Regeneration of Exhausted Palladium-Based Membranes: Recycling Process and Economics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recycling Treatment
2.1.1. Samples Main Characteristics
2.1.2. Leaching Treatment
2.2. Solid Residues Treatment
3. Results and Discussion
3.1. Leaching Results
3.2. Solid Residues Treatment Results
3.3. Effect of Operative Conditions on Leaching Yields
3.4. Characterization of Treated Samples
3.5. Process Transferability on Metal-Supported Membranes
- -
- Nitric acid concentration: 4 M;
- -
- Temperature: 60 °C;
- -
- Reaction time: 4.5 h;
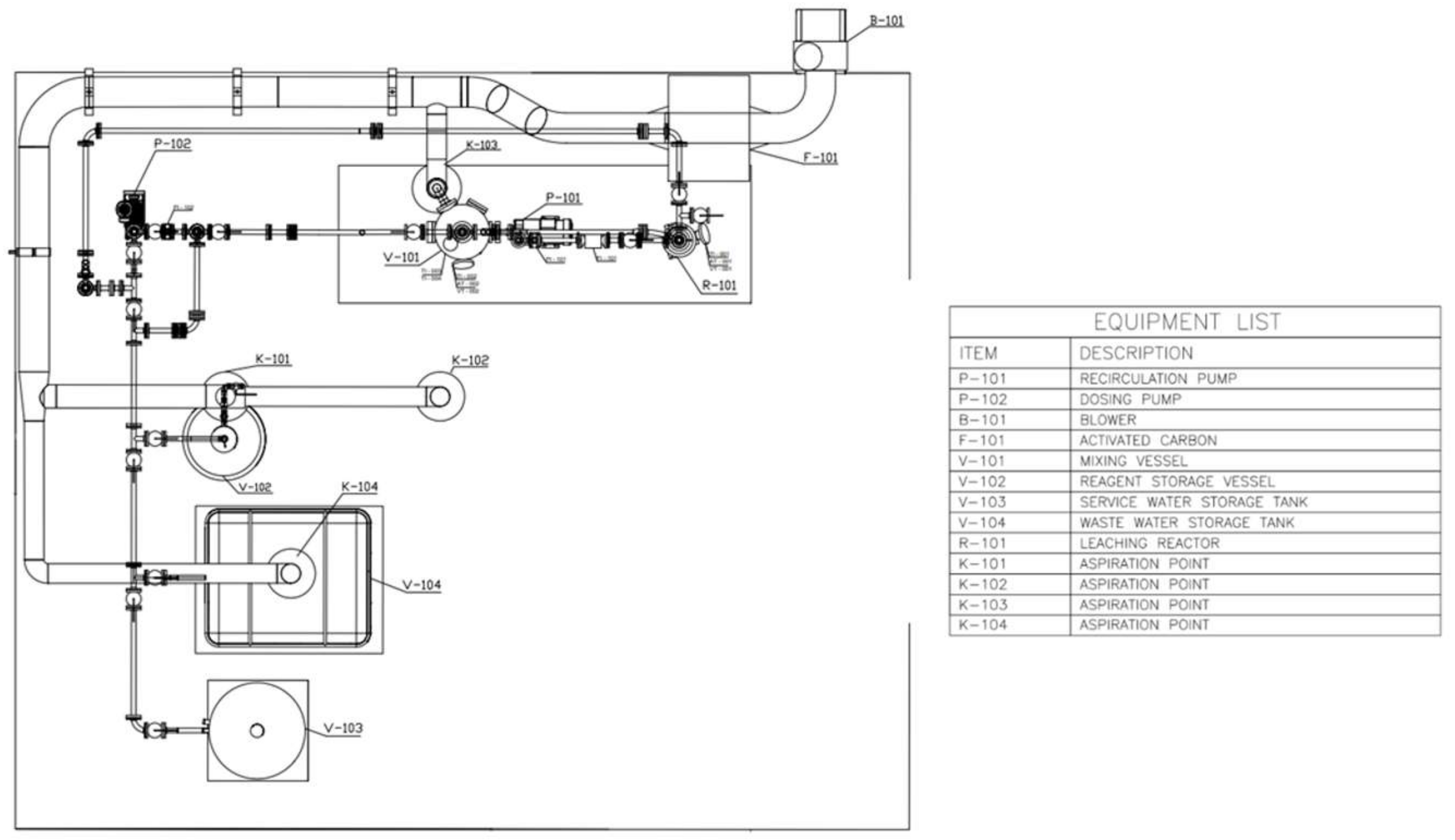
4. Prototype Plant and Process Scale-Up
5. Economic Analysis
6. Conclusions
- Leaching agent: nitric acid 3 M;
- Temperature: 60 °C;
- Reaction time: 3.5 h;
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EUROSTAT: Energy Statistics—An Overview. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Energy_statistics_-_an_overview (accessed on 13 June 2022).
