Abstract
The purification of hydrogen (H2) has been a vital step in H2 production processes such as steam–methane reforming. By first-principle calculations, we revealed the potential applications of holey TMC6 (TM = Mo and W) membranes in H2 purification. The adsorption and diffusion behaviors of five gas molecules (including H2, N2, CO, CO2, and CH4) were compared on TMC6 membranes with different phases. Though the studied gas molecules show weak physisorption on the TMC6 membranes, the smaller pore size makes the gas molecules much more difficult to permeate into h-TMC6 rather than into s-TMC6. With suitable pore sizes, the s-TMC6 structures not only show an extremely low diffusion barrier (around 0.1 eV) and acceptable permeance capability for the H2 but also exhibit considerably high selectivity for both H2/CH4 and H2/CO2 (>1015), especially under relatively low temperature (150–250 K). Moreover, classical molecular dynamics simulations on the permeation process of a H2, CO2, and CH4 mixture also validated that s-TMC6 could effectively separate H2 from the gas mixture. Hence, the s-MoC6 and s-WC6 are predicted to be qualified H2 purification membranes, especially below room temperature.
1. Introduction
With the increasing environmental pollution issues and global energy crisis, more and more attention has been paid to green energy resources, particularly hydrogen (H2) energy [1,2]. Though lots of methods have been developed, steam–methane reforming is still the main approach for industrial production of H2 [3]. During the steam–methane reforming process, however, the mixture composed of H2, CO2, and CH4 is inevitable, which renders the purification of H2 highly crucial in hydrogen production. The membrane separation technology is one of the most widely accepted methods for H2 separation and purification [4]. Among the reported membranes, two-dimensional (2D) carbon-based membranes have been extensively studied due to their distinct advantages, e.g., low energy consumption and good cyclicity through physical interactions [5,6,7].
Material design by computational methods has been an effective tool to achieve novel 2D carbon-based ultrathin membranes for H2 separation and purification, especially for the ones with intrinsic pores. As one of the most well-known representatives, the porous graphitic carbon nitride (g-C3N4) monolayer has received a lot of attention for its potential as an effective gas separation membrane. Under room temperature, g-C3N4 could exhibit extremely high theoretical selectivity for H2/CH4 in the order of 1046 [8]. Moreover, further theoretical simulations indicated that g-C3N4 is also capable in helium (He) purification from both natural gas and noble gas molecules [9]. With the help of theoretical simulations, the porous C2N monolayer was reported to be suitable for He separation from other gases (Ne, CH4, CO2, etc.) [10]. Wang et al. investigated the diffusion properties of He, Ne, CO2, Ar, N2, CO, and CH4 through a porous monolayer covalent triazine-based framework (CTF) membrane. Calculation results demonstrate that the selectivity for He and H2 against common gas molecules (such as CO2, N2, CO, and CH4) is highly promising for practical applications [11]. Meng et al. theoretically explored the structural and mechanical properties of metal-free fused-ring polyphthalocyanine (H2PPc) and halogenated H2PPc (F-H2PPc and Cl-H2PPc) membranes. It was found that fluorination and chlorination can effectively tune the permeable pores. Particularly, F-H2PPc is fascinating as a separation membrane for H2 purification [12]. Recently, a series of 2D γ-C4X (X = O, S, or Se) membranes with intrinsic pores were theoretically designed, among which γ-C4O shows both low diffusion barriers (0.35 eV) and high permeance (5.0 × 10−7 mol m−2 s−1 Pa−1) for H2. Moreover, γ-C4O is highly promising as a H2 purification membrane from the H2/CH4 mixture with a selectivity of about 1019 [13]. The existing studies indicate that there are abundant possibilities for carbon-based ultrathin membranes with intrinsic pores. Therefore, carbon-based membranes with different pore sizes and termination on the pore edges are indispensable for H2 purification.
Besides the ones entirely composed of non-metal elements, 2D membranes composed of carbon and metal atoms may also play a vital role as gas separation membranes due to the incorporation of metal atoms. In the pioneering work by Li et al., a novel 2D transition metal carbide (h-TMC6, TM = Mo, W) structure was theoretically designed [14]. It was found that the crystal structure of h-TMC6 belongs to the hexagonal Kagome lattice. The stability of h-TMC6 was confirmed by molecular dynamics simulations and phonon spectra calculations. Later, Liu et al. reported other transition-metal carbides with the same composition of TMC6 (TM = Mo, W) but a tetragonal lattice [15], therefore being named s-TMC6. In general, the TMC6 monolayers show triple atomic layer structures with Mo/W atomic layers sandwiched between two carbon atomic layers, with TM atoms coordinated with six nearest neighboring C atoms. More importantly, in both h-TMC6 and s-TMC6, there are intrinsic pores surrounded by the TM atoms and carbon atoms, whose sizes are mainly determined by the lattice structures.
In this work, the capability of holey structures of TMC6 (M = Mo, W) membranes for H2 purification was theoretically explored, both square and hexagonal phases. By comparing the pore size and separation performance against H2 and other gas molecules (N2, CO, CO2, CH4), it was found that s-TMC6 is more promising for H2 purification, especially from H2/(CO2, CH4) mixtures below room temperature (150–250 K). Our work not only predicts the potential applications of the TMC6 membranes but also recommends the novel membrane materials for H2 purification under low temperature.
2. Computational Methods
The Vienna ab initio simulation package (VASP) [16,17] was used for the first-principle calculations with plane-wave basis set and the projector augmented-wave (PAW) [18] method. The Perdew–Burke–Ernzerhof (PBE) [19] functional was adopted, with a cutoff energy of 500 eV. The structural relaxations were considered to be converged until the change in total energy reaches 10−5 eV and the residual force per atom reaches 0.02 eV Å−1. To avoid the interaction between the neighboring periodic images, a vacuum slab around 2 nm was applied for all the structures. The Brillouin zone was sampled with Monkhorst–Pack [20] k-point grids of 11 × 11 × 1 and 5 × 5 × 1 for unit cell of h-TMC6 and s-TMC6, respectively. Furthermore, 4 × 4 × 1 k-point grids were adopted for the 3 × 3 supercell of h-TMC6, while 3 × 3 × 1 k-point grids were adopted for the 2 × 2 supercell of s-TMC6. For accurate description of weak van der Waals (vdW) interactions, Grimme’s dispersion correction (DFT–D3) was included during the adsorption-related calculations [21]. The climbing image nudged elastic band (CI-NEB) method [22] was used to search the minimum energy pathway (MEP) and confirm the transition state (TS) during the diffusion of gas molecules. The diffusion barriers (Eb) were calculated to evaluate the capability of gas molecules passing through the intrinsic pores in the TMC6 membranes by the following definition:
where the ETS and EIS denote the energy of transition state (TS) and initial state (IS), respectively. Additionally, the adsorption energies (Ead) of gas molecules on 2D TMC6 were calculated by
where , , and stand for the energy of adsorption system, isolated molecule, and 2D TMC6, respectively. Herein, a negative Ead value means that the adsorption is favorable as exothermic process.
