New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hybrid Membranes Preparation
2.3. Membrane Characterization
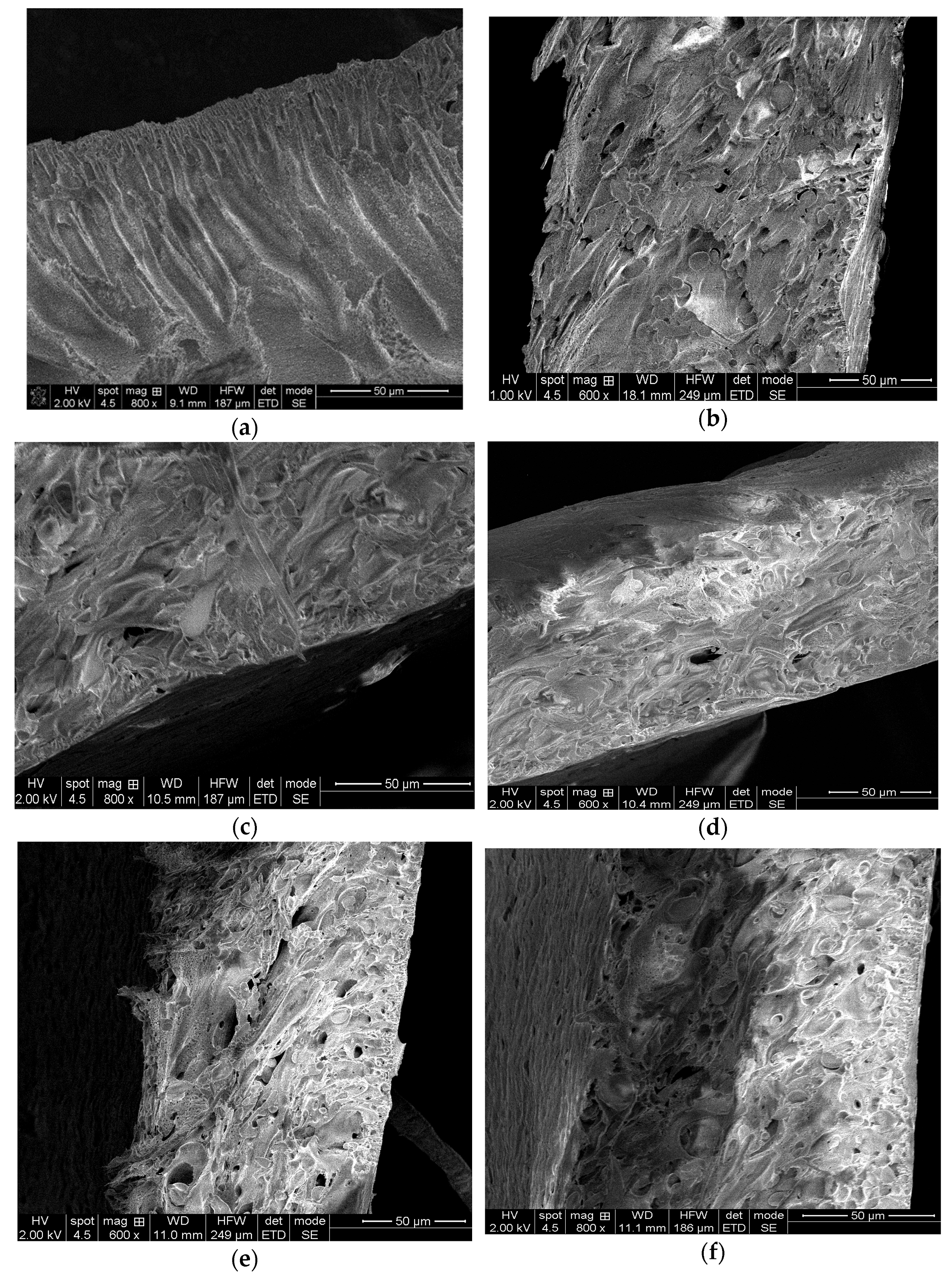
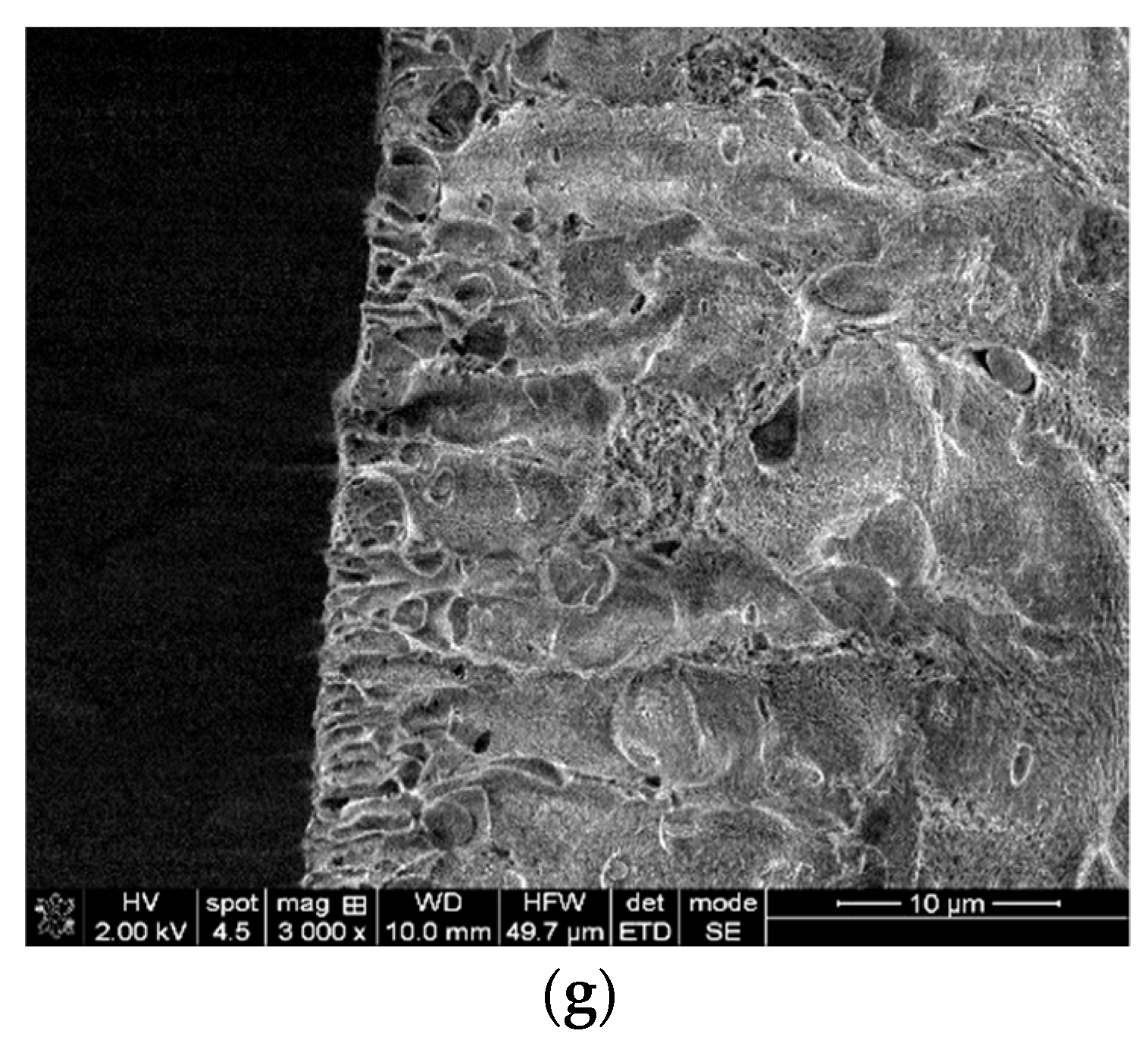
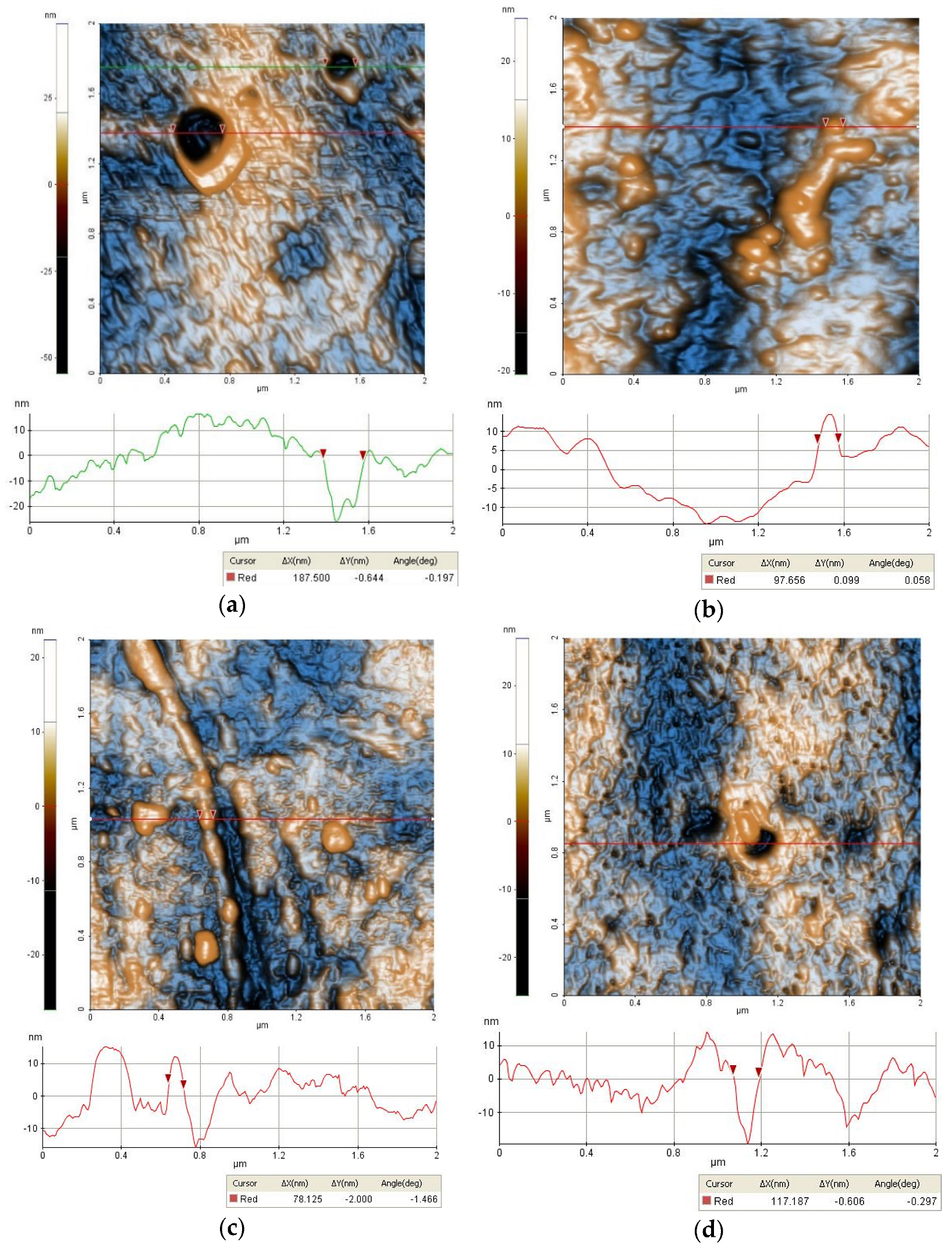
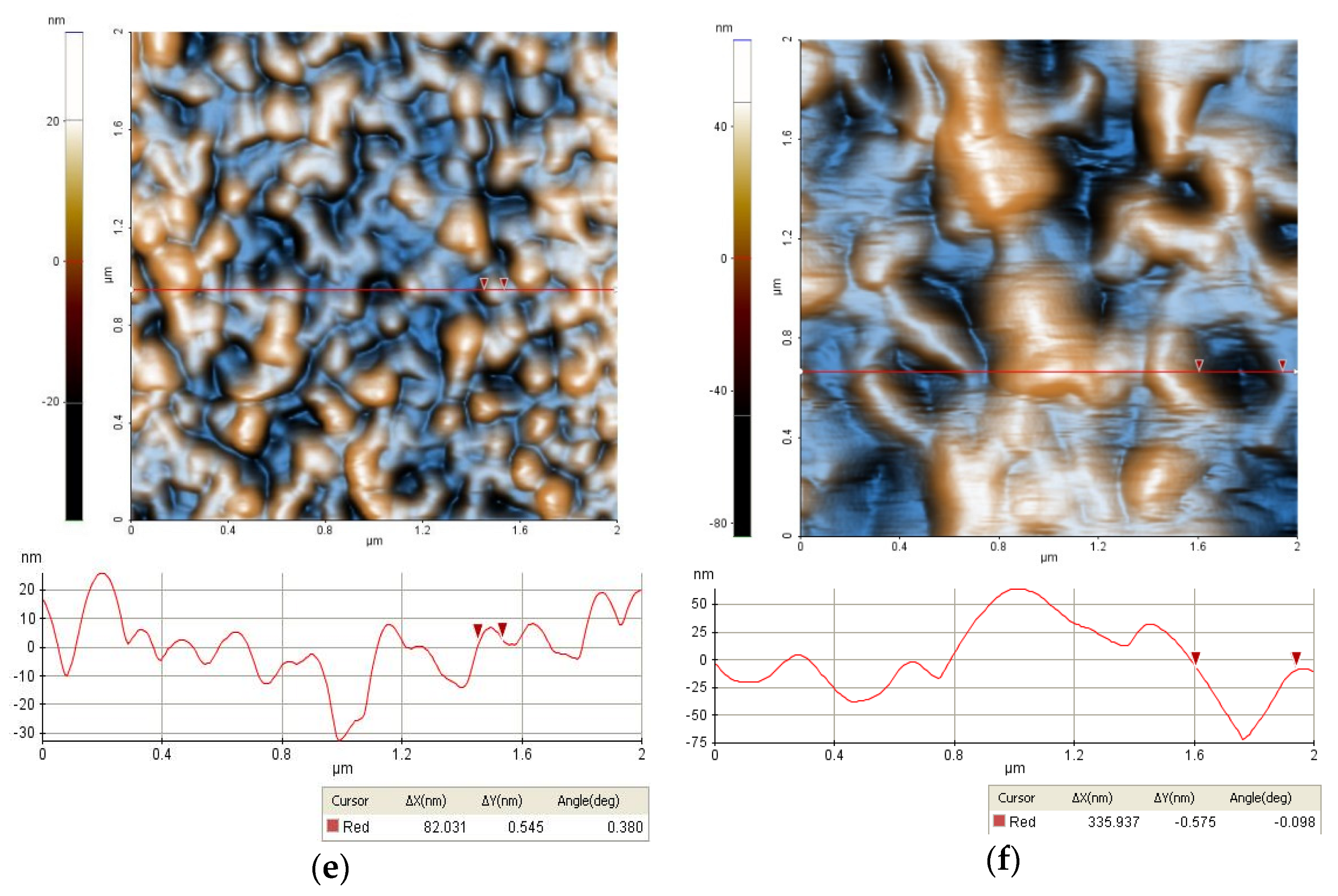
2.3.1. Morphology
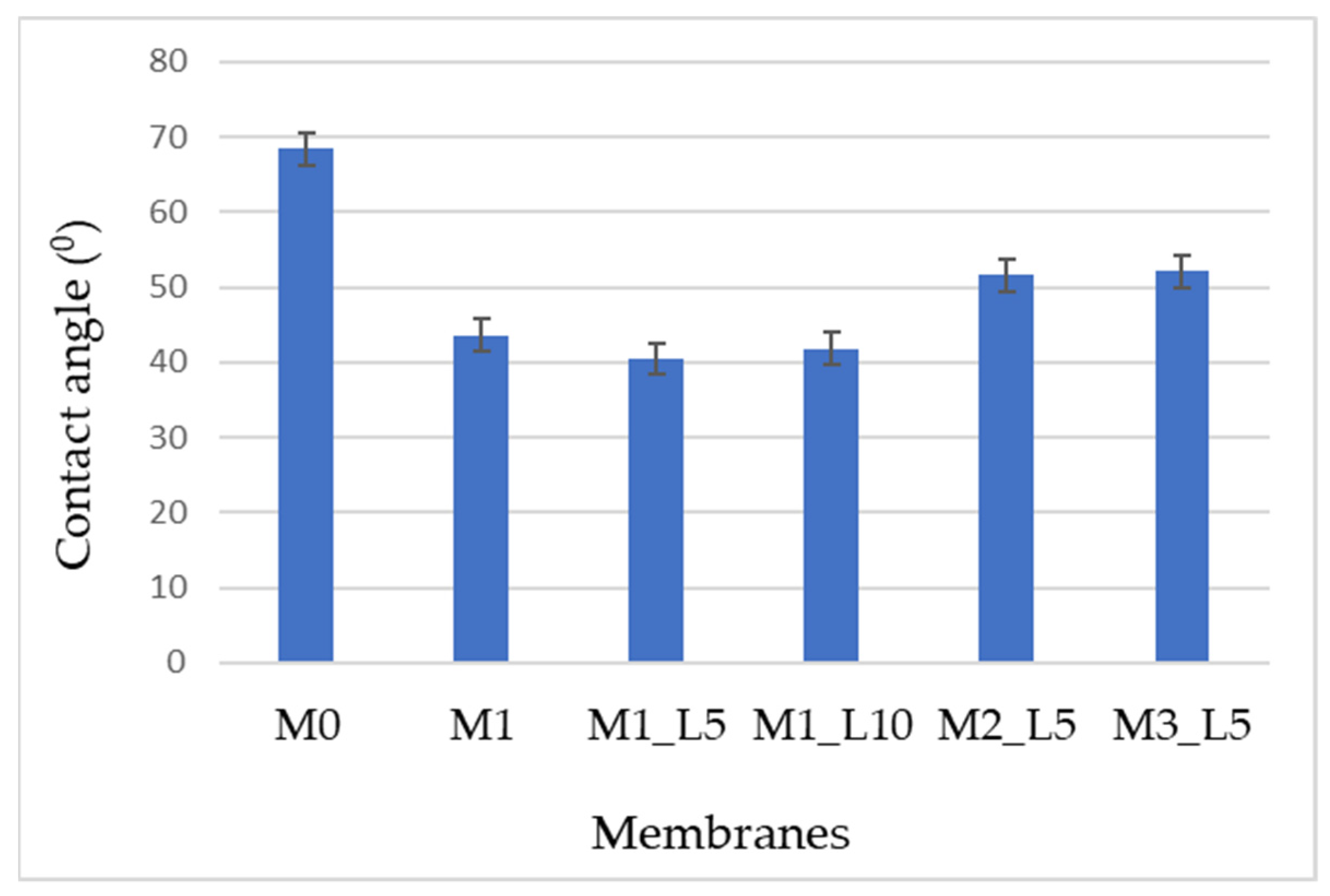
2.3.2. Surface Wetting Properties
2.3.3. Membrane Molecular Weight Cut-Off
2.4. Evaluation of Membrane Performance
2.4.1. Preparation of Feed Solution
2.4.2. Membrane Antifouling Ability
2.4.3. Permeability and Selectivity of Membranes
2.5. Analytical Methods
2.5.1. Total Phenols, Flavones, and Antioxidant Potential Analyses
2.5.2. HPLC Determination of Polyphenols
3. Results
3.1. Characterizations of the Nanofiltration Membranes
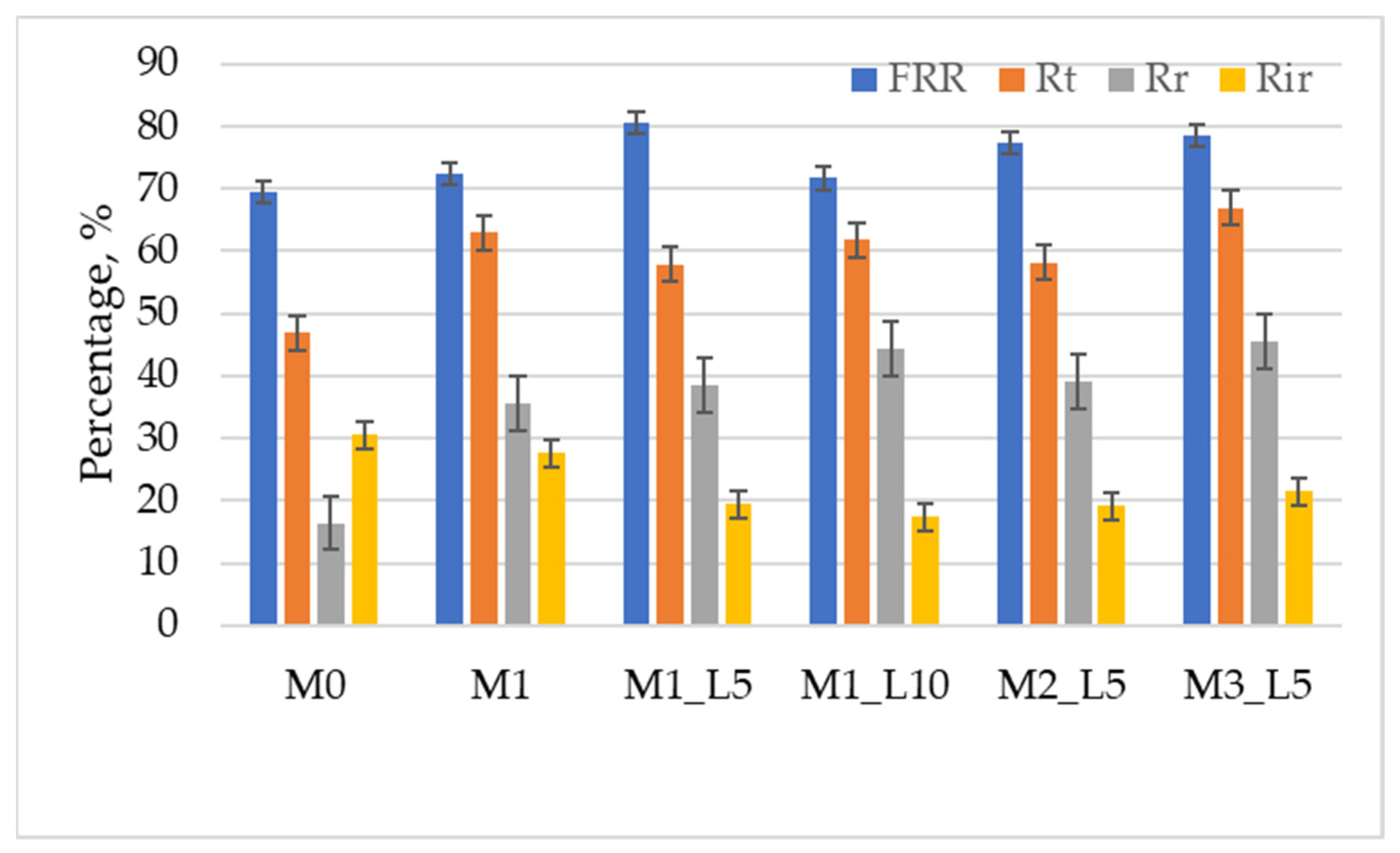
3.2. Antifouling Performance of Membranes
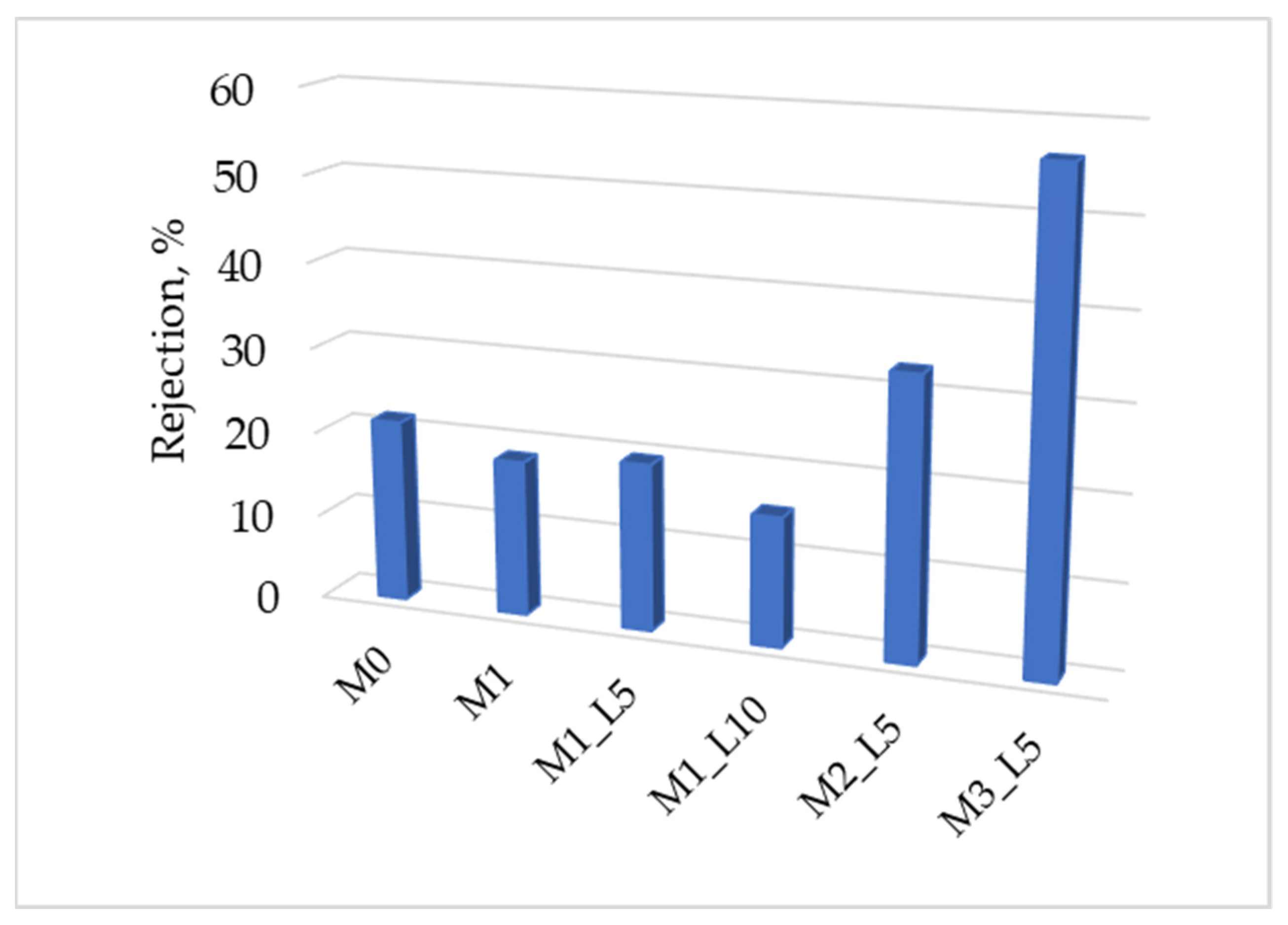
3.3. Hybrid Nanofiltration Membrane Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [Green Version]
- Paun, G.; Neagu, E.; Albu, C.; Savin, S.; Radu, G.L. In vitro evaluation of antidiabetic and anti-inflammatory activities of polyphenolic-rich extracts from anchusa officinalis and melilotus officinalis. ACS Omega 2020, 5, 13014–13022. [Google Scholar] [CrossRef]
- Vitor Pereira, D.T.; Barrales, F.M.; Pereira, E.; Viganó, J.; Iglesias, A.H.; Reyes Reyes, F.G.; Martínez, J. Phenolic compounds from passion fruit rinds using ultrasound-assisted pressurized liquid extraction and nanofiltration. J. Food Eng. 2022, 325, 110977. [Google Scholar] [CrossRef]
