Enhanced MEA Performance for an Intermediate-Temperature Fuel Cell with a KH5(PO4)2-Doped Polybenzimidazole Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Electrolyte Membrane
2.3. Characterization of Membranes
2.4. Preparation of MEA
2.5. Single-Cell Test and EIS Analysis
3. Results and Discussion
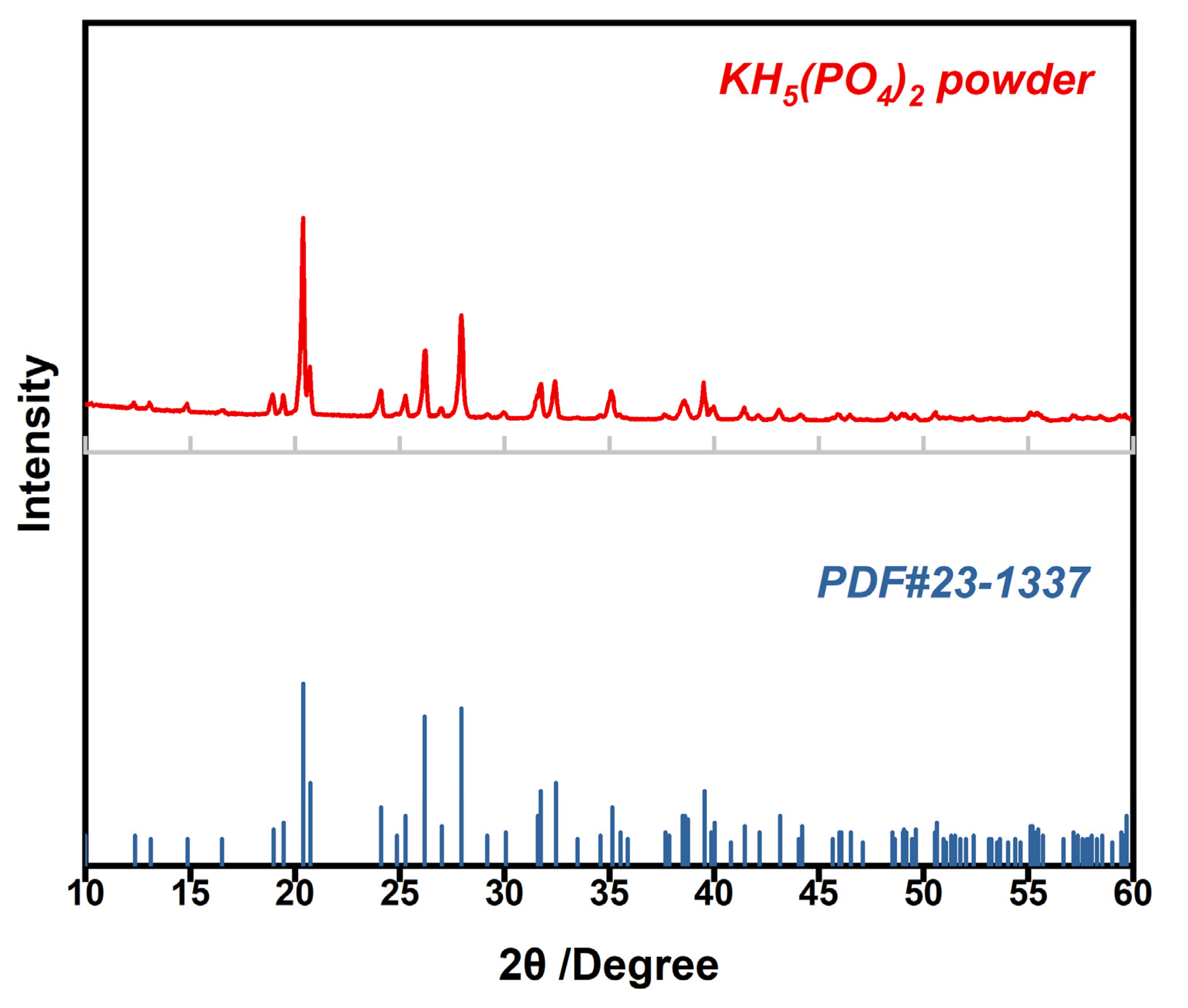
3.1. Characterization of KH5(PO4)2 and Cross-Sectional Morphology of KH5(PO4)2-Doped PBI Membrane
3.2. The Swelling Ratio of KH5(PO4)2-Doped PBI Membrane
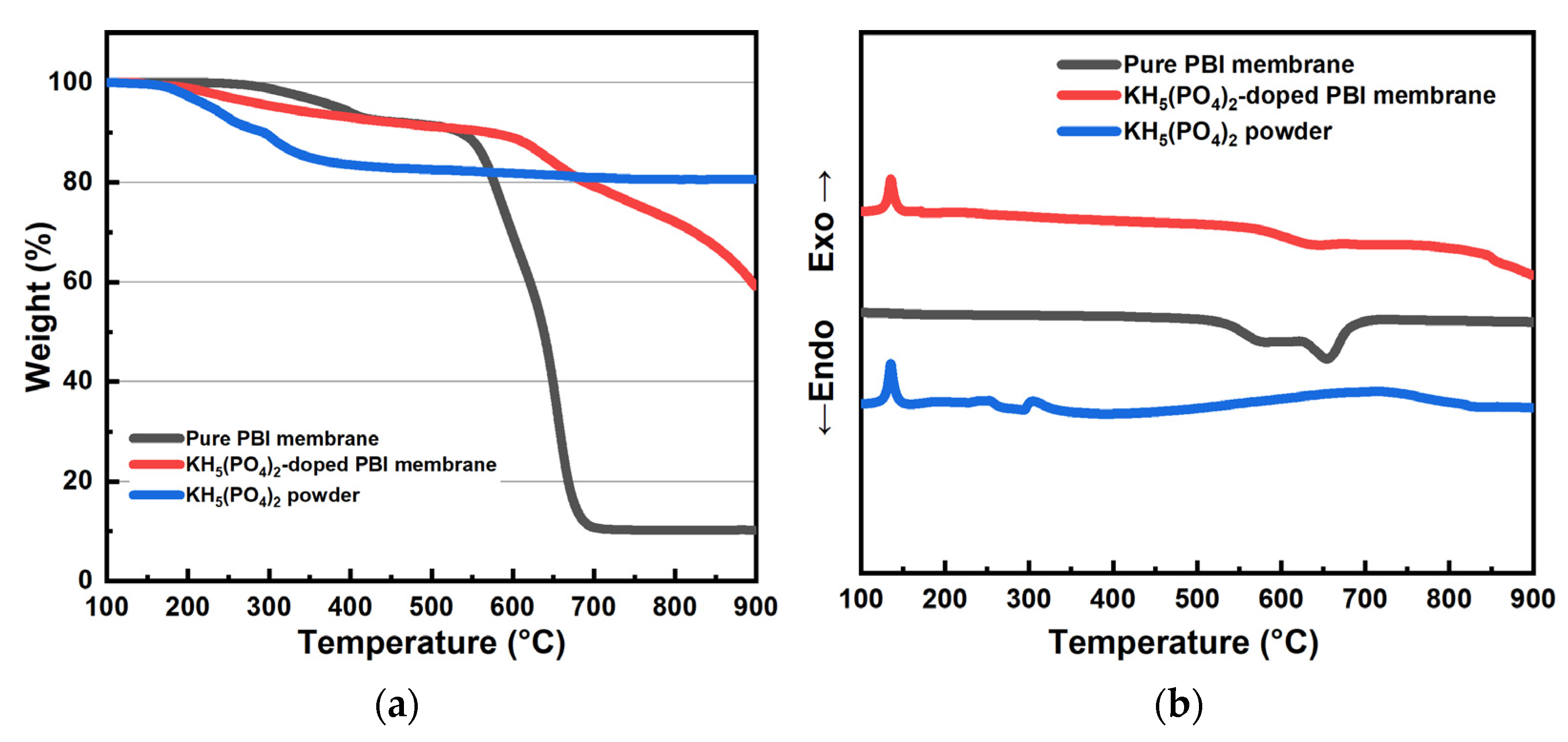
3.3. Thermal and Mechanical Properties of KH5(PO4)2-Doped PBI Membrane
3.4. Proton Conductivity of KH5(PO4)2-Doped PBI Membrane
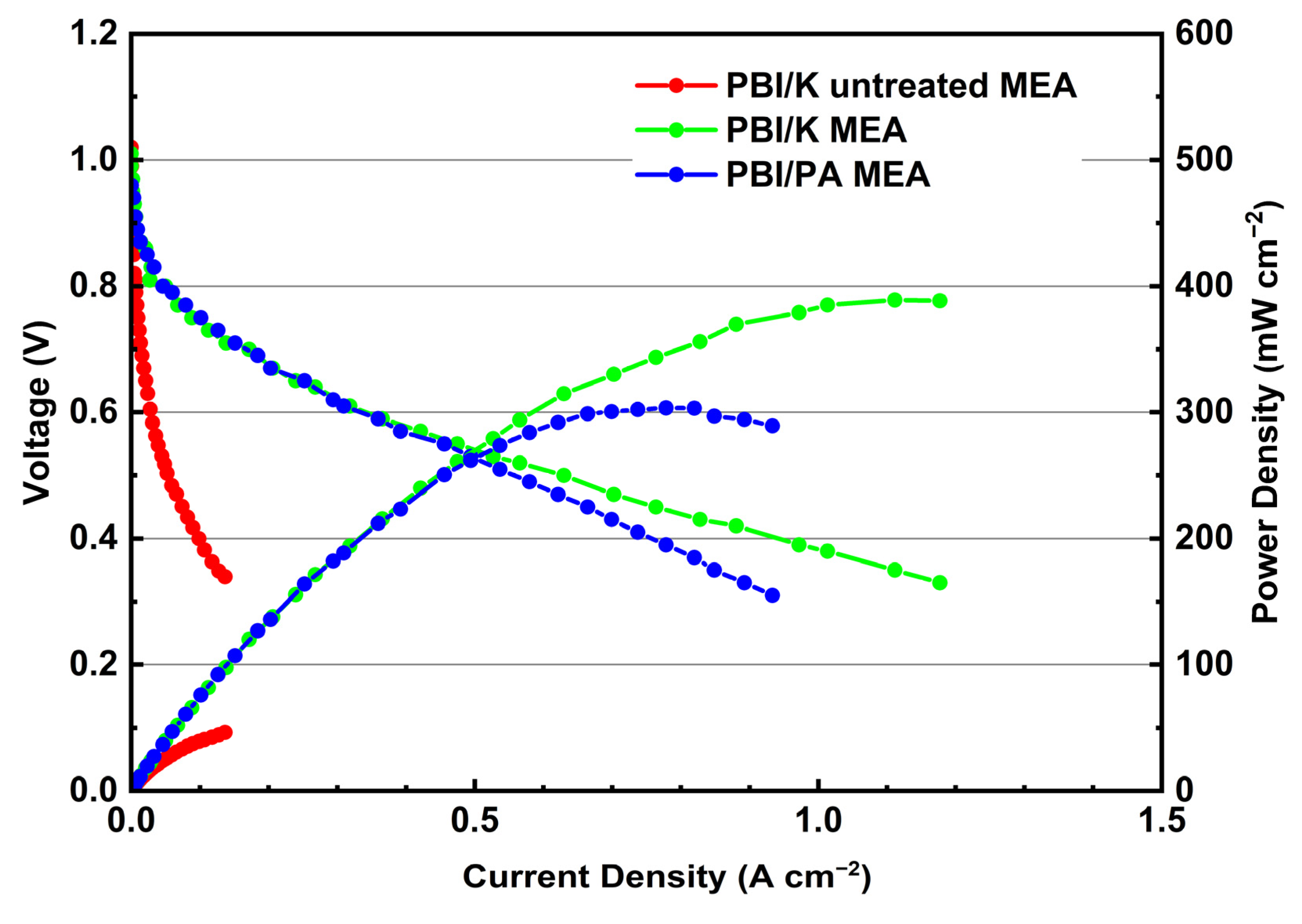
3.5. Performance of MEA before and after Treating GDEs with PA
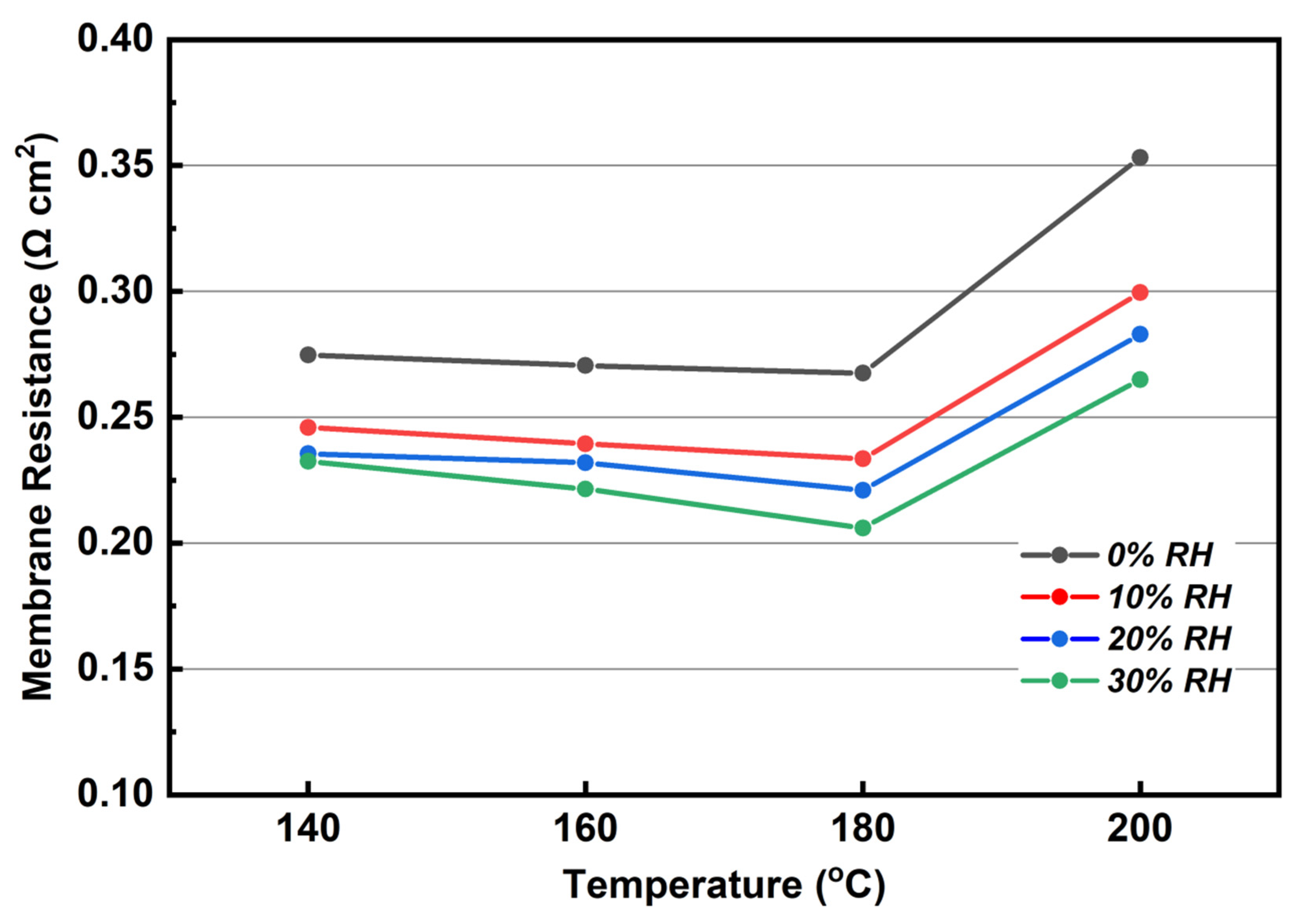
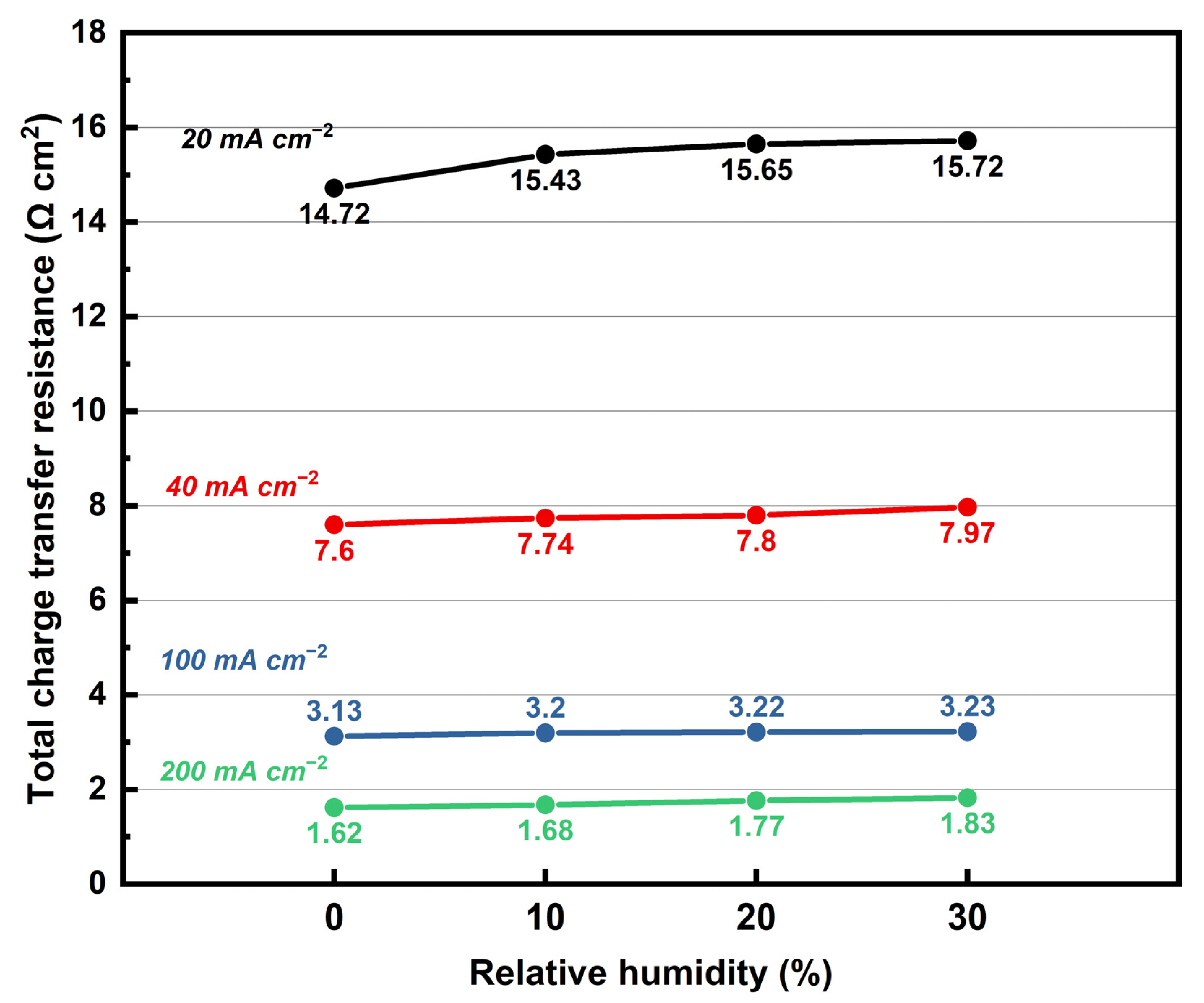
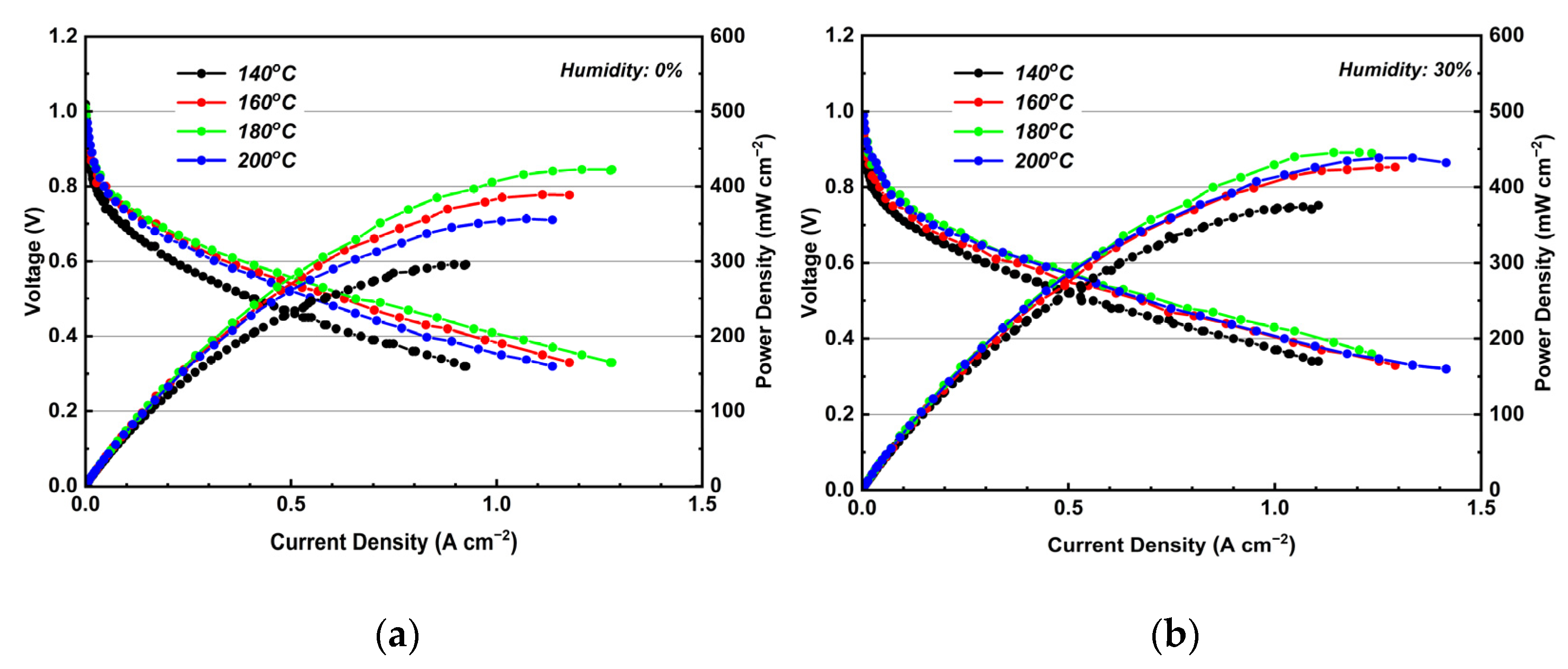
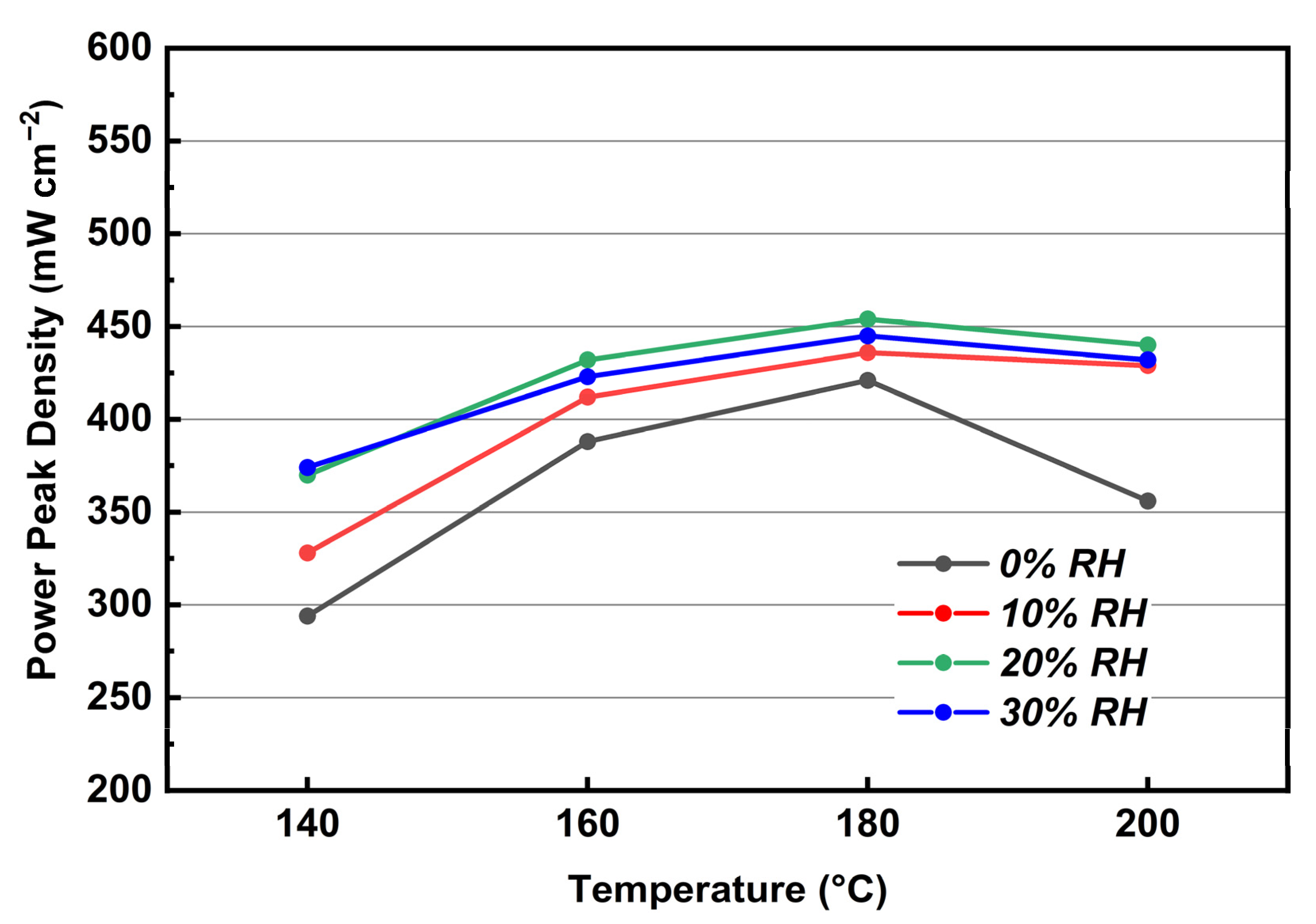
3.6. Performance and EIS Measurements of MEA Assembled with KH5(PO4)2-Doped PBI Membrane
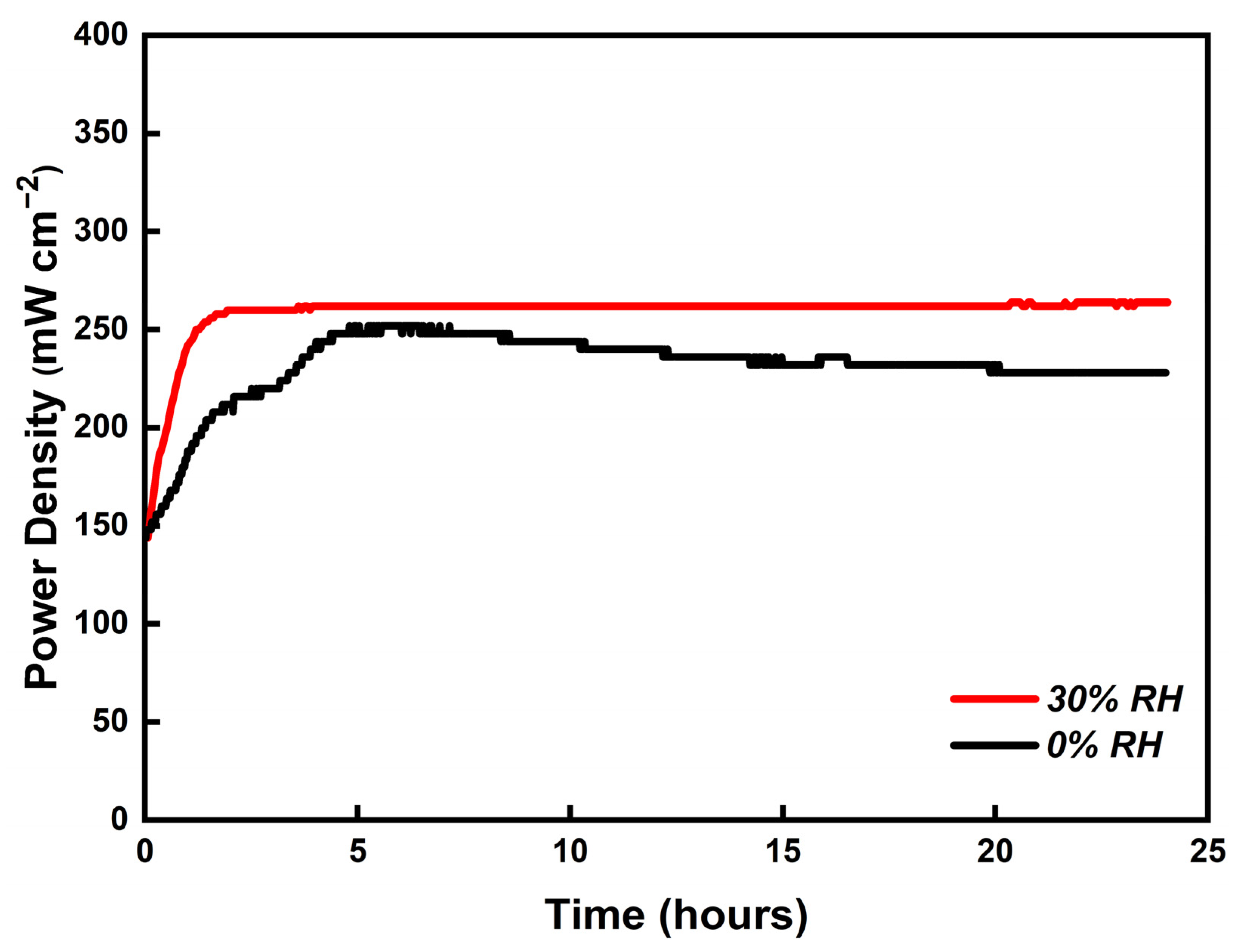
3.7. Durability of PBI/K MEA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.-S.; Maurya, S.; Kim, Y.S.; Kreller, C.R.; Wilson, M.S.; Larsen, D.; Elangovan, S.E.; Mukundan, R. Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte. Energy Environ. Sci. 2018, 11, 979–987. [Google Scholar] [CrossRef]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. A review on synthesis and characterization of solid acid materials for fuel cell applications. J. Power Sources 2016, 322, 77–92. [Google Scholar] [CrossRef]
