Formation of Nanochannels Using Polypropylene and Acetylcellulose for Stable Separators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
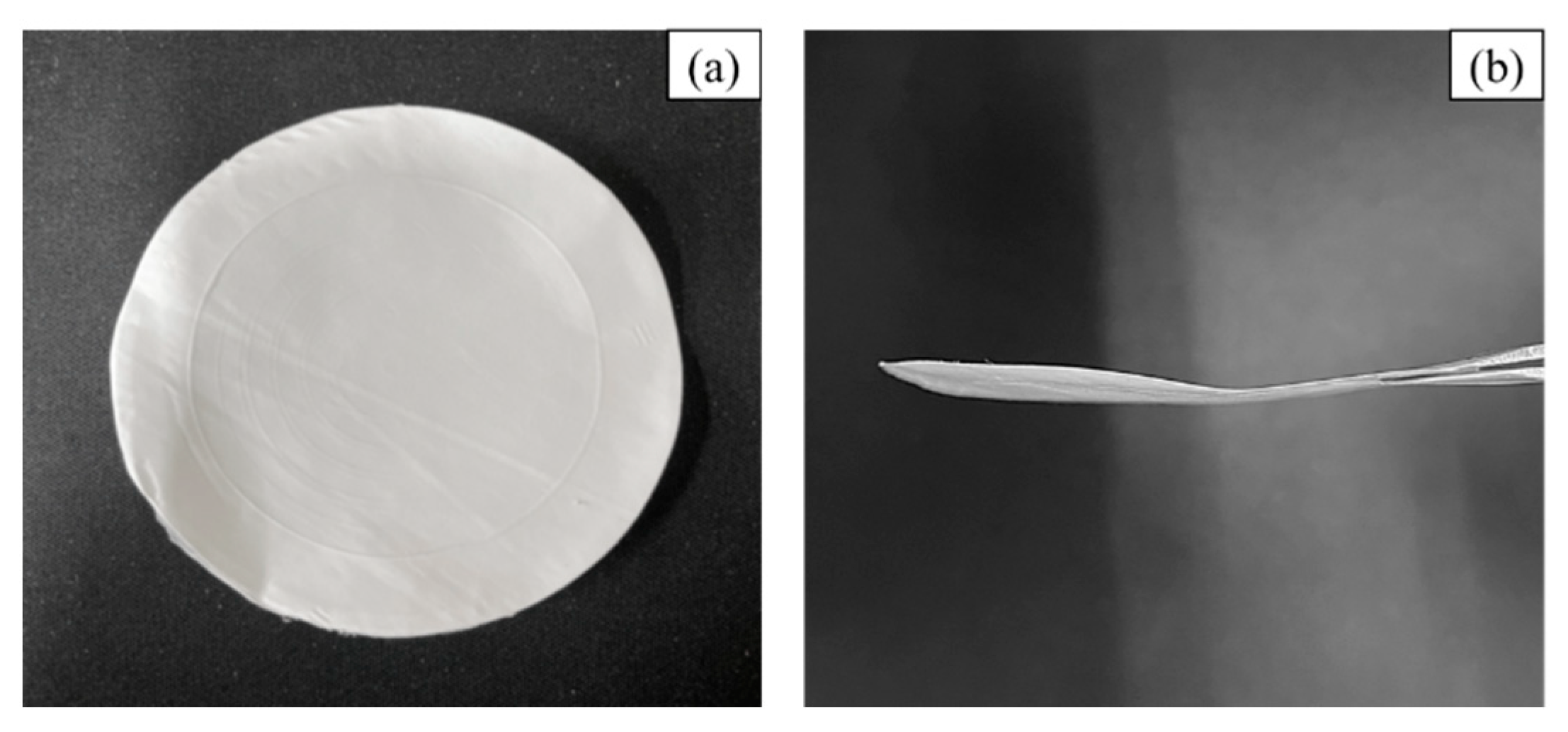
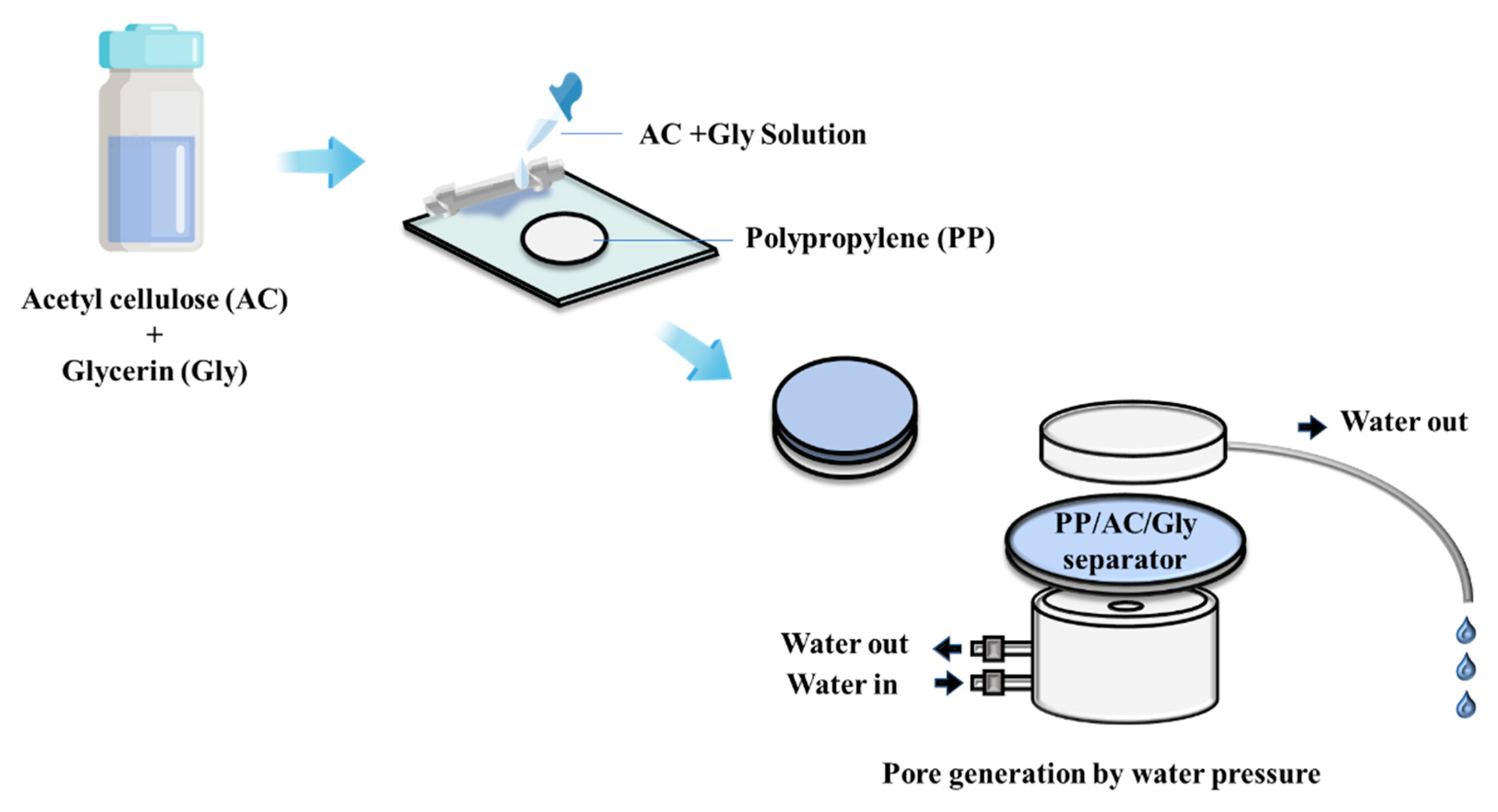
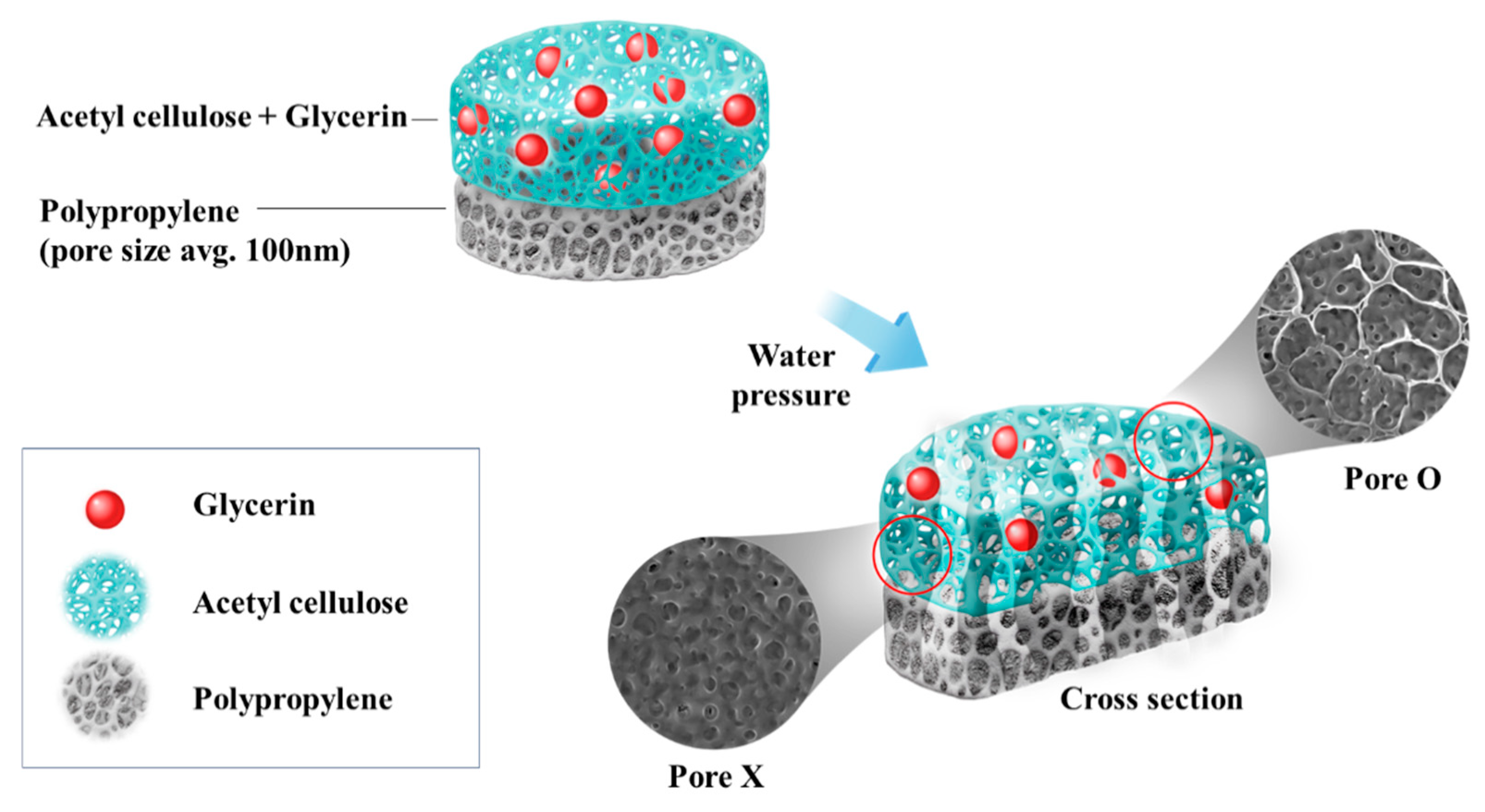
2.2.1. Separator Preparation
2.2.2. Characterization
3. Results and Discussion
3.1. Water Flux Data
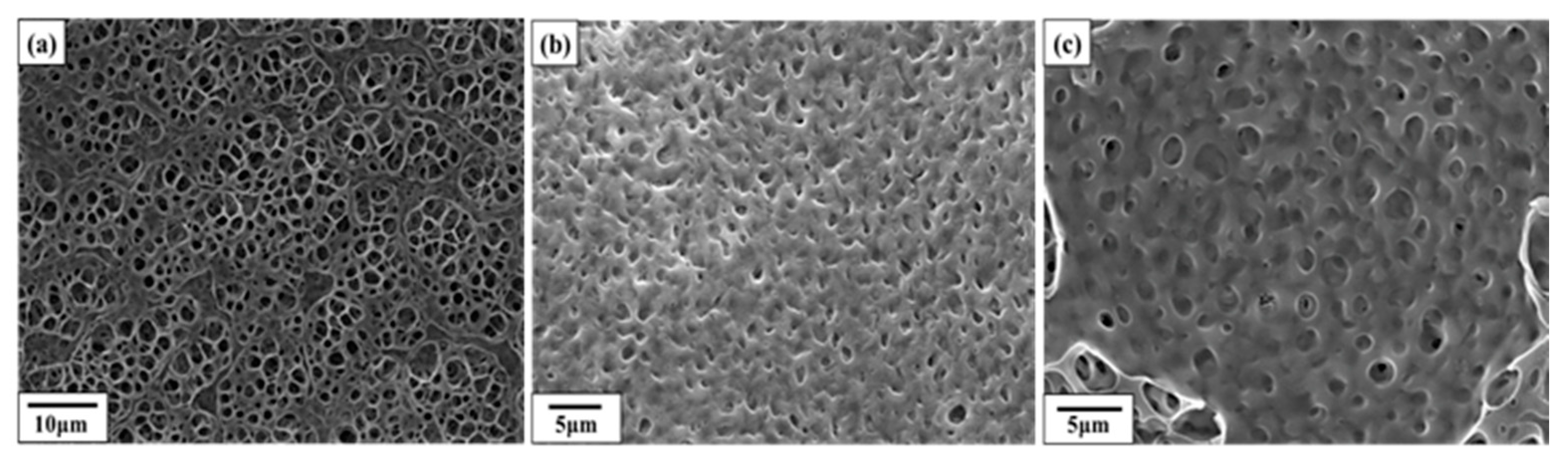
3.2. SEM Images
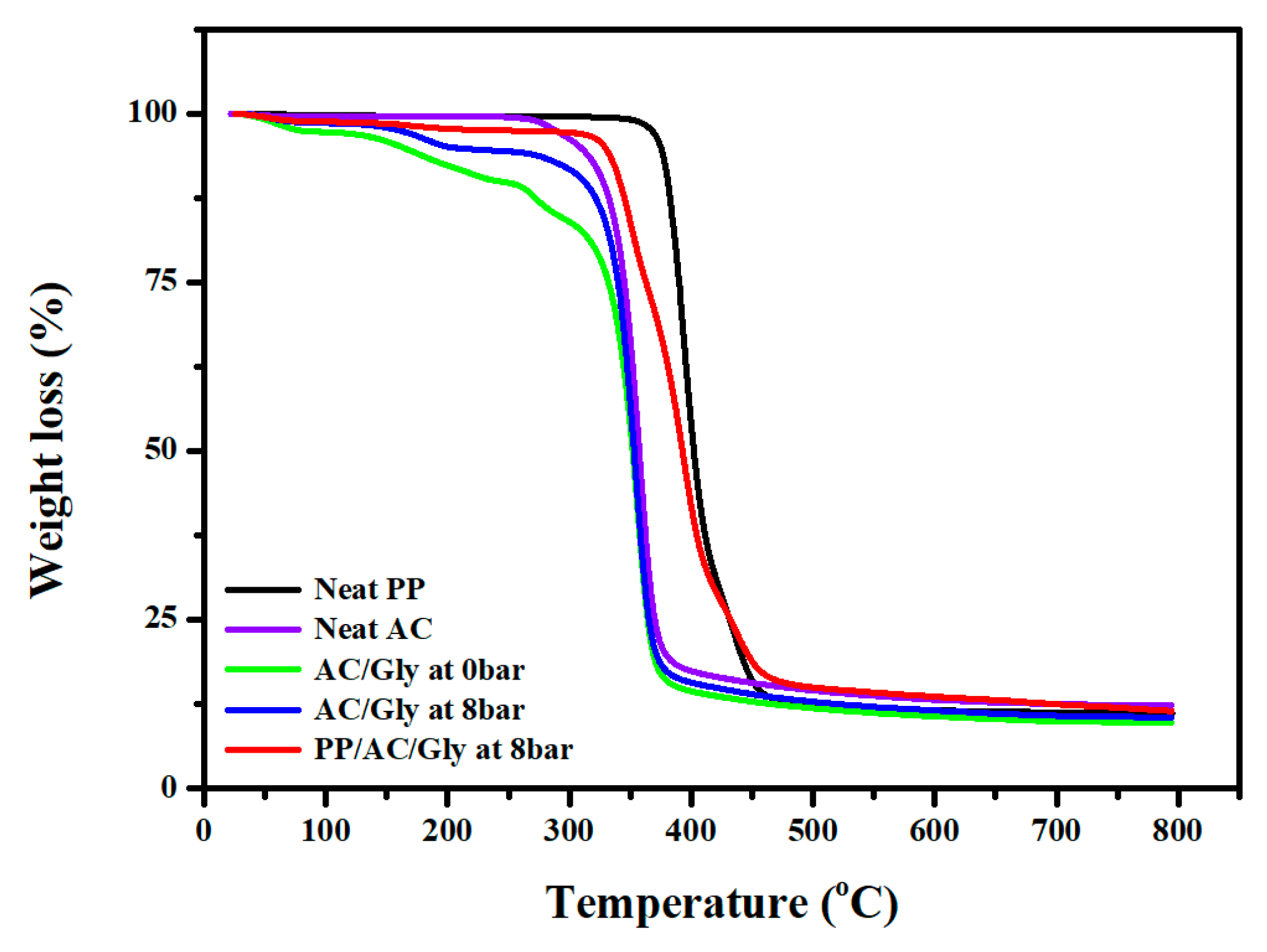
3.3. TGA Data
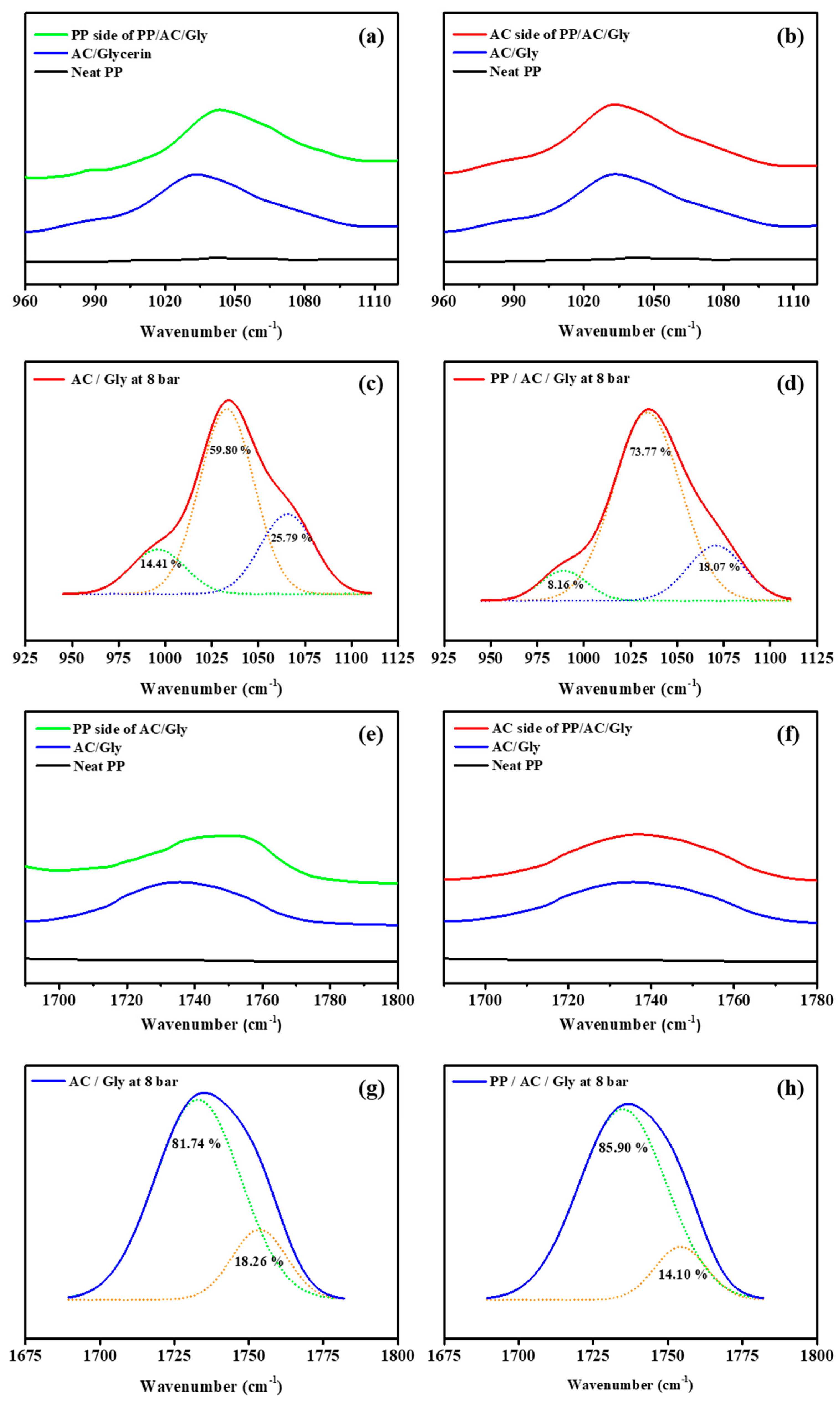
3.4. FT-IR Data
3.5. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Campo, F.J.D.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar]
- Wen, W.; Yang, S.; Zhou, P.; Gao, S.Z. Impacts of COVID-19 on the electric vehicle industry: Evidence from China. Renew. Sustain. Energy Rev. 2021, 144, 111024. [Google Scholar] [CrossRef]
- Pan, S.; Fulton, L.M.; Roy, A.; Jung, J.; Choi, Y.; Gao, H.O. Shared use of electric autonomous vehicles: Air quality and health impacts of future mobility in the United States. Renew. Sustain. Energy Rev. 2021, 149, 111380. [Google Scholar] [CrossRef]