The classical molecular dynamics (MD) simulations on permeation process of H2, CO2, and CH4 mixture were implemented by Forcite module available in the Materials Studio software package. Gas molecules were interspersed between the TMC6 membranes, and the initial condition was 250 K with a total simulation time of 5000 ps. The NVT ensemble and universal force field [23] were employed during the simulation with a time step of 1 fs.
3. Results and Discussion
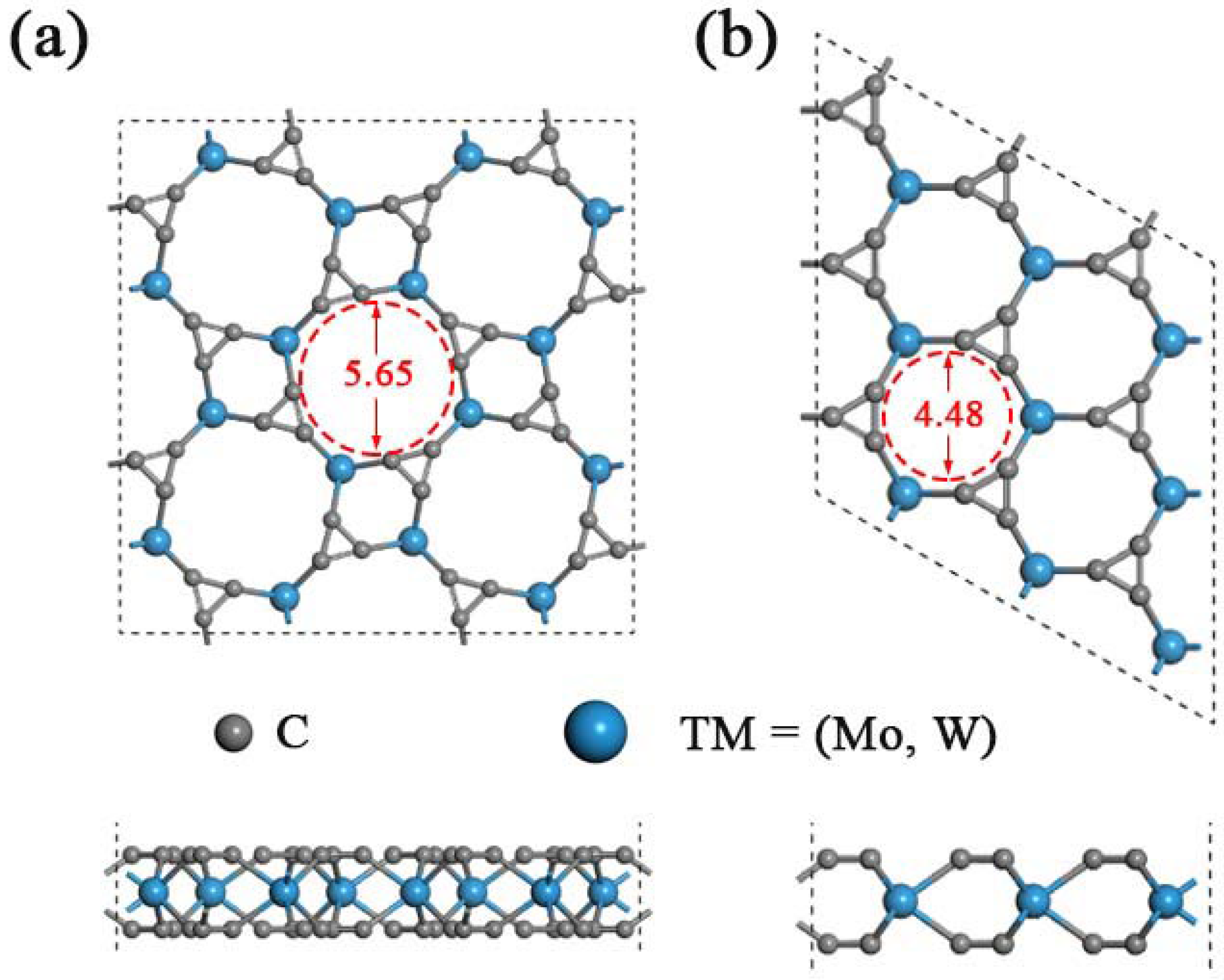
As predicted in previous work, the single-layered TMC6 (M = Mo, W) includes two different phases with holey structure, namely, the square phase (s-TMC6) and the hexagonal phase (h-TMC6). s-TMC6 shows P4/mbm symmetry with a lattice constant of 8.541 and 8.543 Å for s-MoC6 and s-WC6, respectively. There are 4 TM atoms and 24 carbon atoms in the unit cell of s-TMC6. Due to the extremely close lattice parameters, s-MoC6 and s-WC6 exhibit intrinsic pores with the same diameter of about 5.65 Å, which were obtained by directly measuring from the optimized atomic positions of carbon in the edge of pores. The intrinsic pores are composed of four TM atoms and eight C atoms, as displayed in Figure 1a. Differently, the h-TMC6 exhibits Pm2 symmetry with the lattice constant of 4.381 and 4.383 Å for h-MoC6 and h-WC6, respectively. The unit cell of h-TMC6 contains one TM atom and six carbon atoms. Due to the more compact atomic configurations of h-TMC6, their intrinsic pores have smaller sizes when compared with s-TMC6. As indicated in Figure 1b, the pores in h-TMC6 are composed of three TM atoms and six C atoms, whose diameters are about 4.48 Å.
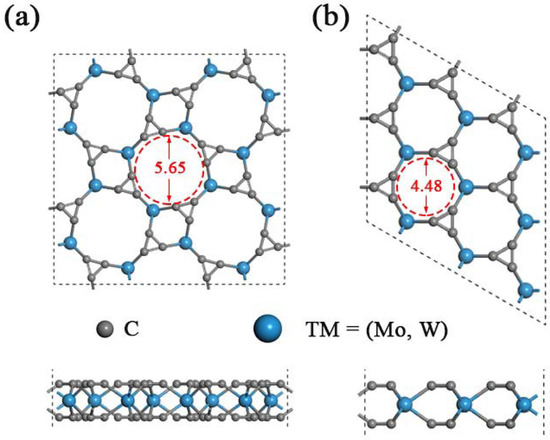
Figure 1.
Schematic illustrations of TMC6 (top view and side view) supercells: (a) Square transition-metal carbides s-TMC6; (b) hexagonal transition-metal carbides h-TMC6. The gray and blue balls represent C and TM atoms, respectively.