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sources 2016, 311, 91–102. [Google Scholar] [CrossRef]
- Boysen, D.A.; Uda, T.; Chisholm, C.; Haile, S.M. High-Performance Solid Acid Fuel Cells Through Humidity Stabilization. Science 2004, 303, 68–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryaprakash, R.; Lohmann, F.; Wagner, M.; Abel, B.; Varga, A. Spray drying as a novel and scalable fabrication method for nanostructured CsH2PO4, Pt-thin-film composite electrodes for solid acid fuel cells. RSC Adv. 2014, 4, 60429–60436. [Google Scholar] [CrossRef]
- Uda, T.; Haile, S.M. Thin-membrane solid-acid fuel cell. Electrochem. Solid-State Lett. 2005, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Goni-Urtiaga, A.; Scott, K.; Cavaliere, S.; Jones, D.J.; Roziére, J. A new fabrication method of an intermediate temperature proton exchange membrane by the electrospinning of CsH2PO4. J. Mater. Chem. A 2013, 1, 10875–10880. [Google Scholar] [CrossRef]
- Muroyama, H.; Kudo, K.; Matsui, T.; Kikuchi, R.; Eguchi, K. Electrochemical properties of MH2PO4/SiP2O7-based electrolytes (M = alkaline metal) for use in intermediate-temperature fuel cells. Solid State Ion. 2007, 178, 1512–1516. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Li, H.; Li, C. Performance of an Intermediate-Temperature Fuel Cell with a CsH5(PO4)2-Doped Polybenzimidazole Membrane. J. Electrochem. Soc. 2022, 169, 024505. [Google Scholar] [CrossRef]