- Goosen, M.; Sablani, S.; Al-Hinai, H.; Al-Obeidani, S.; Al-Belushi, R.; Jackson, A. Fouling of Reverse Osmosis and Ultrafiltration Membranes: A Critical Review. Sep. Sci. Technol. 2005, 39, 2261–2297. [Google Scholar] [CrossRef]
- Weis, A.; Bird, M.R.; Nyström, M.; Wright, C. The influence of morphology, hydrophobicity and charge upon the long-term performance of ultrafiltration membranes fouled with spent sulphite liquor. Desalination 2005, 175, 73–85. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; León, G.; Miguel, B.; Gago, I.; Martínez, P.M. Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes. Membranes 2022, 12, 562. [Google Scholar] [CrossRef]
- Moradi, G.; Rahimi, M.; Zinadini, S. Novel antifouling nanofiltration PES membranes incorporating with C-KIT-6 for heavy metal ions removal. Polym. Adv. Technol. 2021, 32, 4110–4125. [Google Scholar] [CrossRef]
- Asatekin, A.; Olivetti, E.A.; Mayes, A.M. Fouling resistant, high flux nanofiltration membranes from polyacrylonitrile-graft-poly(ethylene oxide). J. Membr. Sci. 2009, 332, 6–12. [Google Scholar] [CrossRef]
- Nayak, V.; Cuhorka, J.; Mikulášek, P. Separation of Drugs by Commercial Nanofiltration Membranes and Their Modelling. Membranes 2022, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, N.A.; Ramadan, A.R.; Esawi, A.M.K. Studying the Effect of Shortening Carbon Nanotubes via Ball Milling on Cellulose Acetate Nanocomposite Membranes for Desalination Applications. Membranes 2022, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sachan, S.; Upadhyay, M. Preparation and application of SPPEES-TiO2 composite micro-porous UF membrane form refinery effluent treatment. Int. J. Environ. Res. Dev. 2014, 4, 147–152. [Google Scholar]