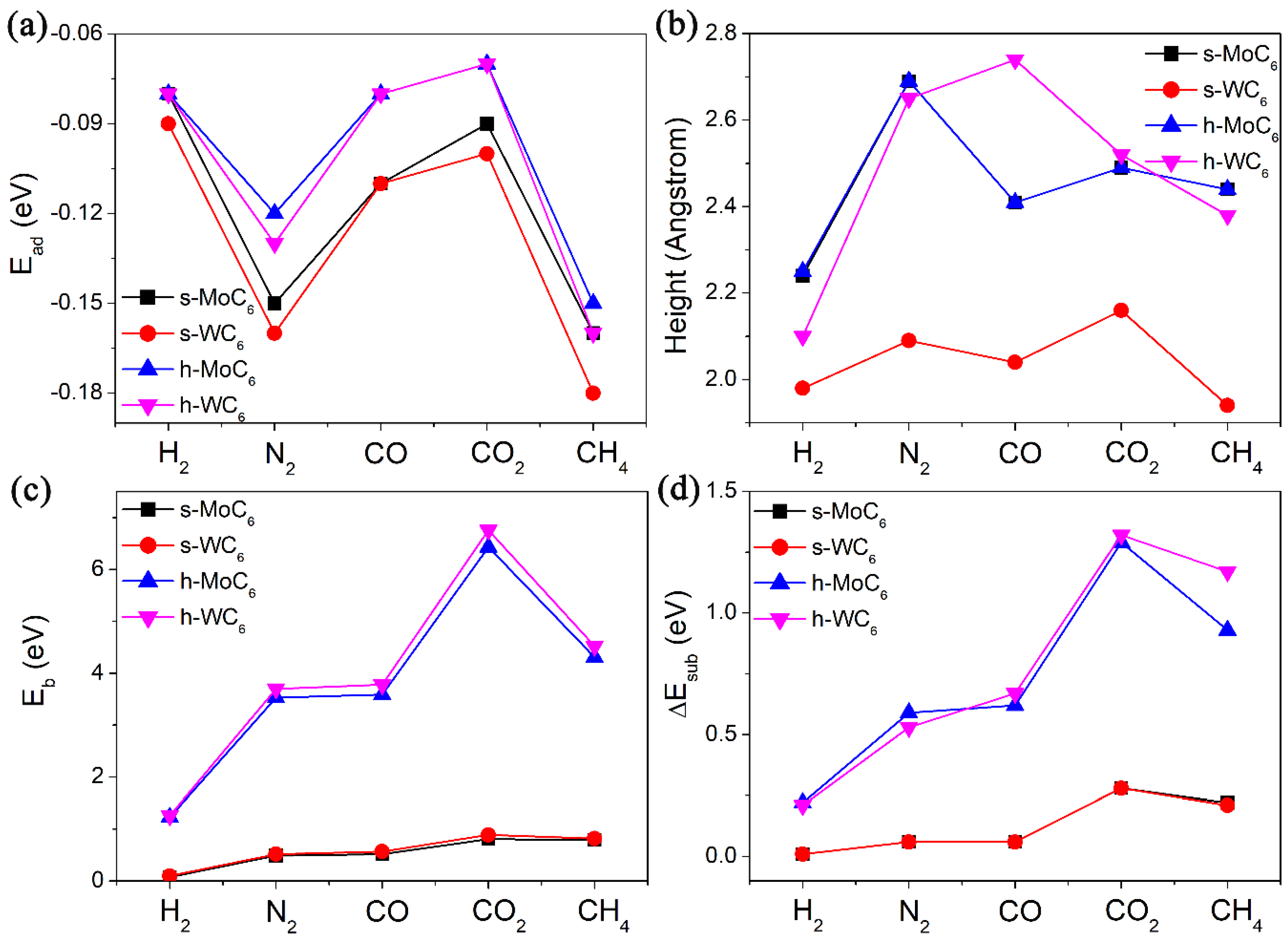
After the confirmation of pore structures in the 2D TMC6, we then tested their performance in gas adsorption and diffusion. In this work, five different kinds of gas molecules (containing H2, N2, CO, CO2, and CH4) were tested, which are the main components of the gas mixture in the steam–methane reforming process. All the adsorption configurations of gas molecules were fully relaxed. The adsorption energies (Ead), equilibrium adsorption heights (h), and diffusion barriers (Eb) are systematically summarized in Table 1. In the equilibrium configurations, the adsorption heights between the gas molecules and substrate are mostly in the range of 2.0–2.5 Å, along with adsorption energies within −0.18 eV, as shown in Figure 2a,b. The large adsorption heights and weak adsorption strength evidently indicate physisorption through van der Waals interaction between the gas molecules and TMC6 substrates. Generally speaking, the Ead of the same gas molecule on MoC6 and WC6 is almost the same, implying the negligible influence of TM atoms on the gas adsorption. Meanwhile, the Ead values of a gas molecule on s-TMC6 will be slightly smaller than those on h-TMC6. However, it is interesting to see that the diffusion of the same molecule on h-TMC6 will be much more difficult than that on s-TMC6 due to the much greater Eb values (see Figure 2c). Another finding is that for s-MoC6 and s-WC6, due to the relatively small Eb values, the difference between Eb of the same gas molecule on them is also very small (<0.1 eV). For h-MoC6 and h-WC6, a significant difference between Eb of the same gas molecule on them arises (up to 0.33 eV). Therefore, though the gas molecules have close adsorption interaction on the TMC6 membranes, their diffusion performance is highly distinguished.

Table 1.
The adsorption energies (Ead, eV), equilibrium adsorption heights (h, Å), diffusion barriers for the studied gases (H2, N2, CO, CO2, and CH4) passing through the intrinsic pore (Eb, eV) of 2D TMC6, energy differences (ΔEsub, eV) of the TMC6 membranes between free-standing state and TS without adsorbates, the bond lengths (l1 and l2, Å) of the gas molecules with free-standing state and TS.
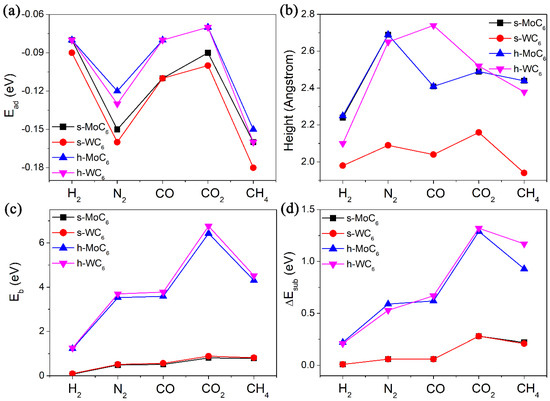
Figure 2.
(a) The adsorption energies (Ead, eV), (b) equilibrium adsorption heights (h, Å), (c) diffusion barriers for the studied gases (H2, N2, CO, CO2, and CH4) passing through the intrinsic pore (Eb, eV) of 2D TMC6, and (d) energy differences (ΔEsub, eV) of the TMC6 membranes between free-standing state and TS without adsorbates.