- Muroyama, H.; Matsui, T.; Kikuchi, R.; Eguchi, K. Influence of the supporting matrix on the electrochemical properties of CsH5(PO4)2 composites at intermediate temperatures. J. Phys. Chem. C 2008, 112, 15532–15536. [Google Scholar] [CrossRef]
- Qing, G.; Kikuchi, R.; Takagaki, A.; Sugawara, T.; Oyama, S.T. Stability of CsH5(PO4)2-based composites at fixed temperatures and during heating–cooling cycles for solid-state intermediate temperature fuel cells. J. Power Sources 2016, 306, 578–586. [Google Scholar] [CrossRef]
- Yoshimi, S.; Matsui, T.; Kikuchi, R.; Eguchi, K. Temperature and humidity dependence of the electrode polarization in intermediate-temperature fuel cells employing CsH2PO4/SiP2O7-based composite electrolytes. J. Power Sources 2008, 179, 497–503. [Google Scholar] [CrossRef]
- Heo, P.; Kim, T.Y.; Ha, J.; Choi, K.H.; Chang, H.; Kang, S. Intermediate-temperature fuel cells with amorphous Sn0.9In0.1P2O7 thin film electrolytes. J. Power Sources 2012, 198, 117–121. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Chen, X.; Lei, X. Electrolyte membranes based on molten KH5(PO4)2 for intermediate temperature fuel cells. Fuel Cells 2019, 19, 280–288. [Google Scholar] [CrossRef]
