Towards Biohybrid Lung Development: Establishment of a Porcine In Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Porcine Endothelial Cell Isolation and Cultivation
2.1.1. Cell Isolation
2.1.2. Cell Cultivation on Tissue Culture Plastic (TCP)
2.2. Characterization of the Isolated pCECs
2.2.1. Flow Cytometry Analysis
2.2.2. Staining of pCECs on TCP for Fluorescence Microscopy Assessment
2.2.3. Gene Expression Analysis via RT-PCR and Quantitative Real-Time qRT-PCR
2.3. Endothelialization of Gas Exchange HFMs with pCECs
2.3.1. Immunofluorescence Microscopy of pCECs on HFMs
2.3.2. Gene Expression Analysis via Real-Time qRT-PCR
2.4. Assessment of Endothelialized HFMs Exposed to Flow Conditions Using Culture Medium
2.4.1. Fluorescence Microscopy of Endothelialized HFMs after Flow
2.4.2. Gene Expression Analysis via Real-Time qRT-PCR
2.5. Hemocompatibility Testing of Non-Endothelialized versus Endothelialized HFMs
2.5.1. Fluorescence Microscopy of Endothelialized HFMs after Blood Flow
2.5.2. Thrombus Formation Assessment via Photographs and Scanning Electron Microscopy (SEM)
2.5.3. Quantification of Blood Parameters Associated with Thrombus Formation
2.6. Statistical Analysis
3. Results
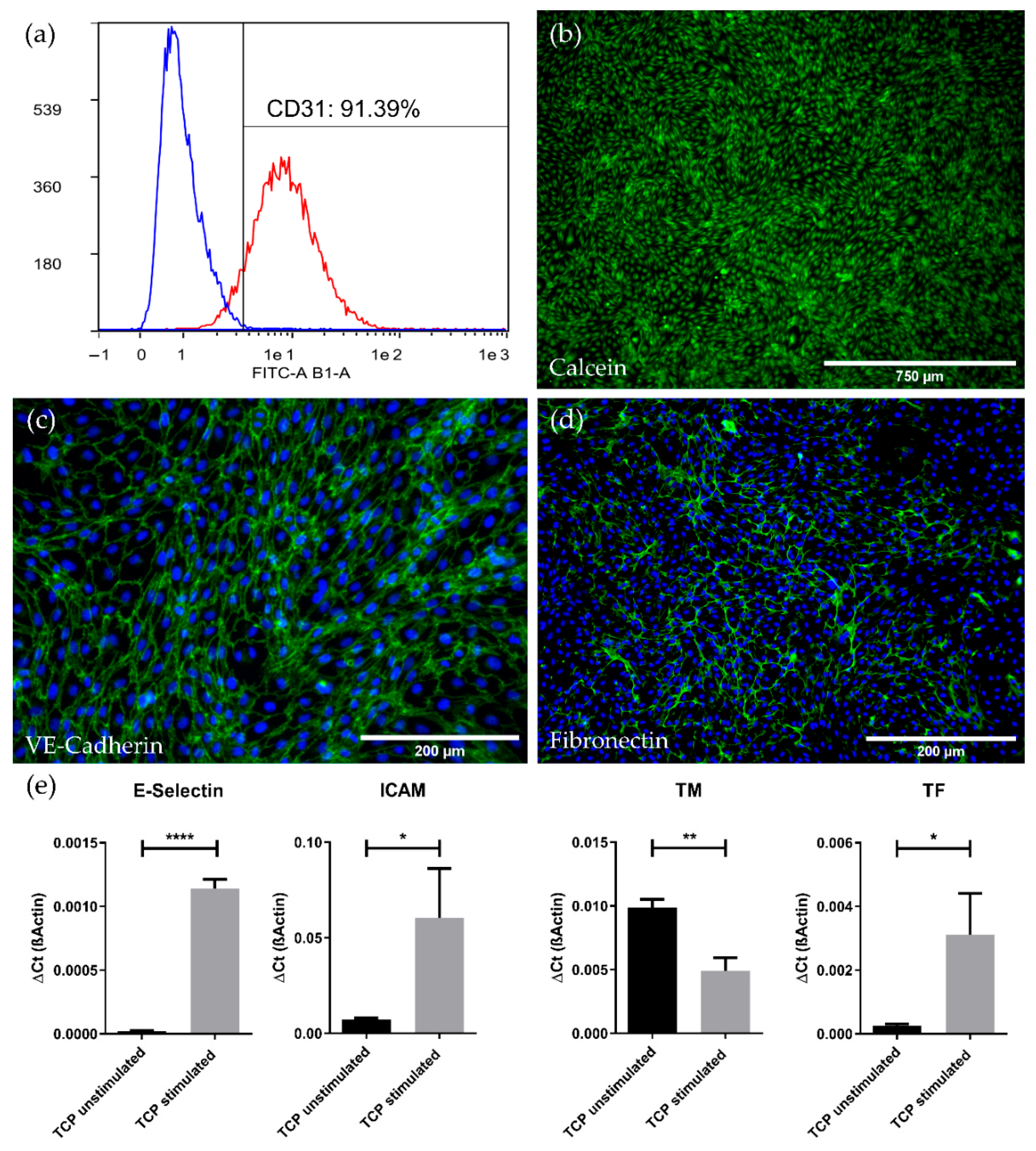
3.1. High Expansion Capacity and Purity of Endothelial Cells Isolated from Porcine Carotid Artery (pCECs)
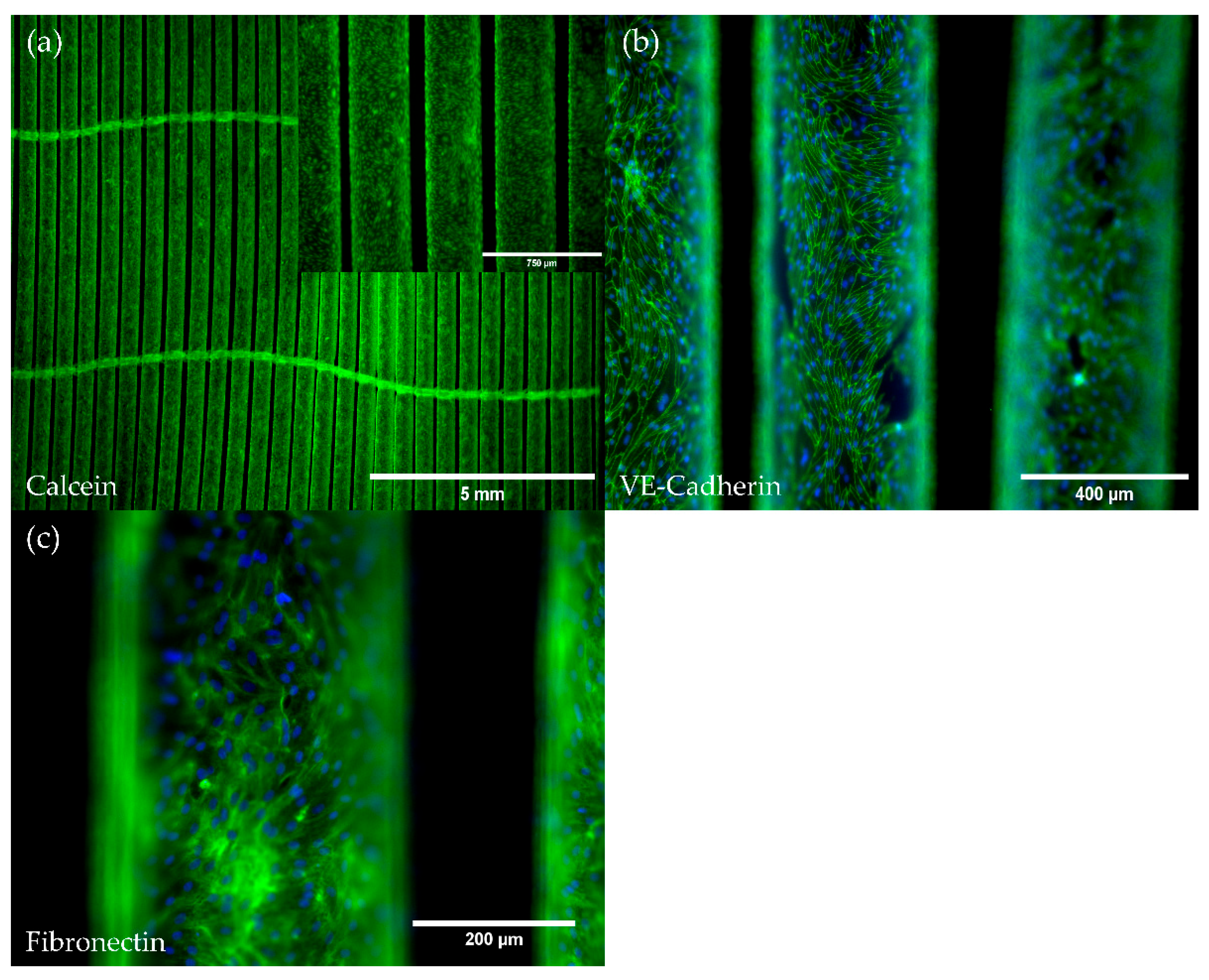
3.2. pCECs Form a Confluent and Non-Activated Neo-Endothelium on HFMs
3.2.1. pCECs Adherent to HFMs Grow to Confluence and Generate Extracellular Matrix
3.2.2. pCECs on HFMs Remain in the Non-Inflammatory and Non-Thrombogenic Genotype
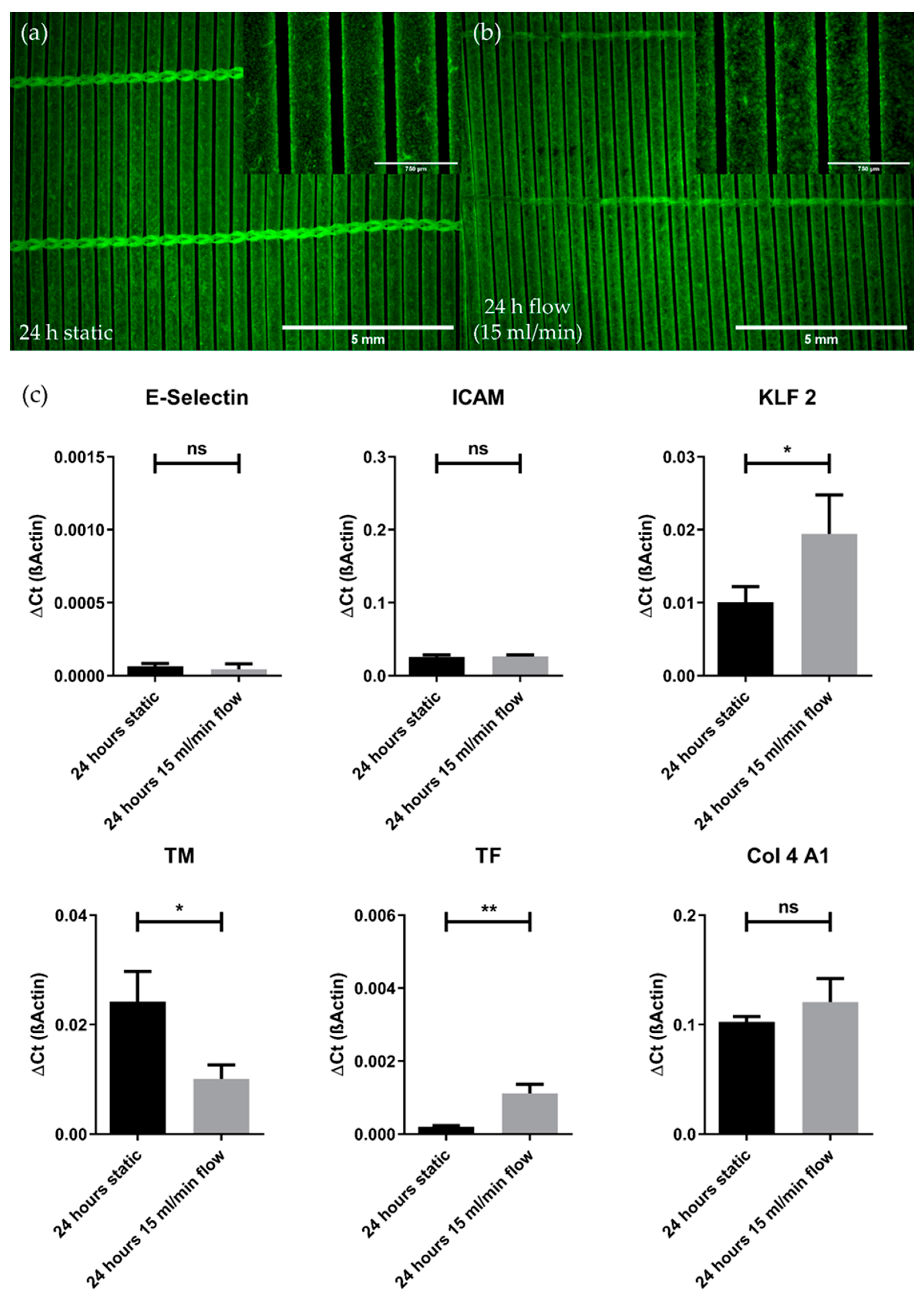
3.3. pCEC Monolayers on HFMs Resist Flow Conditions and Show Typical Physiological Responses on Gene Expression Level
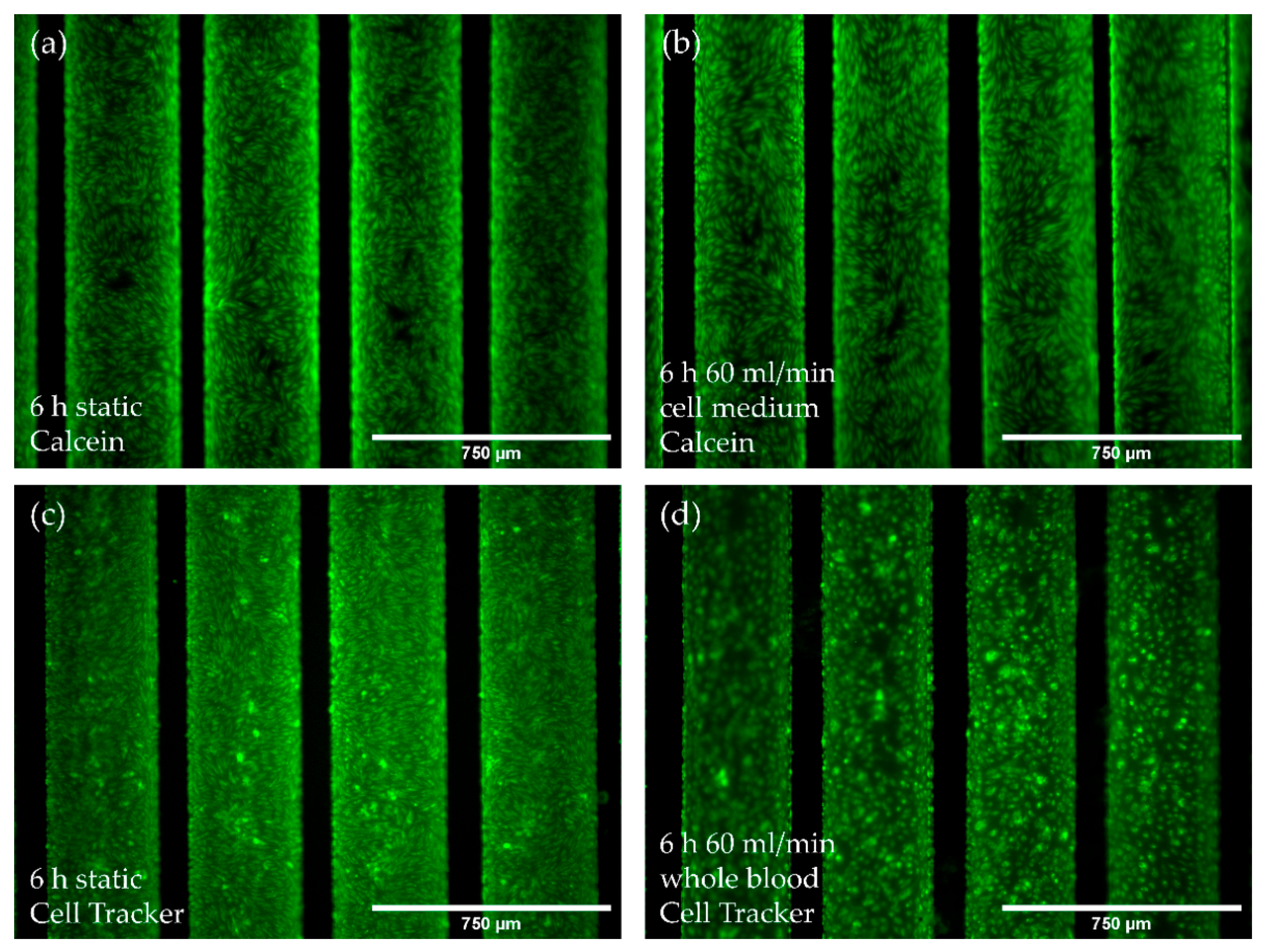
3.3.1. Flow Conditions Do Not Affect Monolayer Integrity
3.3.2. Flow Conditions Lead to Physiological Gene Expression Changes While Not Promoting the Pro-Inflammatory Genotype