- Haustein, S.; Jensen, A.F.; Cherchi, E. Battery electric vehicle adoption in Denmark and Sweden: Recent changes, related factors and policy implications. Energy Policy 2021, 149, 112096. [Google Scholar] [CrossRef]
- Olabi, A.G.; Adil, M.; Sayed, E.T.; Iqbal, A.; Rodriguez, C.; Abdelkareem, M.A. Lithium-Ion Batteries. In Anonymous Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Scrosati, B. Recent advances in lithium ion battery materials. Electrochim. Acta 2000, 45, 2461–2466. [Google Scholar] [CrossRef]
- Jamil, S.; Yousaf, A.B.; Yoon, S.H.; Han, D.S.; Yang, L.; Kasak, P.; Wang, X. Dual cationic modified high Ni-low co layered oxide cathode with a heteroepitaxial interface for high energy-density lithium-ion batteries. Chem. Eng. J. 2021, 416, 129118. [Google Scholar] [CrossRef]
- Casino, S.; Niehoff, P.; Börner, M.; Winter, M. Protective coatings on silicon particles and their effect on energy density and specific energy in lithium ion battery cells: A model study. J. Energy Storage 2020, 29, 101376. [Google Scholar] [CrossRef]
- Zoller, F.; Böhm, D.; Luxa, J.; Döblinger, M.; Sofer, Z.; Semenenko, D.; Bein, T.; Fattakhova-Rohlfing, D. Freestanding LiFe0.2Mn0.8PO4/rGO nanocomposites as high energy density fast charging cathodes for lithium-ion batteries. Mater. Today Energy 2020, 16, 100416. [Google Scholar] [CrossRef]
- Xia, L.; Miao, H.; Zhang, C.; Chen, G.Z.; Yuan, J. Review—Recent advances in non-aqueous liquid electrolytes containing fluorinated compounds for high energy density lithium-ion batteries. Energy Storage Mater. 2021, 38, 542–570. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A review of battery fires in electric vehicles. Fire Technol. 2020, 56, 1411. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Yuen, A.C.Y.; Wang, W.; Cordeiro, D.; Miguel, I.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A Review on Lithium-Ion Battery Separators towards Enhanced Safety Performances and Modelling Approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Costa, C.M.; Lanceros-Mendez, S. Recent advances on battery separators based on poly(vinylidene fluoride) and its copolymers for lithium-ion battery applications. Curr. Opin. Electrochem. 2021, 29, 100752. [Google Scholar] [CrossRef]