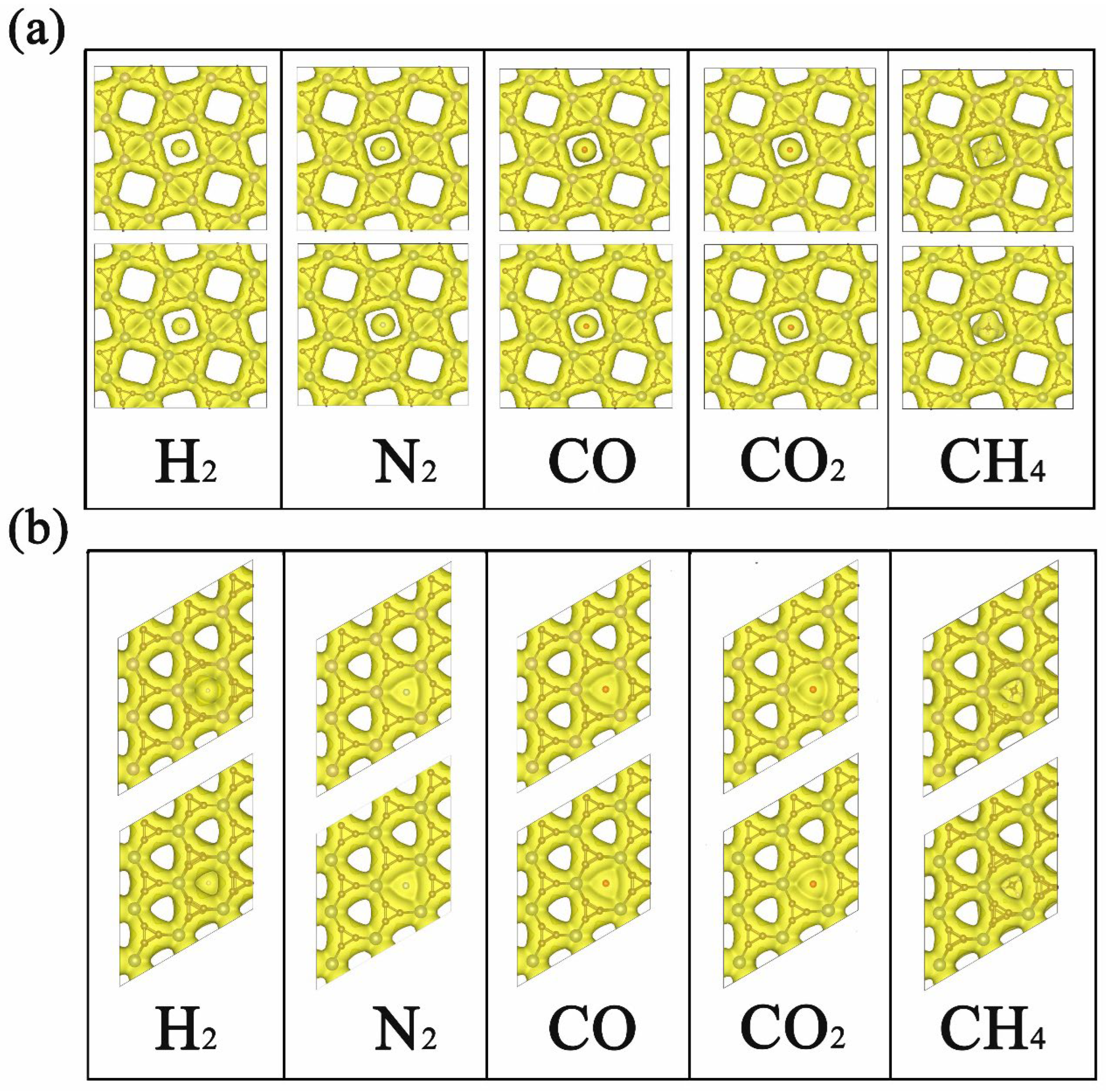
After the comparison of adsorption and diffusion performance of different gas molecules on 2D TMC6, we then turned to the microscopic insights. As displayed in Figure 3, the electron density isosurfaces of H2, N2, CO, CO2, and CH4 at their corresponding transition states are provided with the same isosurface value of 0.015 e Å−3. For s-TMC6, it is evident that the smaller pores are fully occupied by the electron density isosurfaces, and the unoccupied parts only appear in larger pores with square shapes. Meanwhile, for h-TMC6, the triangle shapes appear in the pores as unoccupied parts, whose area is significantly smaller than that in s-TMC6. As we can see, under the transition states, there is significant overlapping between the electron density distribution of h-TMC6 and gas molecules, while the overlapping between s-TMC6 and gas molecules is lower, especially for H2, N2, CO, and CO2. The overlapping of electron density distribution will cause significant electrostatic interactions. The electrostatic interaction plays a leading part during the permeation process because higher overlapping of electron density distribution corresponds to larger Eb values. In this work, the electrostatic interaction mainly originates from the different pore sizes of 2D TMC6. As a useful tool to quantitatively understand the selectivity of TMC6 against different gases, a comparison between the measured diameters of the cross section in the van der Waals (vdW) surface (Dc) of different gas molecules and the pore size of unoccupied vdW surface in 2D TMC6 was also performed. As measured, the unoccupied vdW diameters of pore in s-MoC6 and s-WC6 are 2.29 and 2.32 Å, significantly higher than those of g-C3N4 (about 1.70 Å) [8] which results in significantly smaller Eb values for gas molecules permeating g-C3N4 when compared with s-TMC6. As reported, the Dc of H2, N2, CO, CO2, and CH4 is 2.44, 3.20, 3.46, 3.44, and 3.78 Å, respectively. Therefore, the pore size of s-TMC6 is very close to the Dc of H2 but much smaller than those of N2, CO, CO2, and CH4. This finding means that s-TMC6 will have considerable selectivity against H2, attributed to the suitable pore size. Notably, though the pore size of s-WC6 is slightly larger than that of s-MoC6, it causes higher Eb for the same gas molecule. Hence, the metal species has relatively less influence on the diffusion of gas molecules, but it is not negligible. In the meantime, the unoccupied vdW diameters of pore in h-TMC6 are 0, which explains why h-TMC6 exhibits very high diffusion barriers for all the studied gas molecules.
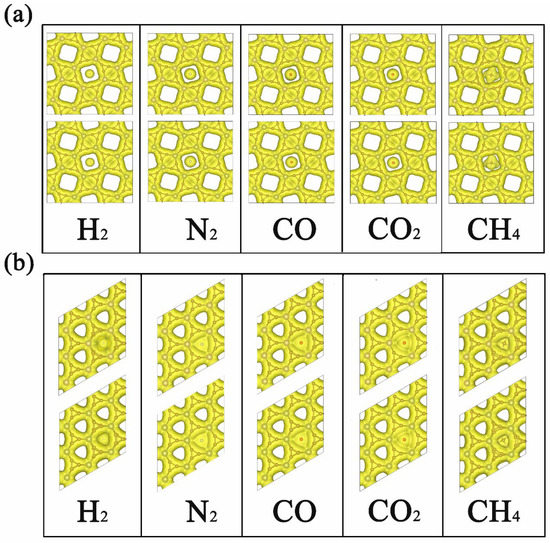
Figure 3.