- Ashokkumar, M.; Sangeetha, D. Evaluation of polyphenylene ether ether sulfone/nanohydroxyapatite nanofiber composite as a biomaterial for hard tissue replacement. Prog. Biomater. 2013, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Paun, G.; Parvulescu, V.; Neagu, E.; Albu, C.; Ionita, L.; Maxim, M.E.; Munteanu, A.; Ciobanu, M.; Radu, G.L. Nanofiltration composite membranes based on KIT-6 and functionalized KIT-6 nanoparticles in a polymeric matrix with enhanced performances. Membranes 2021, 11, 300. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Z.; Ince, J.S.; Economy, J. Preparation of nanofiltration membranes from polyacrylonitrile ultrafiltration membranes. J. Membr. Sci. 2006, 286, 333–341. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 42042. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Feng, C.; Matsuura, T. The art of surface modification of synthetic polymeric membranes. J. Appl. Polym. Sci. 2010, 115, 855–895. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Membr. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Balta, S.; Darvishmanash, S.; Kim, J.; Van der Bruggen, B. Nanofiltration membranes enhanced with TiO2 nanoparticles: A comprehensive study. Desalin. Water Treat. 2011, 34, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.M.; Afshari, M.; Fazlali, A.R.; Koudzari Farahani, S.; Bandehali, S.; Van der Bruggen, B.; Bagheripour, E. Mixed matrix PES-based nanofiltration membrane decorated by (Fe3O4–polyvinylpyrrolidone) composite nanoparticles with intensified antifouling and separation characteristics. Chem. Eng. Res.Des. 2019, 147, 390–398. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Performance of a novel ZrO2/PES membrane for wastewater filtration. J. Membr. Sci. 2010, 352, 222–230. [Google Scholar] [CrossRef]