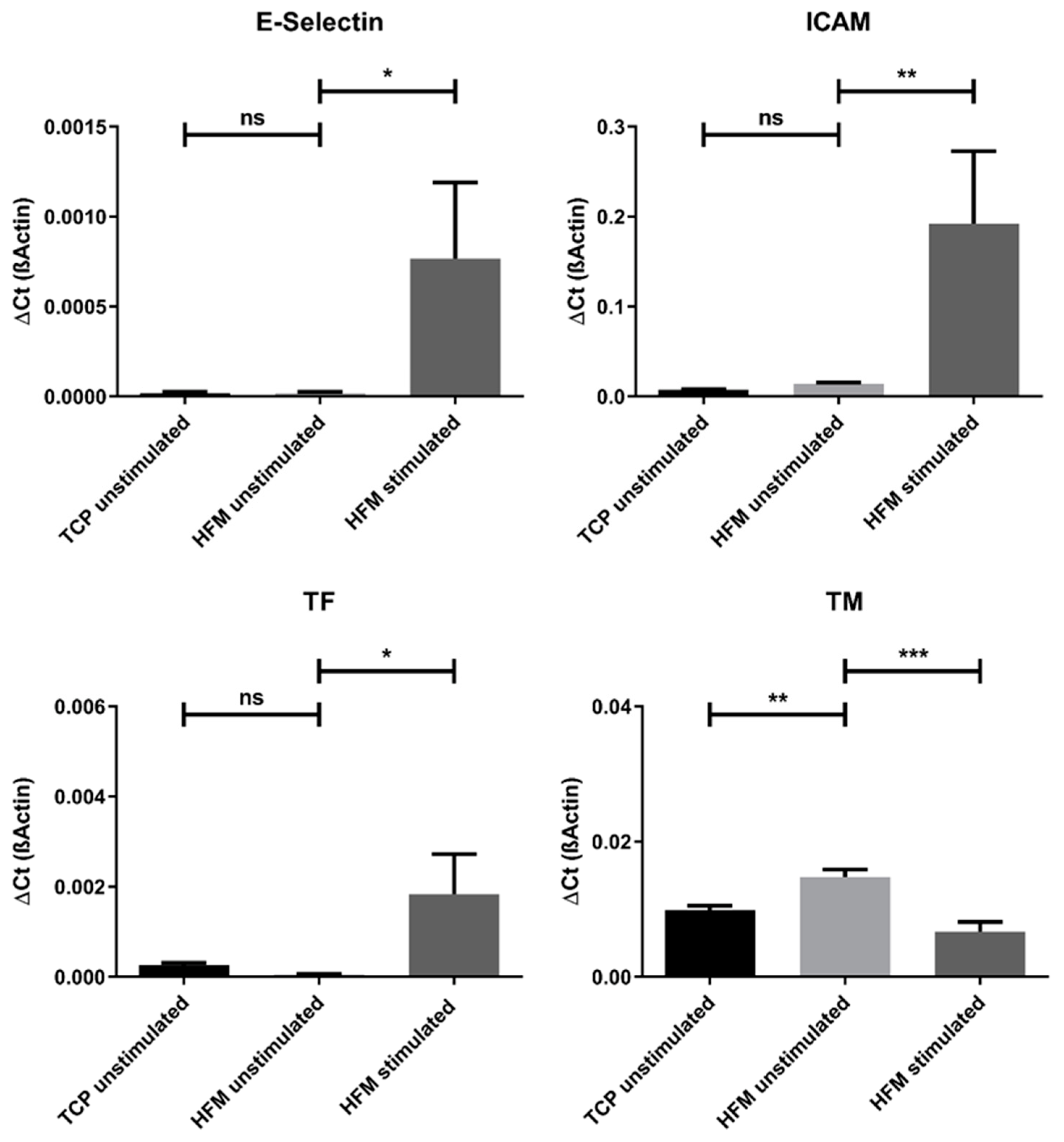
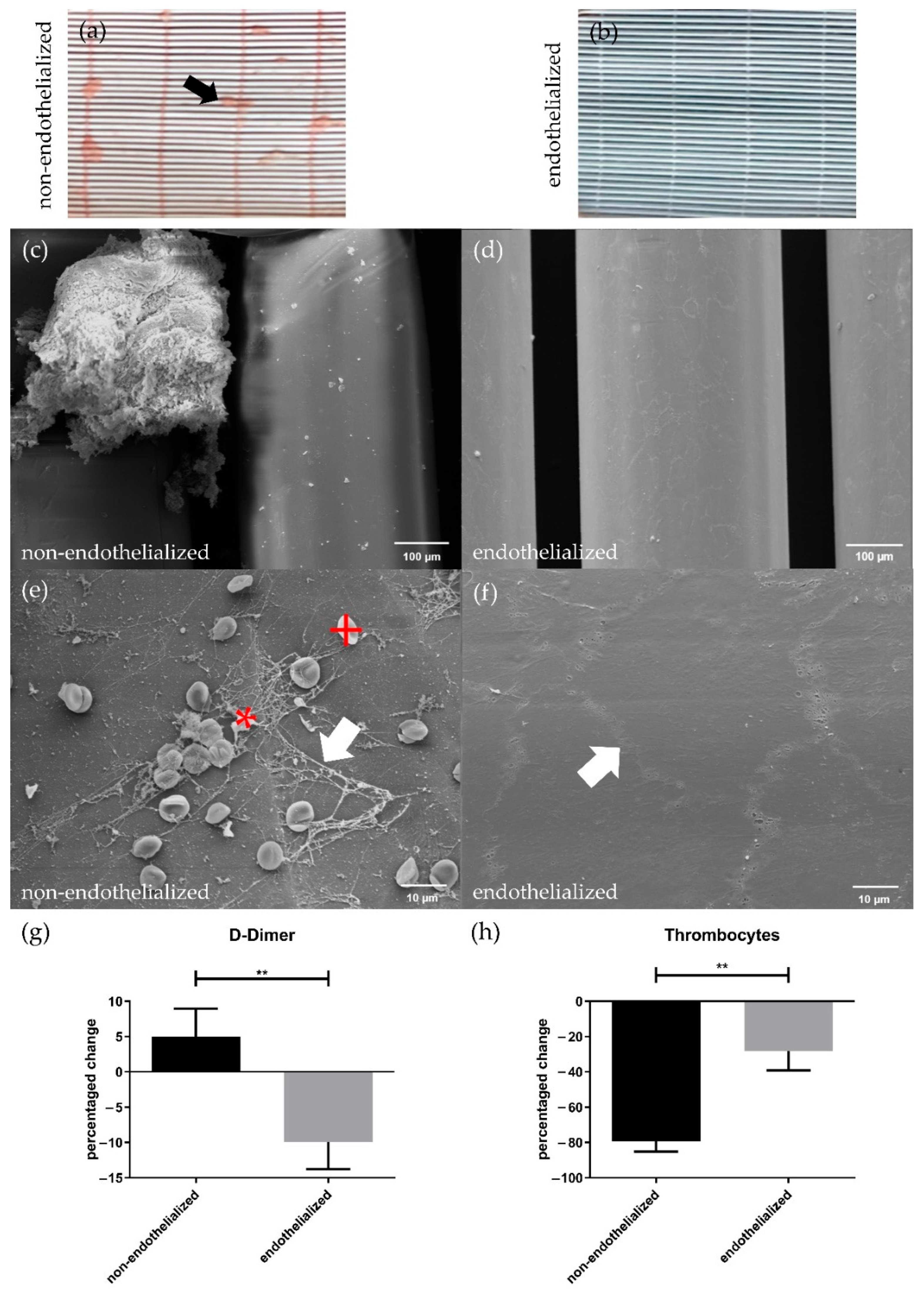
3.4. The pCEC Monolayer on HFMs Is Capable to Withstand Clinically Relevant Blood Flow Conditions
3.5. Endothelialization Significantly Improves HFM Hemocompatibility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Kahan, E.S.; Petersen, G.; Gaughan, J.P.; Criner, G.J. High Incidence of Venous Thromboembolic Events in Lung Transplant Recipients. J. Heart Lung Transplant. 2007, 26, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Kotloff, R.M.; Ahya, V.N.; Tino, G.; Pochettino, A.; Gaughan, C.; DeMissie, E.; Kimmel, S.E. The Effect of Primary Graft Dysfunction on Survival after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2005, 171, 1312–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weill, D.; Benden, C.; Corris, P.A.; Dark, J.H.; Davis, R.D.; Keshavjee, S.; Lederer, D.J.; Mulligan, M.J.; Patterson, G.A.; Singer, L.G.; et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; Vos, R.; Van Raemdonck, D.E.; Verleden, G.M. Survival in adult lung transplantation: Where are we in 2020? Curr. Opin. Organ Transplant. 2020, 25, 268–273. [Google Scholar] [CrossRef]
- Abouna, G.M. Organ Shortage Crisis: Problems and Possible Solutions. Transplant. Proc. 2008, 40, 34–38. [Google Scholar] [CrossRef]
- Andreoli, M.C.C.; Totoli, C. Peritoneal Dialysis. Rev. Assoc. Med. Bras. 2020, 66, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, M.; Kyo, S.; Yamakawa, S.; Ono, M.; Kinugawa, K.; Nishimura, T. Destination therapy: The new gold standard treatment for heart failure patients with left ventricular assist devices. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 111–117. [Google Scholar] [CrossRef]
- Rojas, S.V.; Hanke, J.S.; Avsar, M.; Ahrens, P.R.; Deutschmann, O.; Tümler, K.A.; Uribarri, A.; Rojas-Hernández, S.; Sánchez, P.L.; González-Santos, J.M.; et al. Left Ventricular Assist Device Therapy for Destination Therapy: Is Less Invasive Surgery a Safe Alternative? Rev. Española Cardiol. Engl. Ed. 2018, 71, 13–17. [Google Scholar] [CrossRef]