- Our World in Data, CO₂ Emissions Dataset: Our Sources and Methods. Available online: https://ourworldindata.org/co2-dataset-sources (accessed on 13 June 2022).
- E-Education, Products of Combustion. Available online: https://www.e-education.psu.edu/egee102/node/1951 (accessed on 13 June 2022).
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. Post-combustion CO2 capture by coupling [emim] cation based ionic liquids with a membrane contactor; Pseudo-steady-state approach. Int. J. Greenh. Gas Control 2020, 99, 103076. [Google Scholar]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Gomez-Coma, L.; Garea, A.; Rouch, J.; Savart, T.; Lahitte, J.-F.; Remigy, J.-C.; Irabien, A. Membrane modules for CO2 capture based on PVDF hollow fibers with ionic liquids immobilized. J. Membr. Sci. 2016, 498, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-Combustion CO2 Capture Technologies from Coal-Fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Sarioglan, S. Recovery of Palladium from Spent Activated Carbon-supported Palladium Catalysts. Platin. Met. Rev. 2013, 57, 289–296. [Google Scholar] [CrossRef]
- Barakat, M.; Mahmoud, M.; Mahrous, Y. Recovery and separation of palladium from spent catalyst. Appl. Catal. A Gen. 2006, 301, 182–186. [Google Scholar] [CrossRef]
- De Aberasturi, D.J.; Pinedo, R.; De Larramendi, I.R.; De Larramendi, J.R.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Khaleghi, A.; Ghader, S.; Afzali, D. Ag recovery from copper anode slime by acid leaching at atmospheric pressure to synthesize silver nanoparticles. Int. J. Min. Sci. 2013, 24, 251–257. [Google Scholar] [CrossRef]
- Muscetta, M.; Minichino, N.; Marotta, R.; Andreozzi, R.; Di Somma, I. Zero-valent palladium dissolution using NaCl/CuCl2 solutions. J. Hazard. Mater. 2021, 404, 124184. [Google Scholar] [CrossRef]