Electron density isosurfaces of H2, N2, CO, CO2, and CH4 on (a) s-TMC6 and (b) h-TMC6 at their corresponding transition states (TSs). The upper panels mark MoC6, while the lower panels denote WC6. The isosurface value is 0.015 e Å−3.
In addition to the comparison between pore sizes, the steric deformation of 2D TMC6 caused by electrostatic repulsion was also checked. We measured the main bond lengths of different molecules in isolated state (l1) and TS (l2), that is, H-H bond length for H2, N-N bond length for N2, C-O bond length for CO and CO2, and C-H bond length for CH4, taking in consideration the non-polarized structures of CO2 and CH4. The comparison between l1 and l2 (seeing Table 1) indicates that the structural changes of the gas molecules under TS are negligible, except for CO and N2 on h-TMC6. Then, by removing the gas molecule in its TS structure and calculating the single-point energy (SPE) of the 2D TMC6, we can obtain the energy differences (ΔEsub) of the 2D TMC6 between free-standing state and TS without adsorbates, as listed in Table 1. It can be summarized that the ΔEsub is directly proportional to the corresponding Eb. For H2, N2, and CO, the electrostatic repulsion takes the proportion of 10–18% in the Eb, and the proportion increases to 20–30% for CO2 and CH4. It is revealed that CO2 and CH4 have more significant electrostatic repulsion with the pore edge of 2D-TMC6 when compared with other smaller gas molecules. Moreover, as indicated by Figure 2d, the ΔEsub in CO2 involved TS is obviously larger than that in the corresponding CH4 involved TS, implying stronger electrostatic repulsion of CO2 to the substrate. This finding explains the origin of the high Eb values of CO2 permeating into the pores of 2D TMC6.
To evaluate the gas separation performance quantitatively, we calculated the permeability and selectivity based on diffusion energy barriers. For the permeability, gas kinetic theory was employed under the ideal gas approximation. Herein, the permeance (P, in mol m−2 s−1 Pa−1) of the penetrated gases is determined by [12]
In Equation (1), Ap denotes the area of the pores, and the total number of collisions per unit time per area (N) is described as , where p, m, kB, and T stand for pressure, the mass of the molecule, the Boltzmann constant, and temperature, respectively. Hence, ApN could be viewed as the number of molecules that collide with the pore area per unit time. The portion of molecules with a speed large enough to overcome the diffusion barrier through the pore (i.e.,) is counted as the penetrant portion. Am, , and represent the total area of the membrane, the pressure difference (absolute value) between the two sides of the membrane, and the Maxwell velocity distribution, respectively. It is worth noting that Am is explicit for a 2D membrane, while Ap is related to the pore shapes and the effective radii (Reff) of the atoms at the pore rim. Here, Reff is calculated as, where RvdW denotes the vdW radius. The feed pressure and the pressure difference are Pa and Pa as provided in previous work [24].
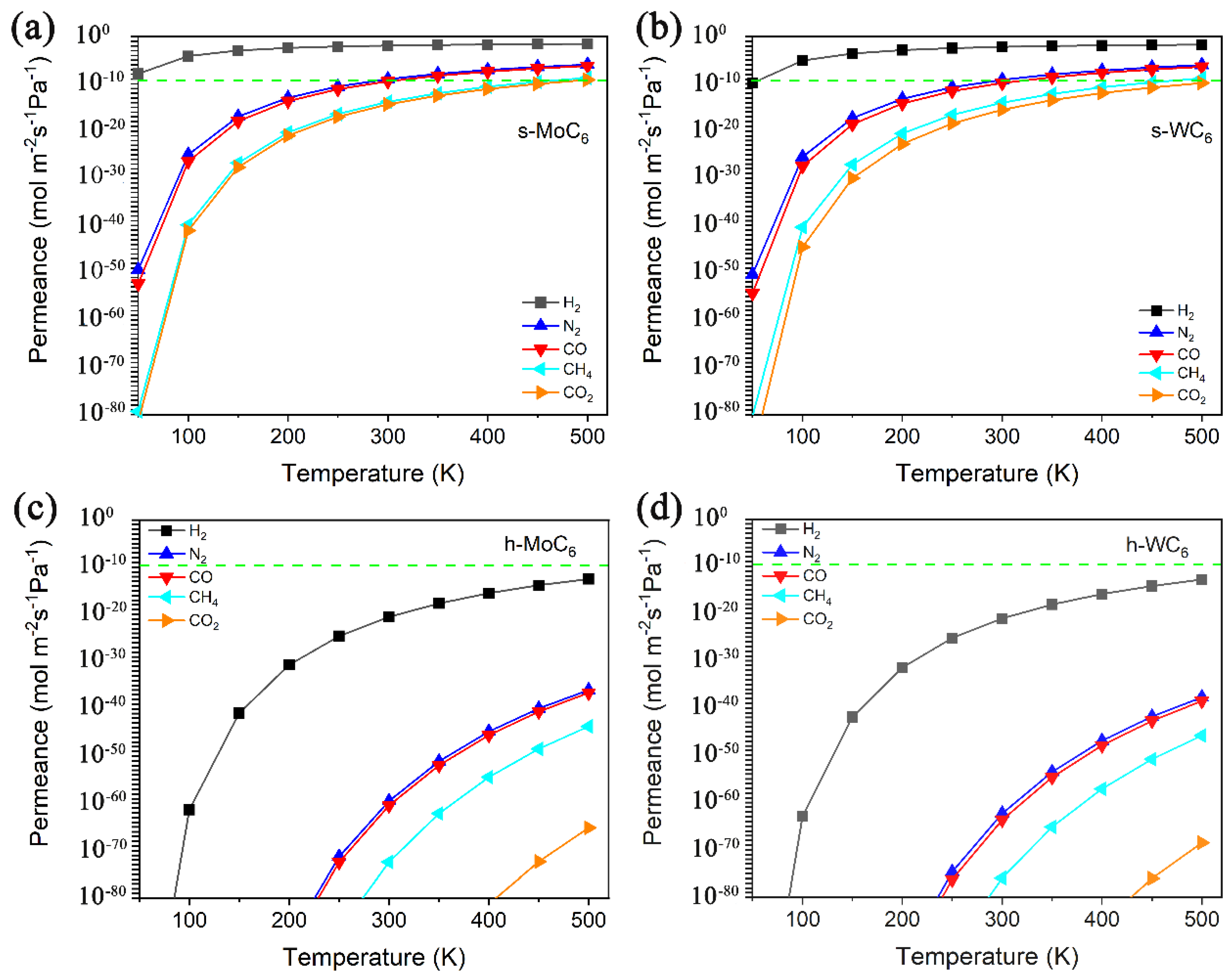
The calculated permeance vs. temperature for H2, N2, CO, CO2, and CH4 passing through the intrinsic pore of s-TMC6 and h-TMC6 are displayed in Figure 4. The green dashed line indicates the industrially acceptable permeance capability for gas separation (green dashed line, 6.7 × 10−9 mol m−2 s−1 Pa−1). Over the temperature range of 100–500 K, the permeance values for each gas molecule through s-TMC6 are evidently larger than those through h-TMC6. s-TMC6 shows good permeance capability against hydrogen, while h-TMC6 has low permeance capability for the different gas molecules. Specifically, in the temperature range of 100–300 K, the permeance values of H2 through s-TMC6 are always higher than the industrially acceptable one for gas separation. Meanwhile, the permeance values of CO2 and CH4 are always lower than the standard. For N2 and CO, the permeance values will not be higher than the standard until the temperature is higher than 300 K. It is suggested that s-TMC6 could be a potential H2 purification membrane to separate H2 from a mixture composed of N2, CO, CO2, or CH4 below room temperature (100–300 K).