- Peek, G.J.; Killer, H.M.; Reeves, R.; Sosnowski, A.W.; Firmin, R.K. Early Experience with a Polymethyl Pentene Oxygenator for Adult Extracorporeal Life Support. ASAIO J. 2002, 48, 480–482. [Google Scholar] [CrossRef]
- Thomas, J.; Kostousov, V.; Teruya, J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin. Thromb. Hemost. 2017, 44, 20–29. [Google Scholar] [CrossRef]
- Fuehner, T.; Kuehn, C.; Haverich, A.; Hoeper, M.M.; Warnecke, G.; Hadem, J.; Wiesner, O.; Gottlieb, J.; Tudorache, I.; Olsson, K.M.; et al. Extracorporeal membrane oxygenation in awake patients as a bridge to lung transplantation. Am. J. Respir. Crit. Care Med. 2012, 185, 763–768. [Google Scholar] [CrossRef]
- McGuigan, A.P.; Sefton, M.V. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 2007, 28, 2547–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegmann, B.; von Seggern, H.; Höffler, K.; Korossis, S.; Dipresa, D.; Pflaum, M.; Schmeckebier, S.; Seume, J.; Haverich, A. Developing a biohybrid lung-sufficient endothelialization of poly-4-methly-1-pentene gas exchange hollow-fiber membranes. J. Mech. Behav. Biomed. Mater. 2016, 60, 301–311. [Google Scholar] [CrossRef]
- Zwirner, U.; Höffler, K.; Pflaum, M.; Korossis, S.; Haverich, A.; Wiegmann, B. Identifying an optimal seeding protocol and endothelial cell substrate for biohybrid lung development. J. Tissue Eng. Regen. Med. 2018, 12, 2319–2330. [Google Scholar] [CrossRef]
- Pflaum, M.; Kühn-Kauffeldt, M.; Schmeckebier, S.; Dipresa, D.; Chauhan, K.; Wiegmann, B.; Haug, R.J.; Schein, J.; Haverich, A.; Korossis, S. Endothelialization and characterization of titanium dioxide-coated gas-exchange membranes for application in the bioartificial lung. Acta Biomater. 2017, 50, 510–521. [Google Scholar] [CrossRef]
