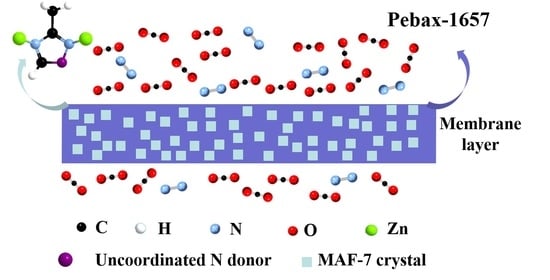
Preparation of Pebax 1657/MAF-7 Mixed Matrix Membranes with Enhanced CO2/N2 Separation by Active Site of Triazole Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MAF-7 Particles
2.3. Preparation of Pebax 1657/MAF-7 Mixed Matrix Membranes
2.4. Characterization Techniques
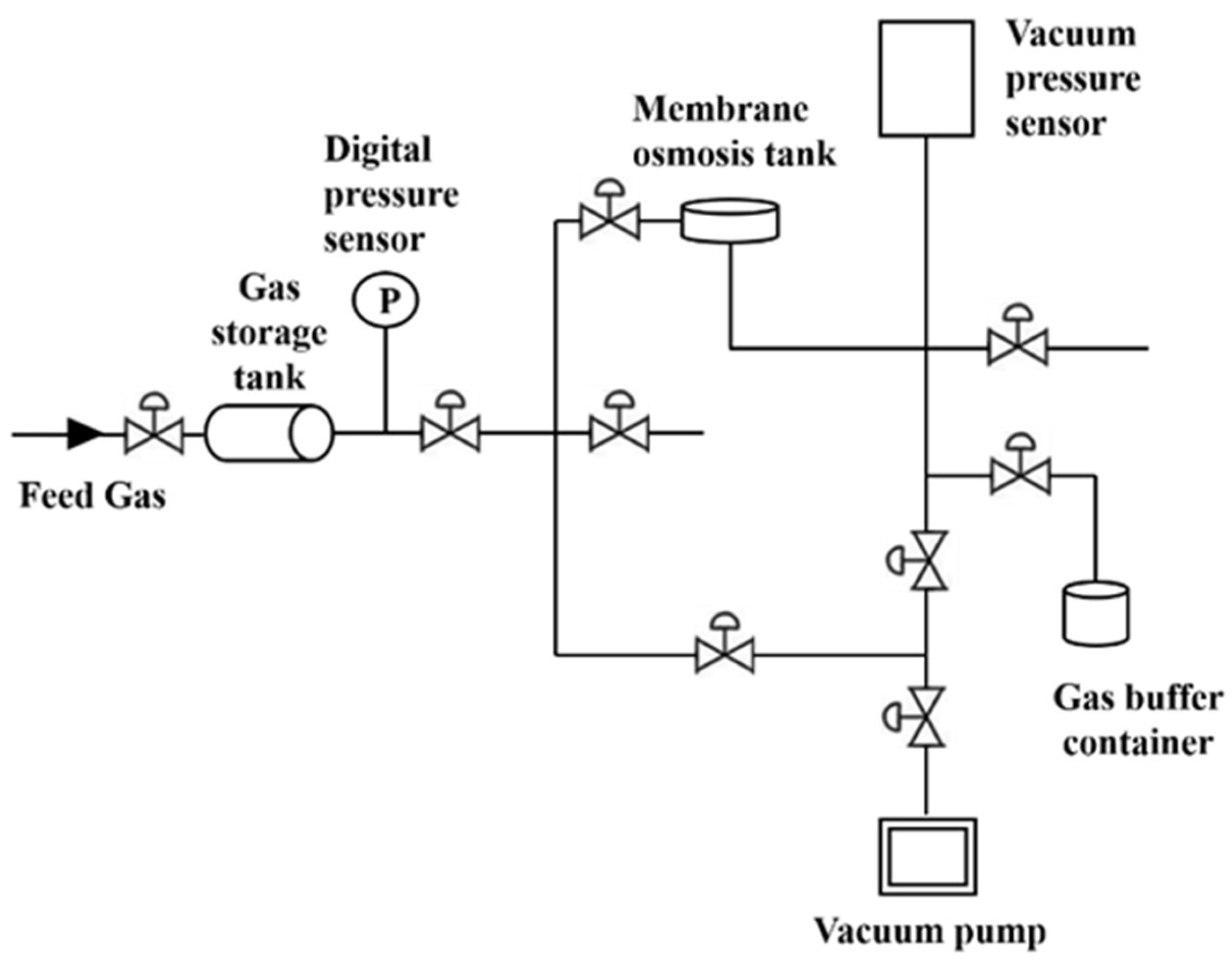
2.5. Gas Permeation Test
3. Results and Discussion
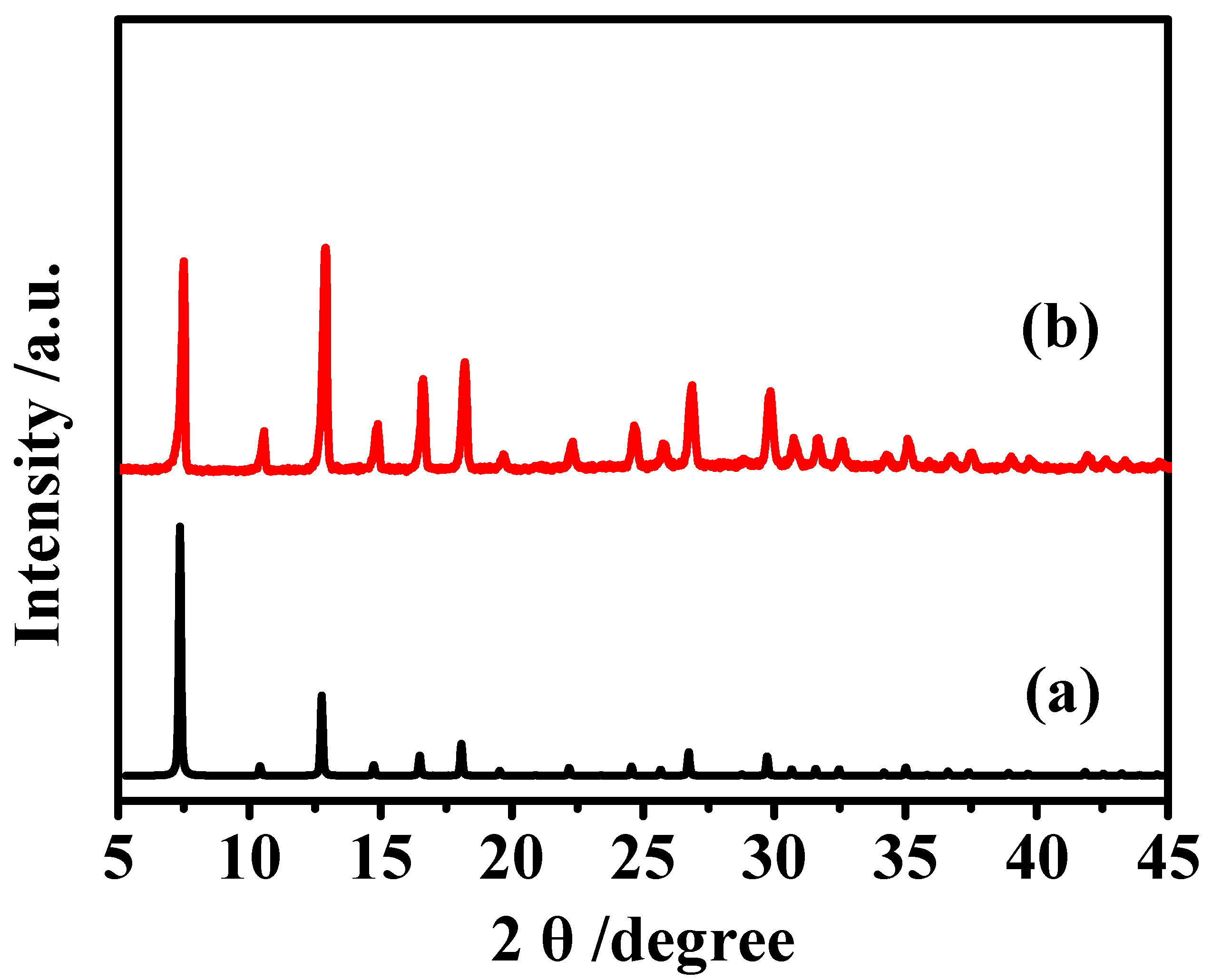
3.1. XRD Patterns and SEM Image of MAF-7 Powders