- Kim, S.; Marand, E. High permeability nano-composite membranes based on mesoporous MCM-41 nanoparticles in a polysulfone matrix. Micropor. Mesopor. Mat. 2008, 114, 129–136. [Google Scholar] [CrossRef]
- Jomekian, A.; Pakizeh, M.; Shafiee, A.R.; Mansoori, S.A.A. Fabrication or preparation and characterization of new modified MCM-41/PSF nanocomposite membrane coated by PDMS. Sep. Purif. Technol. 2011, 80, 556–565. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V. High selective mixed membranes based on mesoporous MCM-41 and MCM-41-NH2 particles in a polysulfone matrix. Front. Chem. 2019, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Vatankhah, G.; Aminshahidy, B. Investigation of the silica pore size effect on the performance of polysulfone (PSf) mixed matrix membranes (MMMs) for gas separation. J. Polym. Eng. 2021, 41, 627–636. [Google Scholar] [CrossRef]
- Shen, J.N.; Ruan, H.M.; Wu, L.G.; Gao, C.J. Preparation and characterization of PES-SiO2 organic-inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Al-Furaiji, M.H.; Kalash, K.R.; Kadhom, M.A.; Alsalhy, Q.F. Evaluation of polyethersulfone microfiltration membranes embedded with mcm-41 and SBA-15 particles for turbidity removal. Desalin. Water Treat. 2021, 215, 50–59. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Preparation, characterization and performance studies of polysulfone membranes using PVP as an additive. J. Membr. Sci. 2008, 315, 36–47. [Google Scholar] [CrossRef]