- Pflaum, M.; Jurmann, S.; Katsirntaki, K.; Mälzer, M.; Haverich, A.; Wiegmann, B. Towards Biohybrid Lung Development—Fibronectin-Coating Bestows Hemocompatibility of Gas Exchange Hollow Fiber Membranes by Improving Flow-Resistant Endothelialization. Membranes 2021, 12, 35. [Google Scholar] [CrossRef]
- Hess, C.; Wiegmann, B.; Maurer, A.N.; Fischer, P.; Möller, L.; Martin, U.; Hilfiker, A.; Haverich, A.; Fischer, S. Reduced Thrombocyte Adhesion to Endothelialized Poly 4-Methyl-1-Pentene Gas Exchange Membranes—A First Step Toward Bioartificial Lung Development. Tissue Eng. Part A 2010, 16, 3043–3053. [Google Scholar] [CrossRef]
- Klein, S.; Hesselmann, F.; Djeljadini, S.; Berger, T.; Thiebes, A.L.; Schmitz-Rode, T.; Jockenhoevel, S.; Cornelissen, C.G. EndOxy: Dynamic Long-Term Evaluation of Endothelialized Gas Exchange Membranes for a Biohybrid Lung. Ann. Biomed. Eng. 2020, 48, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Menzel, S.; Finocchiaro, N.; Donay, C.; Thiebes, A.L.; Hesselmann, F.; Arens, J.; Djeljadini, S.; Wessling, M.; Schmitz-Rode, T.; Jockenhoevel, S.; et al. Towards a Biohybrid Lung: Endothelial Cells Promote Oxygen Transfer through Gas Permeable Membranes. BioMed Res. Int. 2017, 2017, 5258196. [Google Scholar] [CrossRef] [Green Version]
- Pflaum, M.; Jurmann, S.; Katsirntaki, K.; Mälzer, M.; Haverich, A.; Wiegmann, B. Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization. Micromachines 2021, 12, 981. [Google Scholar] [CrossRef]
- Wiegmann, B.; Figueiredo, C.; Gras, C.; Pflaum, M.; Schmeckebier, S.; Korossis, S.; Haverich, A.; Blasczyk, R. Prevention of rejection of allogeneic endothelial cells in a biohybrid lung by silencing HLA-class I expression. Biomaterials 2014, 35, 8123–8133. [Google Scholar] [CrossRef] [PubMed]
- Pflaum, M.; Merhej, H.; Peredo, A.; De, A.; Dipresa, D.; Wiegmann, B.; Wolkers, W.; Haverich, A.; Korossis, S. Hypothermic preservation of endothelialized gas-exchange membranes. Artif. Organs 2020, 44, e552–e565. [Google Scholar] [CrossRef] [PubMed]