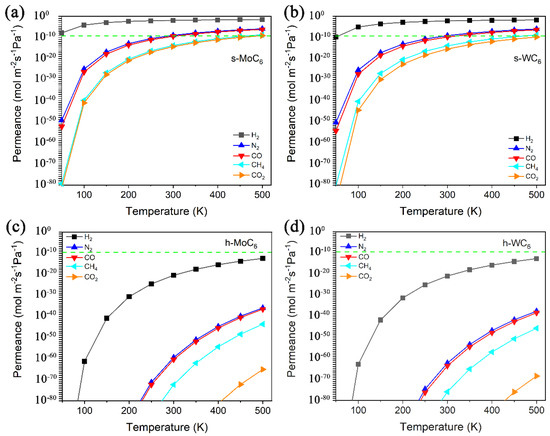
Figure 4.
Permeance versus temperature for H2, N2, CO, CO2, and CH4 passing through the intrinsic pore of (a) s-MoC6, (b) s-WC6, (c) h-MoC6, and (d) h-WC6.
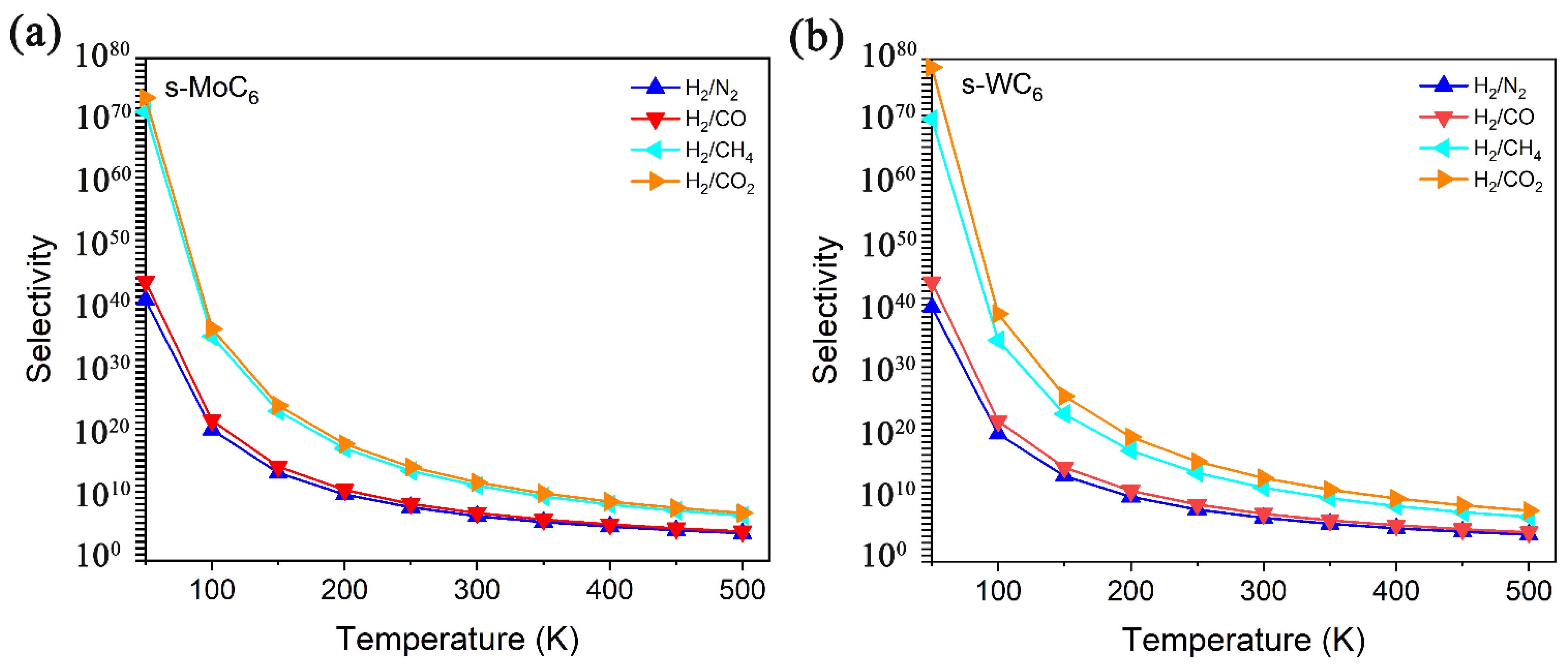
It is well accepted that the performance of a separation membrane is characterized by both the permeance capability and selectivity. Herein, the selectivity between two gas species is defined as the ratio of the diffusion rates, Sgas-1/gas-2 = Agas-1/Agas-2, which comes from the Arrhenius equation:
In Equation (2), the A0 means the diffusion prefactor that can be taken as the same value for all gases (1011 s−1). Based on the definition, the selectivity gradually decreases with the increase in temperature. In Figure 5, the selectivity versus temperature for H2/N2, H2/CO, H2/CH4, and H2/CO2 separation by s-MoC6 and s-WC6 are illustrated. It is easy to find that the s-TMC6 membranes mainly exhibit excellent selectivity for H2/CH4 and H2/CO2 due to the great differences in Eb between H2 and CH4/CO2. Different from other carbon-based 2D membranes, under room temperature (300 K), the selectivity of s-TMC6 for H2 against other gas molecules is not ideal enough, with 1011 and 1013 for H2/CH4 and H2/CO2, respectively. It is suggested that s-TMC6 could be applied as H2 purification membranes under low temperatures (100–250 K), with the selectivity of H2/(CO2, CH4) larger than 1015. As indicated by the selectivity, s-WC6 possesses better H2 purification ability when compared with s-MoC6.
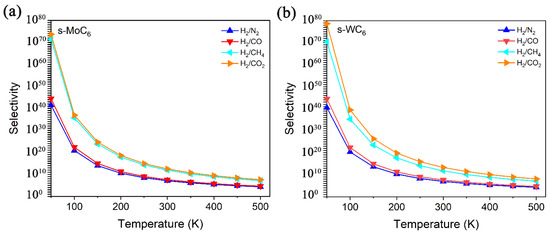
Figure 5.