- Peng, L.; Kong, X.; Li, H.; Wang, X.; Shi, C.; Hu, T.; Liu, Y.; Zhang, P.; Zhao, J. A Rational Design for a High-Safety Lithium-Ion Battery Assembled with a Heatproof–Fireproof Bifunctional Separator. Adv. Funct. Mater. 2021, 31, 2008537. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, H.; Lee, Y.; Esken, D.; Dehe, D.; Song, H.A.; Kim, D. Nanostructured reactive alumina particles coated with water-soluble binder on the polyethylene separator for highly safe lithium-ion batteries. J. Power Sources 2021, 506, 230119. [Google Scholar] [CrossRef]
- Costa, C.M.; Kundu, M.; Dias, J.C.; Nunes-Pereira, J.; Botelho, G.; Silva, M.M.; Lanceros-Méndez, S. Mesoporous poly(vinylidene fluoride-co-trifluoroethylene) membranes for lithium-ion battery separators. Electrochim. Acta 2019, 301, 97–106. [Google Scholar] [CrossRef]
- Prasanna, K.; Subburaj, T.; Lee, W.J.; Lee, C.W. Polyethylene separator: Stretched and coated with porous nickel oxide nanoparticles for enhancement of its efficiency in Li-ion batteries. Electrochim. Acta 2014, 137, 273–279. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Kong, Q.; Zhang, C.; Pang, S.; Yue, L.; Wang, X.; Yao, J.; Cui, G. Renewable and superior thermal-resistant cellulose-based composite nonwoven as lithium-ion battery separator. ACS Appl. Mater. Interfaces 2013, 5, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yanilmaz, M.; Lu, Y.; Zhu, J.; Zhang, X. Silica/polyacrylonitrile hybrid nanofiber membrane separators via sol-gel and electrospinning techniques for lithium-ion batteries. J. Power Sources 2016, 313, 205–212. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, Y.; Kang, S.W. Porous Cellulose acetate membranes prepared by water pressure-assisted process for water-treatment. J. Ind. Eng. Chem. 2019, 78, 421–424. [Google Scholar] [CrossRef]
- Lee, W.G.; Kang, S.W. Control of pore in cellulose acetate containing Mg salt by water pressure treatment for applications to separators. J. Ind. Eng. Chem. 2019, 70, 103–106. [Google Scholar] [CrossRef]
- Lee, W.G.; Kang, S.W. Eco-friendly process for facile pore control in thermally stable cellulose acetate utilizing zinc(II) nitrate for water-treatment. J. Ind. Eng. Chem. 2020, 81, 88–92. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, Y.; Kang, S.W. Development of low-cost process for pore generation in cellulose acetate by utilizing calcium salts. J. Ind. Eng. Chem. 2021, 94, 419–424. [Google Scholar] [CrossRef]