- Behnamfard, A.; Salarirad, M.M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Chieng, P. Recovery of Silver from Lead/Zinc Flotation Tailings by Thiosulfate Leaching. Master’s Thesis, School of Engineering, The University of Queensland, St Lucia, QL, USA, 2006. [Google Scholar]
- Li, Y.; Ding, W.; Jin, X.; Yu, J.; Hu, X.; Huang, Y. Toward extensive application of Pd/ceramic membranes for hydrogen separation: A case study on membrane recycling and reuse in the fabrication of new membranes. Int. J. Hydrogen Energy 2014, 40, 3528–3537. [Google Scholar] [CrossRef]
- Hu, X. Toward low-cost Pd/ceramic composite membranes for hydrogen separation: A case study on reuse of the recycled porous Al2O3 substrates in membrane fabrication. Int. J. Hydrogen Energy 2011, 36, 15794–15802. [Google Scholar] [CrossRef]
- Toro, L.; Pagnanelli, F.; Moscardini, E.; Baldassari, L.M.; Altimari, P.; Palo, E.; Salladini, A.; Iaquaniello, G.; Vegliò, F.; Zueva, S.; et al. Process for Recovery and Recycling of Materials Constituting Membranes for Separation of Hydrogen. European Patent WO 2017/017647A1, 2 February 2017. [Google Scholar]
- Arratibel, A.; Tanaka, A.P.; Laso, I.; Annaland, M.V.S. Development of Pd-based double-skinned membranes for hydrogen production in fluidized bed membrane reactors. J. Membr. Sci. 2018, 550, 536–544. [Google Scholar] [CrossRef]
- Arratibel, A.; Medrano, J.A.; Melendez, J.; Tanaka, D.A.P.; Annaland, M.V.S.; Gallucci, F. Attrition-resistant membranes for fluidized-bed membrane reactors: Double-skin membranes. J. Membr. Sci. 2018, 563, 419–426. [Google Scholar] [CrossRef]


| Parameter | MEMBR1 | MEMBR2 |
|---|---|---|
| Ceramic support | α-Al2O3 | α-Al2O3 |
| Pore size | 100 nm | 100 nm |
| Interdiffusion barrier | No | No |
| Selective layer | Pd/Ag (~4–5%wt Ag) | Pd/Ag (~4–5%wt Ag) |