- Chen, X.; Tang, B.; Luo, J.; Wan, Y. Towards high-performance polysulfone membrane: The role of PSF-b-PEG copolymer additive. Micropor. Mesopor. Mat. 2017, 241, 355–365. [Google Scholar] [CrossRef]
- Slepička, P.; Michaljaničová, I.; Švorčík, V. Controlled biopolymer roughness induced by plasma and excimer laser treatment. Express Polym. Lett. 2013, 7, 950–959. [Google Scholar] [CrossRef]
- Mohammadtaheri, S.; Jaleh, B.; Mohazzab, B.F.; Eslamipanah, M.; Nasrollahzadeh, M.; Varma, R.S. Greener hydrophilicity improvement of polypropylene membrane by ArF excimer laser treatment. Surf. Coat. Technol. 2020, 399, 126198. [Google Scholar] [CrossRef]
- Sims, G.E.C.; Snape, T.J. A method for the estimation of polyethylene glycol in plasma protein fractions. Anal. Biochem. 1980, 107, 60–63. [Google Scholar] [CrossRef]
- Febriasari, A.; Ananto, A.H.; Suhartini, M.; Kartohardjono, S. Polysulfone–Polyvinyl Pyrrolidone Blend Polymer Composite Membranes for Batik Industrial Wastewater Treatment. Membranes 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2006, 101, 140–147. [Google Scholar] [CrossRef]
- Alecu, A.; Albu, C.; Litescu, C.S.; Eremia, S.A.V.; Radu, G.L. Phenolic and Anthocyanin Profile of Valea Calugareasca Red Wines by HPLC-PDA-MS and MALDI-TOF Analysis. Food Anal. Methods 2016, 9, 300–310. [Google Scholar] [CrossRef]
- Saffar, A.P.; Jaleh, B.; Parvin, P.; Wanichapichart, P.; Farshchi-Tabrizi, M. Surface Modification and Dielectric Response Investigation of Cellulose Acetate Membrane Treated by ArF Excimer Laser. OALib. J. 2014, 1, 1–9. [Google Scholar] [CrossRef]