- Wannek, C.; Konradi, I.; Mergel, J.; Lehnert, W. Redistribution of phosphoric acid in membrane electrode assemblies for high-temperature polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2009, 34, 9479–9485. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, T.Y.; Yoo, D.Y.; Hong, S.-G.; Park, J.O. Maximization of high-temperature proton exchange membrane fuel cell performance with the optimum distribution of phosphoric acid. J. Power Sources 2009, 188, 463–467. [Google Scholar] [CrossRef]
- Wannek, C.; Lehnert, W.; Mergel, J. Membrane electrode assemblies for high-temperature polymer electrolyte fuel cells based on poly (2, 5-benzimidazole) membranes with phosphoric acid impregnation via the catalyst layers. J. Power Sources 2009, 192, 258–266. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Influence of the phosphoric acid-doping level in a polybenzimidazole membrane on the cell performance of high-temperature proton exchange membrane fuel cells. J. Power Sources 2009, 189, 943–949. [Google Scholar] [CrossRef]
- Li, Y.; Kappis, K.; Papavasiliou, J.; Fu, Z.; Chen, L.; Li, H.; Vlachos, D.E.; Avgouropoulos, G. Insights on the electrochemical performance of a molten proton conductor fuel cell with internal methanol reformer. J. Power Sources 2022, 542, 231813. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Ribeiorinha, P.; Li, H.; Kong, X.; Boaventura, M. A proton conductor electrolyte based on molten CsH5(PO4)2 for intermediate-temperature fuel cells. RSC Adv. 2018, 8, 5225–5232. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Aili, D.; Hjuler, H.A.; Jensen, J.O. High Temperature Polymer Electrolyte Membrane Fuel Cells; Springer: Cham, Switzerland, 2016; p. 545. [Google Scholar] [CrossRef] [Green Version]
- Bezmalinović, D.; Strahl, S.; Roda, V.; Husar, A. Water transport study in a high temperature proton exchange membrane fuel cell stack. Int. J. Hydrogen Energy 2014, 39, 10627–10640. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, H.; Bhowmik, S.; Benedictus, R. Process optimization of solvent based polybenzimidazole adhesive for aerospace applications. Int. J. Adhes. Adhes. 2014, 48, 188–193. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, J.; Liu, F.; Wang, X.; Chen, H.; Ni, H.; Wang, Z. Composite membranes based on polybenzimidazole and ionic liquid functional Si–O–Si network for HT-PEMFC applications. Int. J. Hydrogen Energy 2017, 42, 21913–21921. [Google Scholar] [CrossRef]
- Guan, Y.; Pu, H.; Jin, M.; Chang, Z.; Wan, D. Preparation and characterisation of proton exchange membranes based on crosslinked polybenzimidazole and phosphoric acid. Fuel Cells 2010, 10, 973–982. [Google Scholar] [CrossRef]
- Aili, D.; Allward, T.; Alfaro, S.M.; Hartmann-Thompson, C.; Steenberg, T.; Hjuler, H.A.; Li, Q.; Jensen, J.O.; Stark, E.J. Polybenzimidazole and sulfonated polyhedral oligosilsesquioxane composite membranes for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2014, 140, 182–190. [Google Scholar] [CrossRef]