- Plein, T.; Thiebes, A.L.; Finocchiaro, N.; Hesselmann, F.; Schmitz-Rode, T.; Jockenhoevel, S.; Cornelissen, C.G. Towards a Biohybrid Lung Assist Device: N-Acetylcysteine Reduces Oxygen Toxicity and Changes Endothelial Cells’ Morphology. Cel. Mol. Bioeng. 2017, 10, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.N. Organ weights and blood flows of sheep and pig for physiological pharmacokinetic modelling. J. Pharmacol. Toxicol. Methods 2008, 58, 198–205. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Kristian, A.; Alstrup, O.; Hansen, A.K.; Jespersen, J.; Marckmann, P. The pig as a model in blood coagulation and fibrinolysis research. Scand. J. Lab. Anim. Sci. 1999, 26, 214–224. [Google Scholar]
- Osterman, F.A.; Bell, W.R.; Montali, R.J.; Novak, G.R.; White, R.J. Natural History of Autologous Blood Clot Embolization in Swine. Investig. Radiol. 1976, 11, 267–276. [Google Scholar] [CrossRef]
- Velik-Salchner, C.; Schnürer, C.; Fries, D.; Müssigang, P.R.; Moser, P.L.; Streif, W.; Kolbitsch, C.; Lorenz, I.H. Normal values for thrombelastography (ROTEM®) and selected coagulation parameters in porcine blood. Thromb. Res. 2006, 117, 597–602. [Google Scholar] [CrossRef]
- Bloch Münster, A.; Olsen, A.K.; Bladbjerg, E. Usefulness of Human Coagulation and Fibrinolysis Assays in Domestic Pigs. Comp. Med. 2002, 52, 39–43. [Google Scholar]
- Borges, A.M.; Ferrari, R.S.; Thomaz, L.D.G.R.; Ulbrich, J.M.; Félix, E.A.; Silvello, D.; Andrade, C.F. Challenges and perspectives in porcine model of acute lung injury using oleic acid. Pulm. Pharmacol. Ther. 2019, 59. [Google Scholar] [CrossRef]
- Watkins, R.; Perrott, R.; Bate, S.; Auton, P.; Watts, S.; Stoll, A.; Rutter, S.; Jugg, B. Development of chlorine-induced lung injury in the anesthetized, spontaneously breathing pig. Toxicol. Mech. Methods 2021, 31, 257–271. [Google Scholar] [CrossRef]