- Tiaw, K.S.; Goh, S.W.; Hong, M.; Wang, Z.; Lan, B.; Teoh, S.H. Laser Surface Modification of Poly(ε-ca-prolactone) (PLC) Membrane for Tissue Engineering Applications. Biomaterials 2005, 26, 763–769. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.-Q.; Su, Y.-L.; Jiang, Z.-Y. Antifouling ultrafiltration membrane composed of polyethersulfone and sulfobetaine copolymer. J. Membr. Sci. 2006, 280, 343–350. [Google Scholar] [CrossRef]
- Purushothaman, M.; Arvind, V.; Saikia, K.; Vaidyanathan, V.K. Fabrication of highly permeable and anti-fouling performance of poly(ether ether sulfone) nanofiltration membranes modified with zinc oxide nanoparticles. Chemosphere 2022, 286, 131616. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, M.; Vandezande, P.; Van Dael, M.; Vloemans, S.; Noten, B.; Bongers, B.; Porto-Carrero, W.; Muñiz Unamunzaga, M.; Bulut, M.; Lemmens, B. Concentration of phenolic compounds from apple pomace extracts by nanofiltration at lab and pilot scale with a techno-economic assessment. J. Food Process Eng. 2018, 41, 12629. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of apple pomace by extraction of valuable compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef] [Green Version]
- Cassano, A.; Cabri, W.; Mombelli, G.; Peterlongo, F.; Giorno, L. Recovery of bioactive compounds from artichoke brines by nanofiltration. Food Bioprod. Process. 2016, 98, 257–265. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Avram, A.M.; Morin, P.; Brownmiller, C.; Howard, L.R.; Sengupta, A.; Wickramasinghe, S.R. Concentrations of polyphenols from blueberry pomace extract using nanofiltration. Food Bioprod. Process. 2017, 106, 91–101. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, C.; Hu, X. Preparation and properties of polysulfone/polyacrylonitrile blend membrane and its modification with hydrolysis. Desalin. Water Treat. 2013, 51, 3979–3987. [Google Scholar] [CrossRef]