- Leal, J.; Martinez, H.; Martinez, I.; Price, A.; Goos, A.; Botez, C. Stability of the superprotonic conduction of (1-x) CsH2PO4/xSiO2 (0 ≤ x ≤ 0.3) composites under dry and humid environments. Mater. Today Commun. 2018, 15, 11–17. [Google Scholar] [CrossRef]
- Galbiati, S.; Baricci, A.; Casalegno, A.; Marchesi, R. Experimental study of water transport in a polybenzimidazole-based high temperature PEMFC. Int. J. Hydrogen Energy 2012, 37, 2462–2469. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, H.; Zhai, Y.; Liu, G.; Hu, J.; Yi, B. Performance degradation studies on PBI/H3PO4 high temperature PEMFC and one-dimensional numerical analysis. Electrochim. Acta 2006, 52, 394–401. [Google Scholar] [CrossRef]
- Liu, F.; Mohajeri, S.; Di, Y.; Wippermann, K.; Lehnert, W. Influence of the Interaction Between Phosphoric Acid and Catalyst Layers on the Properties of HT-PEFCs. Fuel Cells 2014, 14, 750–757. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lai, W.-H. Effects of temperature and humidity on the cell performance and resistance of a phosphoric acid doped polybenzimidazole fuel cell. J. Power Sources 2010, 195, 7152–7159. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Sun, J.C.; Zhang, J. AC impedance technique in PEM fuel cell diagnosis—A review. Int. J. Hydrogen Energy 2007, 32, 4365–4380. [Google Scholar] [CrossRef]