- Hochhausen, N.; Orschulik, J.; Follmann, A.; Santos, S.A.; Dohmeier, H.; Leonhardt, S.; Rossaint, R.; Czaplik, M. Comparison of two experimental ARDS models in pigs using electrical impedance tomography. PLoS ONE 2019, 14, e0225218. [Google Scholar] [CrossRef]
- Galvão, F.H.F.; Pompeu, E.; De Mello, E.S.; Da Costa Lino Costa, A.; Mory, E.; Dos Santos, R.M.; Santos, V.R.; Machado, M.C.; Bacchella, T. Experimental multivisceral xenotransplantation. Xenotransplantation 2008, 15, 184–190. [Google Scholar] [CrossRef]
- Robson, S.C.; Candinas, D.; Hancock Wayne, W.; Wrighton, C.; Winkler, H.; Bach, F.H. Role of Endothelial Cells in Transplantation. Int. Arch. Allergy Immunol. 1995, 106, 305–322. [Google Scholar] [CrossRef]
- Colburn, P.; Buonassisi, V. Anti-clotting activity of endothelial cell cultures and heparan sulfate proteoglycans. Biochem. Biophys. Res. Commun. 1982, 104, 220–227. [Google Scholar] [CrossRef]
- Marcus, A.J.; Broekman, M.J.; Drosopoulos, J.H.; Islam, N.; Alyonycheva, T.N.; Safier, L.B.; Hajjar, K.A.; Posnett, D.N.; Schoenborn, M.A.; Schooley, K.A.; et al. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J. Clin. Investig. 1997, 99, 1351–1360. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, M.P.; Pober, J.S.; Majeau, G.R.; Cotran, R.S.; Gimbrone, M.A.J. Interleukin 1 induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J. Exp. Med. 1984, 160, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Pober, J.S. The cathepsin B death pathway contributes to TNF plus IFN-gamma-mediated human endothelial injury. J. Immunol. 2005, 175, 1858–1866. [Google Scholar] [CrossRef] [Green Version]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, T.; Yamamoto, K.; Kamiya, A.; Ando, J.; Yoshida, A. Effects of Shear Stress on the Gene Expressions of Endothelial Nitric Oxide Synthase, Endothelin-1, and Thrombomodulin in Human Retinal Microvascular Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8496–8504. [Google Scholar] [CrossRef]