- Nicolini, J.V.; Borges, C.P.; Ferraz, H.C. Selective rejection of ions and correlation with surface properties of nanofiltration membranes. Sep. Purif. Technol. 2016, 171, 238–247. [Google Scholar] [CrossRef]
| Membrane Code | Composition (wt. %) | |||
|---|---|---|---|---|
| PPEES | PAN | PVP | SBA-15 | |
| M0 | 20 | 0 | 1 | 0 |
| M1 | 13 | 7 | 1 | 1 |
| M1-L2 1 | 13 | 7 | 1 | 1 |
| M1-L5 2 | 13 | 7 | 1 | 1 |
| M1-L10 3 | 13 | 7 | 1 | 1 |
| M2-L5 1 | 15 | 5 | 1 | 1 |
| M3-L5 1 | 17 | 3 | 1 | 1 |
| Membrane Type | Permeate Flux a (Lm−2h−1) | Total Polyphenols | Flavonoids | ||
|---|---|---|---|---|---|
| Rejection (%) | Solute Permeability, B | Rejection (%) | Solute Permeability, B | ||
| Millipore | 10.7 ± 0.1 | 57.1 ± 0.4 | 7.21 ± 0.05 | 18.2 ± 0.1 | 43.09 ± 0.3 |
| M0 | 11.1 ± 0.1 | 68.8 ± 0.3 | 4.48 ± 0.03 | 21.4 ± 0.3 | 36.27 ± 0.2 |
| M1 | 11.7 ± 0.1 | 65.9 ± 0.5 | 4.93 ± 0.04 | 15.6 ± 0.1 | 56.16 ± 0.4 |
| M1-L5 | 40.8 ± 0.3 | 74.2 ± 0.6 | 4.89 ± 0.02 | 18.5 ± 0.2 | 68.99 ± 0.6 |
| M1-L10 | 106.1 ± 6.2 | 60.7 ± 0.4 | 23.76 ± 0.1 | 10.8 ± 0.1 | 267.95 ± 1.6 |
| M2-L5 | 25.2 ± 0.1 | 78.6 ± 0.7 | 4.25 ± 0.03 | 28.7 ± 0.2 | 48.70 ± 0.4 |
| M3-L5 | 23.7 ± 0.2 | 97.8 ± 0.9 | 0.41 ± 0.01 | 50.3 ± 0.4 | 18.33 ± 0.2 |
| Compound | Feed, μg/mL | Retentate M0, μg/mL | Retentate M1, μg/mL | Retentate M1-L5, μg/mL | Retentate M1-L10, μg/mL | Retentate M2-L5, μg/mL | Retentate M3-L5, μg/mL |