- Matsui, T.; Kukino, T.; Kikuchi, R.; Eguchi, K. Composite effects of silicon pyrophosphate as a supporting matrix for CsH5(PO4)2 electrolytes at intermediate temperatures. Electrochim. Acta 2006, 51, 3719–3723. [Google Scholar] [CrossRef]
- Sun, H.; Xie, C.; Chen, H.; Almheiri, S. A numerical study on the effects of temperature and mass transfer in high temperature PEM fuel cells with ab-PBI membrane. Appl. Energy 2015, 160, 937–944. [Google Scholar] [CrossRef]
- Zhou, F.; Singdeo, D.; Kær, S.K. Investigation of the Effect of Humidity Level of H2 on Cell Performance of a HT-PEM Fuel Cell. Fuel Cells 2019, 19, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Üregen, N.; Pehlivanoğlu, K.; Özdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Adibi, M. Novel composite membranes based on PBI and dicationic ionic liquids for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2016, 205, 142–152. [Google Scholar] [CrossRef]
- Pinar, F.J.; Canizares, P.; Rodrigo, M.A.; Ubeda, D.; Lobato, J. Titanium composite PBI-based membranes for high temperature polymer electrolyte membrane fuel cells. Effect on titanium dioxide amount. RSC Adv. 2012, 2, 1547–1556. [Google Scholar] [CrossRef]







| Electrolyte | Proton Conductor | Pt Loading for Anode and Cathode | Operating Conditions | Peak Power Density | Open Circuit Voltage | Reference |
|---|---|---|---|---|---|---|
| KH5(PO4)2-doped PBI membrane | KH5(PO4)2 | 1 mg cm−2 | Feeding with H2/O2 at 180 °C | 450 mW cm−2 | 1.0 V | This work |