- Schumer, E.; Höffler, K.; Kuehn, C.C.; Slaughter, M.; Haverich, A.; Wiegmann, B. In-vitro evaluation of limitations and possibilities for the future use of intracorporeal gas exchangers placed in the upper lobe position. J. Artif. Organs 2017, 21, 68–75. [Google Scholar] [CrossRef]
- Fledderus, J.O.; van Thienen, J.V.; Boon, R.A.; Dekker, R.J.; Rohlena, J.; Volger, O.L.; Bijens, A.J.J.; Daemen, M.J.A.P.; Kuiper, J.; van Berkel, T.J.C.; et al. Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood 2007, 109, 4249–4257. [Google Scholar] [CrossRef] [Green Version]
- Dekker, R.J.; van Soest, S.; Fontijn, R.D.; Salamanca, S.; de Groot, P.G.; VanBavel, E.; Pannekoek, H.; Horrevoets, A.J.G. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 2002, 100, 1689–1698. [Google Scholar] [CrossRef]
- Horster, S.; Stemmler, H.; Strecker, N.; Brettner, F.; Hausmann, A.; Cnossen, J.; Parhofer, K.G.; Nickel, T.; Geiger, S. Cardiac Output Measurements in Septic Patients: Comparing the Accuracy of USCOM to PiCCO. Crit. Care Res. Pract. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bleilevens, C.; Grottke, O.; Tillmann, S.; Honickel, M.; Kopp, R.; Arens, J.; Rossaint, R. Twelve Hours In Vitro Biocompatibility Testing of Membrane Oxygenators. ASAIO J. 2015, 61, 548–555. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood Rheology and Hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar]
- Lehle, K.; Philipp, A.; Gleich, O.; Holzamer, A.; Müller, T.; Bein, T.; Schmid, C. Efficiency in Extracorporeal Membrane Oxygenation—Cellular Deposits on Polymethypentene Membranes Increase Resistance to Blood Flow and Reduce Gas Exchange Capacity. ASAIO J. 2008, 54, 612–617. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [Green Version]
| Antibody Name | Dilution | Vendor |
|---|---|---|
| Anti-VE Cadherin (ab33168) | 1:300 | Abcam, Cambridge, UK |
| Anti-Fibronectin (ab45688) | 1:250 | Abcam, Cambridge, UK |
| Rabbit IgG Isotype Control (ab172730) | Abcam, Cambridge, UK | |
| Donkey anti-rabbit Cy2 | 1:100 | Jackson ImmunoResearch, Ely, UK |