|---|---|---|---|---|---|---|---|
| Ellagic acid | 3.55 | 3.66 | 3.76 | 3.78 | 3.64 | 3.71 | 4.97 |
| Chlorogenic acid | 1.21 | 1.61 | 1.47 | 1.49 | 1.92 | 1.39 | 1.68 |
| (+) Catechin | 1.76 | 1.99 | 2.11 | 2.23 | 0.66 | 2.67 | 3.66 |
| Rutin | 0.58 | 0.59 | 0.56 | 0.65 | 0.50 | 1.10 | 1.27 |
| Quercetin-3-β-D-qlucoside | 1.69 | 2.15 | 1.81 | 2.61 | 1.67 | 2.77 | 3.26 |
| Quercitrin | 12.8 | 13.85 | 13.48 | 14.78 | 14.07 | 12.7 | 20.57 |
| Quercetol | 0.73 | 1.00 | 0.98 | 0.77 | 0.75 | 0.95 | 1.07 |
| Phloridzin | 2.65 | 2.69 | 3.13 | 3.08 | 2.90 | 2.84 | 4.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paun, G.; Neagu, E.; Parvulescu, V.; Anastasescu, M.; Petrescu, S.; Albu, C.; Nechifor, G.; Radu, G.L. New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15. Membranes 2022, 12, 689. https://doi.org/10.3390/membranes12070689
Paun G, Neagu E, Parvulescu V, Anastasescu M, Petrescu S, Albu C, Nechifor G, Radu GL. New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15. Membranes. 2022; 12(7):689. https://doi.org/10.3390/membranes12070689
Chicago/Turabian StylePaun, Gabriela, Elena Neagu, Viorica Parvulescu, Mihai Anastasescu, Simona Petrescu, Camelia Albu, Gheorghe Nechifor, and Gabriel Lucian Radu. 2022. "New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15" Membranes 12, no. 7: 689. https://doi.org/10.3390/membranes12070689
APA StylePaun, G., Neagu, E., Parvulescu, V., Anastasescu, M., Petrescu, S., Albu, C., Nechifor, G., & Radu, G. L. (2022). New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15. Membranes, 12(7), 689. https://doi.org/10.3390/membranes12070689