| PBI/GO composite membrane | PA | 1 mg cm−2 | Feeding with H2/air at 165 °C | 380 mW cm−2 | 0.95 V | [36] |
| PBI/dicationic ionic liquids composite membrane | PA | 0.5 mg cm−2 | Feeding with H2/O2 at 180 °C | 700 mW cm−2 | 0.85 V | [37] |
| PBI/TiO2 composite membrane | PA | 0.5 mg cm−2 | Feeding with H2/O2 at 175 °C | 425 mW cm−2 | 0.85 V | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, J.; Papavasiliou, J.; Fu, Z.; Chen, L.; Li, H. Enhanced MEA Performance for an Intermediate-Temperature Fuel Cell with a KH5(PO4)2-Doped Polybenzimidazole Membrane. Membranes 2022, 12, 728. https://doi.org/10.3390/membranes12080728
Li Y, Hu J, Papavasiliou J, Fu Z, Chen L, Li H. Enhanced MEA Performance for an Intermediate-Temperature Fuel Cell with a KH5(PO4)2-Doped Polybenzimidazole Membrane. Membranes. 2022; 12(8):728. https://doi.org/10.3390/membranes12080728
Chicago/Turabian StyleLi, Yifan, Jing Hu, Joan Papavasiliou, Zhiyong Fu, Li Chen, and Haibin Li. 2022. "Enhanced MEA Performance for an Intermediate-Temperature Fuel Cell with a KH5(PO4)2-Doped Polybenzimidazole Membrane" Membranes 12, no. 8: 728. https://doi.org/10.3390/membranes12080728
APA StyleLi, Y., Hu, J., Papavasiliou, J., Fu, Z., Chen, L., & Li, H. (2022). Enhanced MEA Performance for an Intermediate-Temperature Fuel Cell with a KH5(PO4)2-Doped Polybenzimidazole Membrane. Membranes, 12(8), 728. https://doi.org/10.3390/membranes12080728