| Gene Name and ID | Primer 1 | Primer 2 |
|---|---|---|
| ß-Actin XM 003124280.4 | GATCAAGATCATCGCG- CCTCC | GGAATGCAACTAACAG- TCCGCC |
| Endothelium-Selectin (E-Selectin)NM 214268.2 | TCCTGTCAACGGAGTC- GTGA | GTCACAGCTTTACACGT- TGGC |
| ICAM-1 (ICAM) NM 213816.1 | GCTCAGTGTCCTGTAT- GGACC | AGAGCTGGTGGCCTGA-CATT |
| Thrombomodulin (TM) NM 001130732.1 | CAACCAGACTTCGTG- CCCTG | GTAGCCGTTGTTGCAC- TCGT |
| Tissue Factor (TF) NM 213785.1 | TTAGTCAGGGTGAAC- GGCAC | GGTCGTGGCCTTTTTC- TTTCC |
| von Willebrand Factor (vWF) NM 001246221.1 | AGGGGGACCAAAGC- ATCTCC | TGAAAGTTGCCGCTC- CCATC |
| CD31 NM 01010101.01 | CACGGAGGTCTGGAA- CAAAG | TCTGCTCTGCGGTCC- TAAGT |
| VE-Cadherin (VE-Cadh.) NM 001001649.2 | GCGAGTTCACCTTGT- GCGAG | CGAGGAGGGAGATC- ACTGCG |
| Krüppel-like factor 2 (KLF 2) NM 001134351.2 | CGTCTCCGCTGGAGC- TACTA | GTAGGGCTTCTCGCC- TGTAT |
| Collagen 4 subunit A1 (Col 4 A1) XM 021065910.1 | ATGCAACGGGACAA- AGGGTG | CCCAGGTATGTGGCC- GAGTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlör, S.; Pflaum, M.; Höffler, K.; Kühn, C.; Haverich, A.; Wiegmann, B. Towards Biohybrid Lung Development: Establishment of a Porcine In Vitro Model. Membranes 2022, 12, 687. https://doi.org/10.3390/membranes12070687
Schlör S, Pflaum M, Höffler K, Kühn C, Haverich A, Wiegmann B. Towards Biohybrid Lung Development: Establishment of a Porcine In Vitro Model. Membranes. 2022; 12(7):687. https://doi.org/10.3390/membranes12070687
Chicago/Turabian StyleSchlör, Simon, Michael Pflaum, Klaus Höffler, Christian Kühn, Axel Haverich, and Bettina Wiegmann. 2022. "Towards Biohybrid Lung Development: Establishment of a Porcine In Vitro Model" Membranes 12, no. 7: 687. https://doi.org/10.3390/membranes12070687
APA StyleSchlör, S., Pflaum, M., Höffler, K., Kühn, C., Haverich, A., & Wiegmann, B. (2022). Towards Biohybrid Lung Development: Establishment of a Porcine In Vitro Model. Membranes, 12(7), 687. https://doi.org/10.3390/membranes12070687