| Thickness selective layer | 4–5 μm | 4–5 μm |
| Overall length | 22 cm | 25.5 cm |
| Outer diameter | 14.6 mm | 14.3 mm |
| SAMPLE | Size (d × h, mm) | Weight (g) | HNO3 (M) | Temperature (°C) | Time (h) |
|---|---|---|---|---|---|
| M1#2 | 1.46 × 0.32 | 1.029 | 3 | 50 | 3.5 |
| M1#3A | 1.46 × 0.92 | 2.848 | |||
| M1#3B | 1.46 × 0.86 | 2.760 | |||
| M1#1 | 1.46 × 0.31 | 1.018 | 3 | 60 | 3.5 |
| M1#4A | 1.46 × 0.88 | 2.693 | |||
| M1#4B | 1.46 × 0.92 | 2.758 | |||
| M1#5A | 1.46 × 0.93 | 3.049 | 3 | 60 | 5 |
| M1#5B | 1.46 × 0.90 | 2.767 | |||
| M1#6A | 1.46 × 0.88 | 2.556 | 4 | 60 | 7 |
| M1#6B | 1.46 × 0.88 | 2.545 | |||
| M2#1A | 1.43 × 0.90 | 2.882 | 3 | 60 | 7 |
| M2#1B | 1.43 × 0.90 | 2.851 |
| SAMPLE M1 | |
| Length (cm) | 11.4 |
| Weight (g) | 37.54 |
| SAMPLE M2 | |
| Length (cm) | 11.8 |
| Weight (g) | 38.57 |
| Leaching Conditions | |
| Reagent | HNO3 (3 M) |
| Temperature (°C) | 60 °C |
| Solution volume (mL) | M1: 1036 M2: 1072 |
| Stirrer speed (rpm) | 300 |
| Parameter | Type/Value |
|---|---|
| Reagent | Aqua regia |
| Temperature (°C) | 60 |
| Solution Volume (mL) | 50 |
| Test duration (h) | 3 |
| SAMPLE | Pd (mg/g) | Ag (mg/g) |
|---|---|---|
| M1#1 | 2.98 | 0.21 |
| M1#2 | 3.26 | 0.16 |
| M1#3A | 2.80 | 0.18 |
| M1#3B | 2.90 | 0.19 |
| M1#4A | 3.01 | 0.19 |
| M1#4B | 2.93 | 0.17 |
| M1#5A | 3.28 | 0.19 |
| M1#5B | 3.23 | 0.16 |
| M1#6A | 3.04 | 0.19 |
| M1#6B | 3.13 | 0.22 |
| M2#1A | 2.92 | 0.09 |
| M2#1B | 2.79 | 0.15 |
| SAMPLE | Pd (mg/g) | Ag (mg/g) |
|---|---|---|
| M1 | 2.67 | 0.14 |
| M2 | 2.87 | 0.12 |
| SAMPLE | Reagent | Pd (mg/g) |
|---|---|---|
| M1#1 | Aqua regia | 0.42 |
| M1#2 | Aqua regia | 0.04 |
| M1#3A | Aqua regia | 0.41 |
| M1#3B | Aqua regia | 0.39 |
| M1#4A | Aqua regia | 0.26 |
| M1#4B | Aqua regia | 0.35 |
| M1#5A | Aqua regia | 0.20 |
| M1#5B | Aqua regia | 0.32 |
