]
- Togo, K.; Yamamoto, M.; Ono, T.; Imai, M.; Akiyama, K.; Ebine, K.; Yamashita, A.C. Comparison of biocompatibility in polysulfone dialysis membranes with different sterilization. Hemodial. Int. 2018, 22, S10–S14. [Google Scholar] [CrossRef] [Green Version]
- Langsdorf, L.J.; Krankel, L.G.; Zydney, A.L. Effect of blood-membrane interactions on solute clearance during hemodialysis. ASAIO J. 1993, 39, M767–M772. [Google Scholar] [PubMed]
- Morti, S.M.; Zydney, A.L. Protein-membrane interactions during hemodialysis effects on solute transport. ASAIO J. 1998, 44, 319–326. [Google Scholar] [CrossRef]
- Mineshima, M.; Ishimori, I.; Sakiyama, R. Validity of internal filtration-enhanced hemodialysis as a new hemodiafiltration therapy. Blood Purif. 2009, 27, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Boschetti-de-Fierro, A.; Voigt, M.; Storr, M.; Krause, B. MCO membranes: Enhanced selectivity in high-flux class. Sci. Rep. 2015, 5, 18448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.R.; Hadidi, M.; Motevalian, S.P.; Sunohara, T.; Zydney, A.L. Effects of plasma proteins on the transport and surface characteristics of polysulfone/polyethersulfone and asymmetric cellulose triacetate high flux dialyzers. Artif. Organs 2018, 42, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Melchior, P.; Schall, C.; Erlenkötter, A.; Lang, T.; Keller, T.; Stauss-Grabo, M.; Kennedy, J.P. Time-resolving characterization of molecular weight retention changes among three synthetic high-flux dialyzers. Artif. Organs 2022, 46, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kiguchi, T.; Yamashita, A.C. Novel substitution technique in intermittent infusion hemodiafiltration (I-HDF) therapy using back filtration as substitution. J. Artif. Organs 2022, in press. [CrossRef] [PubMed]
- Bonifazi, M.; Aloisi, A.M.; Ceccarelli, I.; Lamponi, S.; Lodi, L.; Scaramuzzino, A.; Stabile, E.; Lupo, C. Platelet adhesion and aggregation on polyethylene: Effect of exhaustive exercise. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68, 53–58. [Google Scholar] [CrossRef]
- Lin, W.C.; Liu, T.Y.; Yang, M.C. Hemocompatibility of polyacrylonitrile dialysis membrane immobilized with chitosan and heparin conjugate. Biomaterials 2004, 25, 1947–1957. [Google Scholar] [CrossRef]
- Liu, T.Y.; Lin, W.C.; Huang, L.Y.; Chen, S.Y.; Yang, M.C. Hemocompatibility and anaphylatoxin formation of protein-immobilizing polyacrylonitrile hemodialysis membrane. Biomaterials 2005, 26, 1437–1444. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hiwatari, M.; Kohori, F.; Sakai, K.; Fukuda, M.; Hiyoshi, T. Membrane fouling and dialysate flow pattern in an internal filtration-enhancing dialyzer. J. Artif. Organs 2005, 8, 198–205. [Google Scholar] [CrossRef]
- Kiguchi, T.; Tomisawa, N.; Yamashita, A.C. Replication of fouling in vitro in hollow fiber dialyzers by albumin immobilization. J. Artif. Organs 2022, in press. [CrossRef]
- Heyrovsky, A. A new method for the determination of inulin in plasma and urine. Clin. Chim. Acta 1956, 1, 470–474. [Google Scholar] [CrossRef]
- Michaels, A.S. Operating parameters and performance criteria for hemodialyzers and other membrane-separation devices. Trans. Am. Soc. Artif. Int. Organs 1966, 12, 387–392. [Google Scholar]
- Kawanishi, H.; Mineshima, M.; Hirakata, H.; Akizawa, T. Committee Report, Performance evaluation for blood purification devices in 2012. J. Jpn. Soc. Dial. Ther. 2012, 45, 435–445. (In Japanese) [Google Scholar] [CrossRef]
- Mineshima, M.; Ishimori, I.; Ishida, K.; Hoshino, T.; Kaneko, I.; Sato, Y.; Agishi, T.; Tamamura, N.; Sakurai, H.; Masuda, T.; et al. Effect of internal filtration on the solute removal efficiency of a dialyzer. ASAIO J. 2000, 46, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yamashita, A.C. Effect of hollow fiber packing density and housing shape on the solute removal performance of CRRT filters for acute blood purification. Int. J. Artif. Organs 2022, 45, 543–549. [Google Scholar] [CrossRef]








| APS | |||||||
|---|---|---|---|---|---|---|---|
| 08SA | 11SA | 13SA | 15SA | 18SA | 21SA | 25SA | |
| Effective length, L [cm] | 27.0 | 27.0 | 27.0 | 27.0 | 27.0 | 27.0 | 27.0 |
| Arithmetic surface area, A0 [cm2] | 8000 | 11,000 | 13,000 | 15,000 | 18,000 | 21,000 | 25,000 |
| Inner diameter of fiber, d [µm] | 183.5 | 183.5 | 183.5 | 183.5 | 183.5 | 183.5 | 183.5 |
| Membrane thickness, Δx [µm] | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Inner diameter of the case, dd [cm] | 2.8 | 3.2 | 3.4 | 3.8 | 4.0 | 4.2 | 4.6 |
| Number of hollow fiber, n [-] | 5139 | 7066 | 8351 | 9636 | 11,563 | 13,490 | 16,060 |
| MFX | ||||
|---|---|---|---|---|
| 11S eco | 15S eco | 21S eco | 25S eco | |
| Effective length, L [cm] | 22.5 | 25.5 | 28.8 | 30.0 |
| Arithmetic surface area, A0 [cm2] | 11,000 | 15,000 | 21,000 | 25,000 |
| Inner diameter of fiber, d [µm] | 200 | 200 | 200 | 200 |
| Membrane thickness, Δx [µm] | 40 | 40 | 40 | 40 |
| Inner diameter of the case, dd [cm] | 3.2 | 3.5 | 3.9 | 4.2 |
| Number of hollow fiber, n [-] | 7781 | 9362 | 11,605 | 13,263 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiguchi, T.; Ito, H.; Yamashita, A.C. Effect of Membrane Surface Area on Solute Removal Performance of Dialyzers with Fouling. Membranes 2022, 12, 684. https://doi.org/10.3390/membranes12070684
Kiguchi T, Ito H, Yamashita AC. Effect of Membrane Surface Area on Solute Removal Performance of Dialyzers with Fouling. Membranes. 2022; 12(7):684. https://doi.org/10.3390/membranes12070684
Chicago/Turabian StyleKiguchi, Takayoshi, Hiromi Ito, and Akihiro C. Yamashita. 2022. "Effect of Membrane Surface Area on Solute Removal Performance of Dialyzers with Fouling" Membranes 12, no. 7: 684. https://doi.org/10.3390/membranes12070684