Selectivity versus temperature for H2/N2, H2/CO, H2/CH4, and H2/CO2 separation by (a) s-MoC6, (b) s-WC6.
The convection–diffusion process is a problem in the field of fluid mechanics. Generally, the finite difference method (FDM) is a major method to treat with the convection–diffusion equation [25] which only applies to macroscopic systems. However, for microscopic systems such as the TMC6 membranes in this work, it is extremely difficult to perform quantitative calculations on convection and diffusion of gas flow. As a result, the MD simulations were widely adopted to visualize the time-dependence diffusion process of the molecules, as well as to assess some parameters such as gas diffusion coefficient and the permeated number of gas molecules [26,27,28,29]. Due to the lack of quantitative results, MD simulations are often applied to confirm the results of DFT calculations.
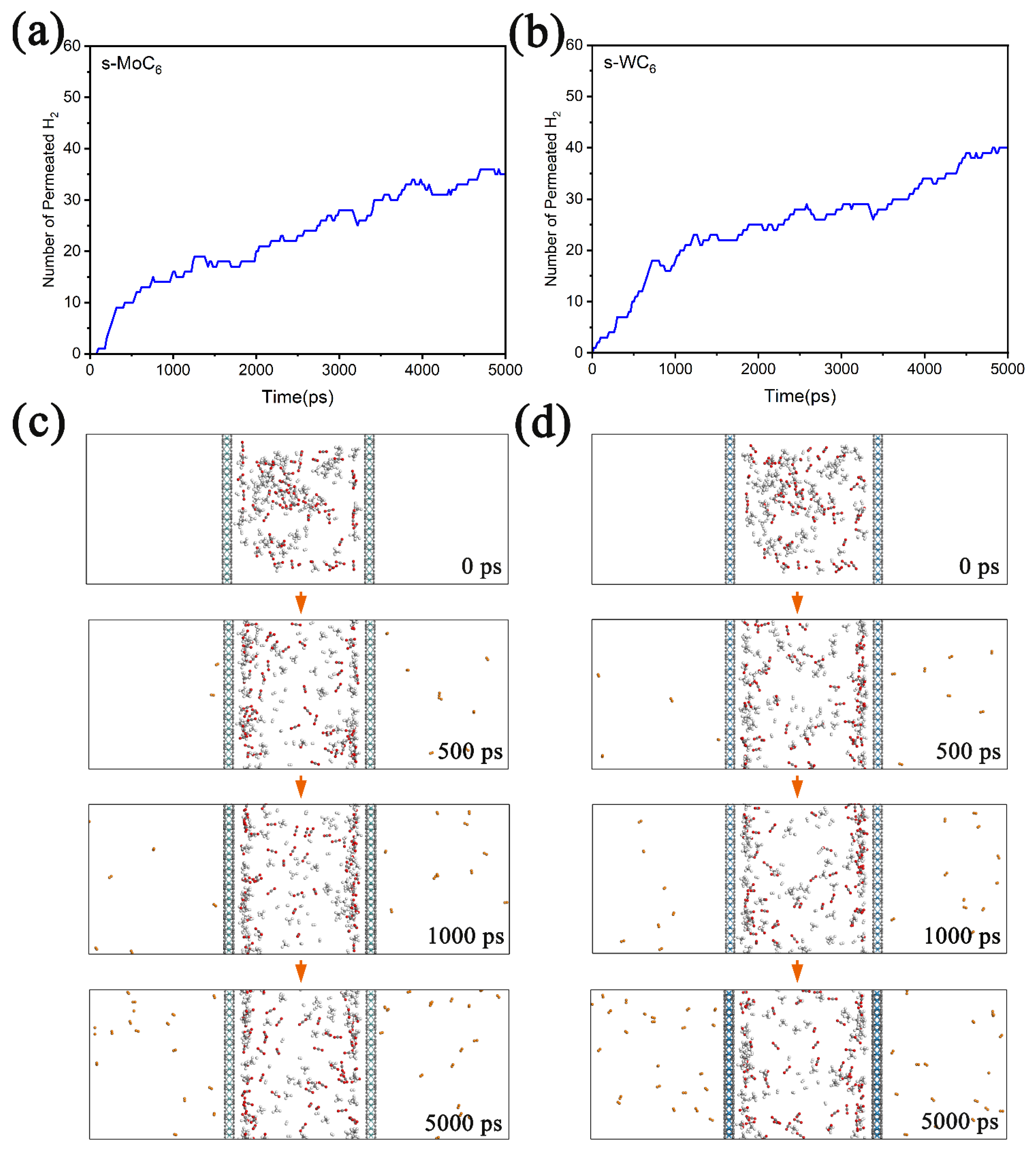
To better understand the transmembrane processes during gas separation, we performed classical MD simulations (at 250 K) on the permeation of gas mixture through the TMC6 membranes. During the MD simulations, a box of about 4 nm × 4 nm × 12 nm was employed; then, 60 H2, 60 CO2, and 60 CH4 molecules were randomly put into a chamber composed of two TMC6 membranes (the distance between the membranes was set as 4 nm). In Figure 6a,b, the number of permeated H2 molecules versus the simulation time for gas mixture in the chamber composed of s-TMC6 are depicted. It is found that after MD simulations of 5000 ps, there are 35 and 40 H2 molecules diffusing outside of the s-MoC6 and s-WC6 membranes, respectively. It is worth noting that the diffusion equilibrium was not achieved within 5000 ps; we can expect that after a long enough time, all the H2 molecules will diffuse through the s-TMC6 membranes into the product chamber. The snapshots of the gas mixture permeating through the s-TMC6 membranes at 0 ps, 500 ps, 1000 ps, and 5000 ps are given in Figure 6c,d. During the permeation process, the H2 molecules gradually migrate from the feed chamber to the product chamber. Moreover, none of CO2 or CH4 is found outside the membranes, clearly indicating that the s-TMC6 membranes could efficiently separate H2 molecules from the H2, CO2, and CH4 mixture. Therefore, the MD simulations could well simulate the transmembrane processes of H2 molecules, which greatly supports our first-principle calculation results.
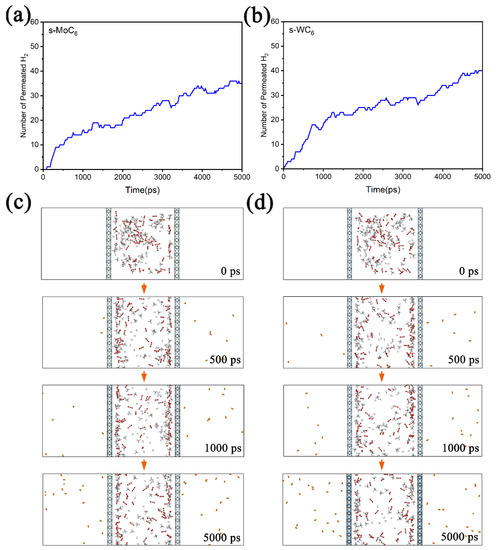
Figure 6.