- Jie, S.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar]
- Lizundia, E.; Costa, C.M.; Alves, R.; Méndez, S.L. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Pan, R.; Cheung, O.; Wang, Z.; Tammela, P.; Huo, J.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Mesoporous Cladophora cellulose separators for lithium-ion batteries. J. Power Sources 2016, 321, 185–192. [Google Scholar] [CrossRef]
- Hong, S.H.; Cho, Y.; Kang, S.W. Formation of water-channel by propylene glycol into polymer for porous materials. Membranes 2021, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Kim, D.H.; Jeon, W.C.; Kwak, S.K.; Kang, S.J.; Kang, S.W. Facile control of nanoporosity in cellulose acetate using Nickel (II) nitrate additive and water pressure treatment for highly efficient battery gel separators. Sci. Rep. 2017, 7, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Neat AC at 10 Bar | Neat PP at 10 Bar | PP/AC/Gly at 10 Bar | |
|---|---|---|---|
| LMH | 0 | Not measurable | 1.42 (±0.5) |
| Peak (cm−1) | Area (%) | |
|---|---|---|
| AC/Gly | PP/AC/Gly | |
| 989–996 | 14.41 | 8.16 |
| 1033–1034 | 59.80 | 73.77 |
| 1066–1071 | 25.79 | 18.07 |
| Peak (cm−1) | Area (%) | |
|---|---|---|
| AC/Gly | PP/AC/Gly | |
| 1732 | 81.74 | 85.90 |
| 1753 | 18.26 | 14.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Cho, Y.; Kang, S.W. Formation of Nanochannels Using Polypropylene and Acetylcellulose for Stable Separators. Membranes 2022, 12, 764. https://doi.org/10.3390/membranes12080764
Lee HJ, Cho Y, Kang SW. Formation of Nanochannels Using Polypropylene and Acetylcellulose for Stable Separators. Membranes. 2022; 12(8):764. https://doi.org/10.3390/membranes12080764
Chicago/Turabian StyleLee, Hye Ji, Younghyun Cho, and Sang Wook Kang. 2022. "Formation of Nanochannels Using Polypropylene and Acetylcellulose for Stable Separators" Membranes 12, no. 8: 764. https://doi.org/10.3390/membranes12080764
APA StyleLee, H. J., Cho, Y., & Kang, S. W. (2022). Formation of Nanochannels Using Polypropylene and Acetylcellulose for Stable Separators. Membranes, 12(8), 764. https://doi.org/10.3390/membranes12080764