| M1#6A | Aqua regia | 0.25 |
| M1#6B | Aqua regia | 0.29 |
| M2#1A | Aqua regia | 0.18 |
| M2#1B | Aqua regia | 0.29 |
| SAMPLE | Pd (mg/g) | Ag (mg/g) | Alloy (% Ag) |
|---|---|---|---|
| M1#1 | 3.39 | 0.21 | 5.77 |
| M1#2 | 3.29 | 0.16 | 4.69 |
| M1#3A | 3.22 | 0.18 | 5.29 |
| M1#3B | 3.29 | 0.19 | 5.41 |
| M1#4A | 3.27 | 0.19 | 5.51 |
| M1#4B | 3.28 | 0.17 | 4.87 |
| M1#5A | 3.48 | 0.19 | 5.12 |
| M1#5B | 3.54 | 0.16 | 4.21 |
| M1#6A | 3.29 | 0.19 | 5.53 |
| M1#6B | 3.42 | 0.22 | 6.03 |
| M2#1A | 3.10 | 0.09 | 2.89 |
| M2#1B | 3.08 | 0.15 | 4.66 |
| SAMPLE | Pd (mg/g) | Ag (mg/g) | Alloy (% Ag) |
|---|---|---|---|
| MEMBR1 | 3.35 | 0.18 | 5.23 |
| MEMBR2 | 3.09 | 0.12 | 3.78 |
| SAMPLE | Method | Aluminium Dissolution (mg/g) |
|---|---|---|
| M1#1 | Aqua regia, 60 °C, 3 h | 0.12 |
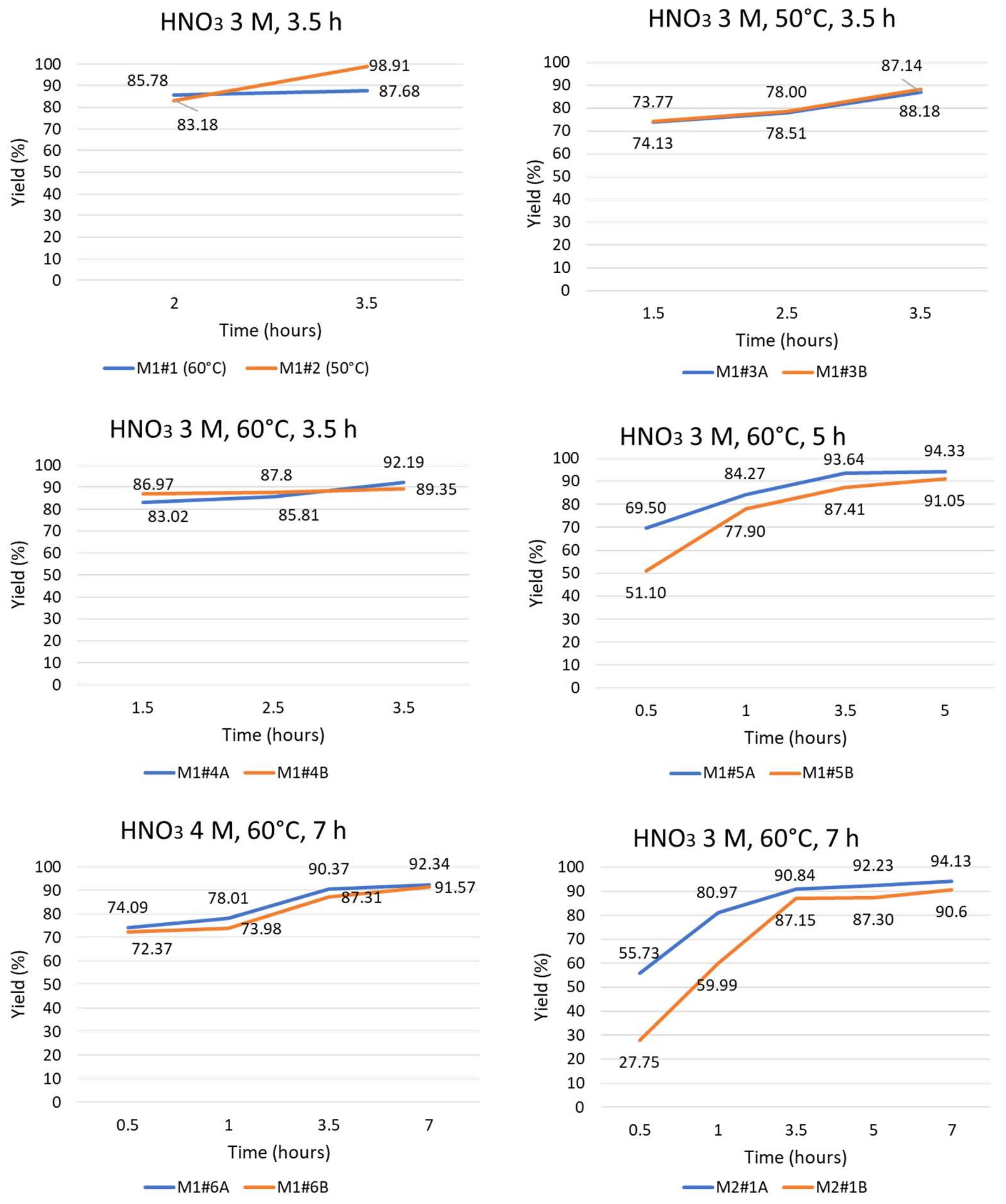
| SAMPLE | HNO3 (M) | Temperature (°C) | Time (h) | Pd Yield (%) |
|---|---|---|---|---|
| M1#2 | 3 | 50 | 3.5 | 98.91 |
| M1#3A | 87.14 | |||
| M1#3B | 88.18 | |||
| M1#1 | 3 | 60 | 3.5 | 87.68 |
| M1#4A | 92.19 | |||
| M1#4B | 89.35 | |||
| M1#5A | 3 | 60 | 5 | 94.33 |
| M1#5B | 91.05 | |||
| M1#6A | 4 | 60 | 7 | 92.34 |
| M1#6B | 91.57 | |||
| M2#1A | 3 | 60 | 7 | 94.1 |
| M2#1B | 90.6 |
| SAMPLE M1 | ||
| Time | Pd (mg/g) | Ag (mg/g) |
| 0.5 h | 1.44 | 0.09 |
| 1 h | 2.13 | 0.12 |
| 3.5 h | 2.67 | 0.14 |
| SAMPLE M2 | ||
| Time | Pd (mg/g) | Ag (mg/g) |
| 0.5 h | 1.20 | 0.07 |
| 1 h | 2.53 | 0.11 |
| 3.5 h | 2.87 | 0.12 |
| SAMPLE | Ra (µm) | Rt (µm) | N2 Permeance (mol m−2 s−1 Pa−1) |
|---|---|---|---|
| M1 | 0.35 ± 0.04 | 3.39 ± 1.41 | 1.29·10−5 |
| M2 | 0.31 ± 0.02 | 2.83 ± 1.49 | 1.49·10−5 |
| α-Al2O3 * | 0.52 ± 0.12 | 6.49 ± 2.75 | >1·10−5 |
| SAMPLE | HNO3 (M) | Temperature (°C) | Time (h) | Pd Yield (%) |
|---|---|---|---|---|
| M3#1A | 4 | 60 | 4.5 | 97.72 |
| M3#1B | 97.52 | |||