The number of permeated H2 molecules versus the simulation time for gas mixture in chamber composed of (a) s-MoC6 membranes and (b) s-WC6 membranes. Corresponding snapshots of the diffusion process (from 0 to 5000 ps) of gas mixture permeating through (c) s-MoC6 membranes and (d) s-WC6 membranes. The H2 molecules that diffuse outside are displayed by different color.
In addition, it should be mentioned that we also performed the same MD simulations on h-TMC6. However, there are no gas molecules that run out from the chamber after 5000 ps at 250 K, indicating that h-TMC6 could not work as effective gas separation membranes.
4. Conclusions
In this work, the potential applications of holey TMC6 membranes in H2 purification were uncovered by comparative first-principle calculations. The adsorption and diffusion behaviors of five gas molecules (including H2, N2, CO, CO2, and CH4) were investigated on h-TMC6 and s-TMC6. All the studied gas molecules showed weak physisorption on the TMC6 membranes, but distinguishing diffusion barriers were obtained for different gas molecules across the pores of TMC6 membranes. The smaller pore size makes the gas molecules much more difficult to permeate into h-TMC6 rather than into s-TMC6. With suitable pore sizes, the s-TMC6 structures not only show an extremely low diffusion barrier and acceptable permeance for the H2 but also exhibit considerably high selectivity for H2/CH4 and H2/CO2, under relatively low temperature (150–250 K). Moreover, classical MD simulations on the permeation process also validated that the s-TMC6 could effectively separate H2 from the gas mixture composed of H2, CO2, and CH4. Therefore, s-MoC6 and s-WC6 are qualified as separation membranes for H2 purification from a gas mixture consisting of H2, CH4, and CO2 below room temperature.
Author Contributions
Conceptualization, H.D.; Formal analysis, J.X. and H.D.; Investigation, J.X. and C.N.; Resources, Z.S.; Validation, Z.S. and Q.L.; Writing—original draft preparation, J.X. and C.N.; Writing—review & editing, H.D., Z.S. and J.Y.; Supervision, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China [grant No. 51972150]; Start-up Foundation for Senior Talents of Jiangsu University [grant No. 21JDG041]. The APC was funded by the National Key Cultivation Engineering Project of Changshu Institute of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant No. 51972150) and the Start-up Foundation for Senior Talents of Jiangsu University (No. 21JDG041). H.D. acknowledges the support from the National Key Cultivation Engineering Project of Changshu Institute of Technology. The theoretical calculations were performed on the A6 Zone of Beijing Super Cloud Computing Center, supported by PARATERA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Unlu, D.; Hilmioglu, N.D. Application of aspen plus to renewable hydrogen production from glycerol by steam reforming. Int. J. Hydrogen Energy 2020, 45, 3509–3515. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Du, A.; Smith, S.C.; Zhu, Z.; Qiao, S.Z. H2 purification by functionalized graphdiyne—Role of nitrogen doping. J. Mater. Chem. A 2015, 3, 6767–6771. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, H.; Yu, X.; Ji, Y.; Hou, T.; Li, Y. Two-dimensional porous polyphthalocyanine (PPc) as an efficient gas-separation membrane for ammonia synthesis. Curr. Appl. Phys. 2017, 17, 1765–1770. [Google Scholar] [CrossRef]
- Wang, S.; Dai, S.; Jiang, D.-E. Continuously Tunable Pore Size for Gas Separation via a Bilayer Nanoporous Graphene Membrane. ACS Appl. Nano Mater. 2018, 2, 379–384. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, H.; Lin, H.; Zhang, L.; Hou, T.; Li, Y. Heptazine-based graphitic carbon nitride as an effective hydrogen purification membrane. RSC Adv. 2016, 6, 52377–52383. [Google Scholar] [CrossRef]
- Li, F.; Qu, Y.; Zhao, M. Efficient helium separation of graphitic carbon nitride membrane. Carbon 2015, 95, 51–57. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, Q.; Li, X.; Wu, T.; Jin, Y.; Xing, W. C2N: An excellent two-dimensional monolayer membrane for He separation. J. Mater. Chem. A 2015, 3, 21351–21356. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Yang, Q.; Zhong, C. Two-Dimensional Covalent Triazine Framework Membrane for Helium Separation and Hydrogen Purification. ACS Appl. Mater. Interfaces 2016, 8, 8694–8701. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhang, Y.; Shi, Q.; Liu, Y.; Du, A.; Lu, R. A remarkable two-dimensional membrane for multifunctional gas separation: Halogenated metal-free fused-ring polyphthalocyanine. Phys. Chem. Chem. Phys. 2018, 20, 18931–18937. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhang, Y.; Wang, J.; Gao, H.; Xiao, C.; Meng, Z.; Dong, H. Theoretically designed two-dimensional gamma-C4O as an effective gas separation membrane for hydrogen purification. Phys. Chem. Chem. Phys. 2020, 22, 19492–19501. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Ma, Y.; Sun, Q.; Wei, W.; Huang, B. Exotic quantum spin Hall effect and anisotropic spin splitting in carbon based TMC 6 (TM = Mo, W) kagome monolayers. Carbon 2016, 109, 788–794. [Google Scholar] [CrossRef]
- Liu, P.F.; Wu, Y.; Bo, T.; Hou, L.; Xu, J.; Zhang, H.J.; Wang, B.T. Square transition-metal carbides MC6 (M = Mo, W) as stable two-dimensional Dirac cone materials. Phys. Chem. Chem. Phys. 2018, 20, 732–737. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Blankenburg, S.; Bieri, M.; Fasel, R.; Müllen, K.; Pignedoli, C.A.; Passerone, D. Porous Graphene as an Atmospheric Nanofilter. Small 2010, 6, 2266–2271. [Google Scholar] [CrossRef]
- Li, C. Finite Difference Method for Convection-Diffusion Equation. In Computing and Data Science; Cao, W., Ozcan, A., Xie, H., Guan, B., Eds.; Springer Nature: Singapore, 2021; pp. 193–203. [Google Scholar]
- Wang, M.; Wang, Z.; Zhou, S.; Wang, J.; Liu, S.; Wei, S.; Guo, W.; Lu, X. Strain-controlled carbon nitride: A continuously tunable membrane for gas separation. Appl. Surf. Sci. 2020, 506, 144675. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, L.; He, D.; Guo, T.; Li, X.; Chang, X.; Xue, Q. Two-dimensional graphene oxide membrane for H2/CH4 separation: Insights from molecular dynamics simulations. Int. J. Hydrogen Energy 2017, 42, 30653–30660. [Google Scholar] [CrossRef]
- Jiang, C.; Hou, Y.; Wang, N.; Li, L.; Lin, L.; Niu, Q.J. Propylene/propane separation by porous graphene membrane: Molecular dynamic simulation and first-principle calculation. J. Taiwan Inst. Chem. Eng. 2017, 78, 477–484. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).