| M3#2A | 4 | 60 | 4.5 | 97.29 |
| M3#2B | 96.52 | |||
| M3#3A | 3.5 | 60 | 7 | 97.73 |
| M3#3B | 97.67 | |||
| M3#4A | 3 | 60 | 7 | 97.15 |
| M3#4B | 92.43 |
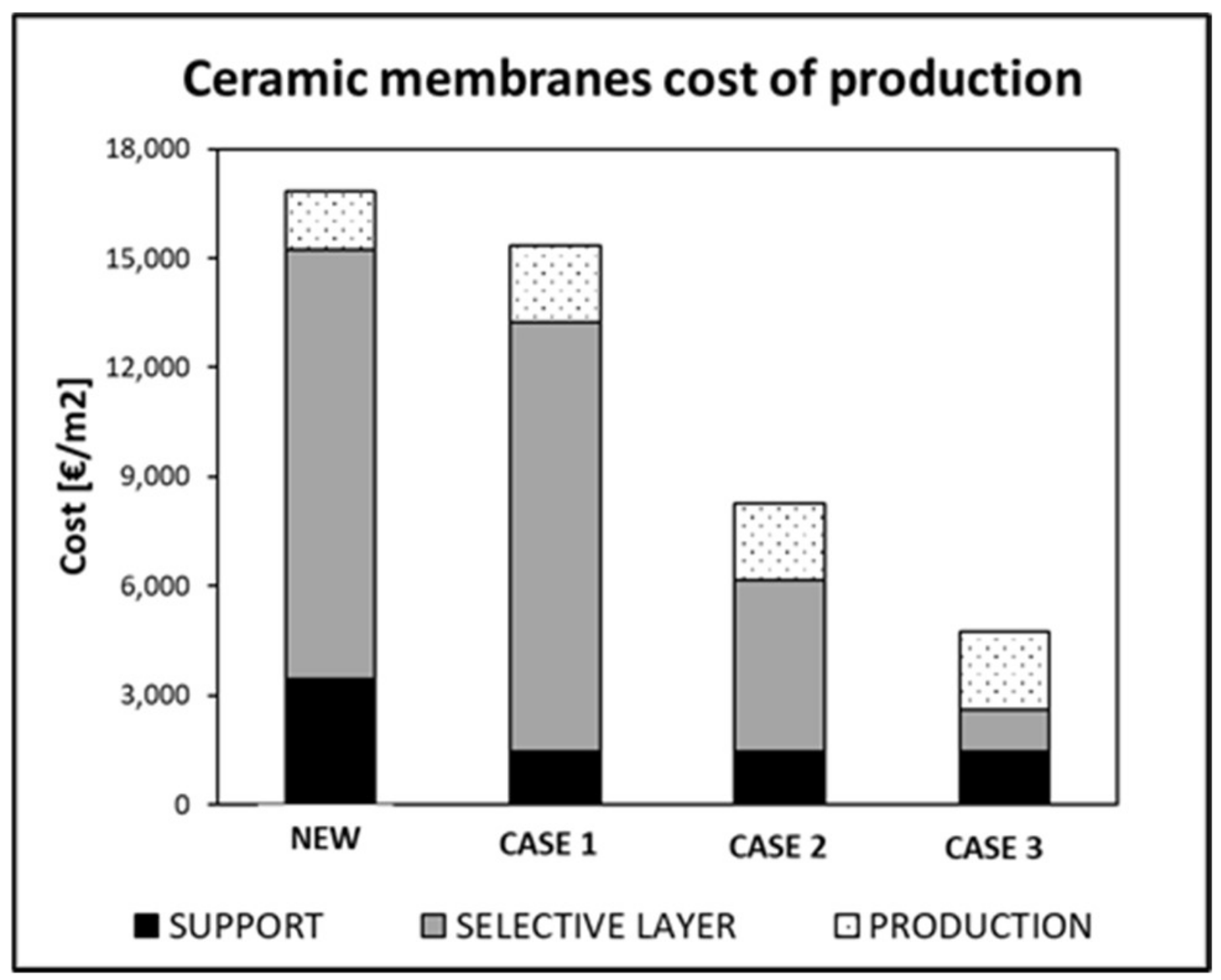
| % Cost (EUR/m2) | |
|---|---|
| OPEX | 15.0% |
| CAPEX | 0.3% |
| Re-deposition selective layer | 84.7% |
| Cost of Production (kEUR/m2) | % of Reduction | |
|---|---|---|
| New ceramic Pd-based membrane | 17.0 | |
| Recycled ceramic Pd-based membrane | 15.0 | 9.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, L.; Moscardini, E.; Baldassari, L.M.; Forte, F.; Coletta, J.; Palo, E.; Cosentino, V.; Angelini, F.; Arratibel Plazaola, A.; Pagnanelli, F.; et al. Regeneration of Exhausted Palladium-Based Membranes: Recycling Process and Economics. Membranes 2022, 12, 723. https://doi.org/10.3390/membranes12070723
Toro L, Moscardini E, Baldassari LM, Forte F, Coletta J, Palo E, Cosentino V, Angelini F, Arratibel Plazaola A, Pagnanelli F, et al. Regeneration of Exhausted Palladium-Based Membranes: Recycling Process and Economics. Membranes. 2022; 12(7):723. https://doi.org/10.3390/membranes12070723
Chicago/Turabian StyleToro, Luigi, Emanuela Moscardini, Ludovica M. Baldassari, Flavia Forte, Jacopo Coletta, Emma Palo, Vittoria Cosentino, Fabio Angelini, Alba Arratibel Plazaola, Francesca Pagnanelli, and et al. 2022. "Regeneration of Exhausted Palladium-Based Membranes: Recycling Process and Economics" Membranes 12, no. 7: 723. https://doi.org/10.3390/membranes12070723
APA StyleToro, L., Moscardini, E., Baldassari, L. M., Forte, F., Coletta, J., Palo, E., Cosentino, V., Angelini, F., Arratibel Plazaola, A., Pagnanelli, F., & Altimari, P. (2022). Regeneration of Exhausted Palladium-Based Membranes: Recycling Process and Economics. Membranes, 12(7), 723. https://doi.org/10.3390/membranes12070723