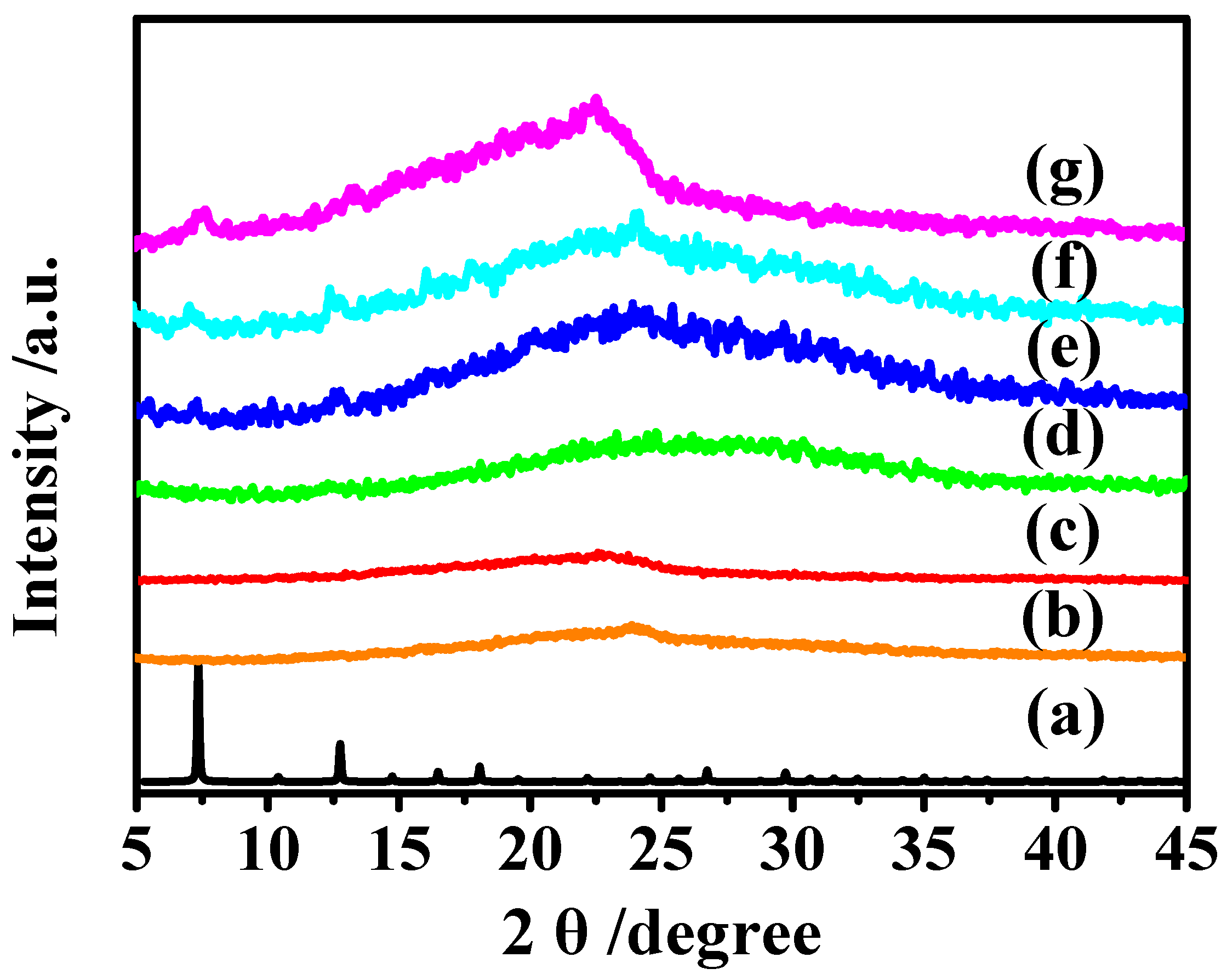
3.2. XRD characterization of MAF-7/Pebax MMMs
3.3. SEM Characterization of Pebax 1657/MAF-7 MMMs
3.4. Thermodynamic Properties of Membranes
3.5. Fourier Transform Infrared Spectroscopy of Membranes
3.6. Gas Permeation Performance
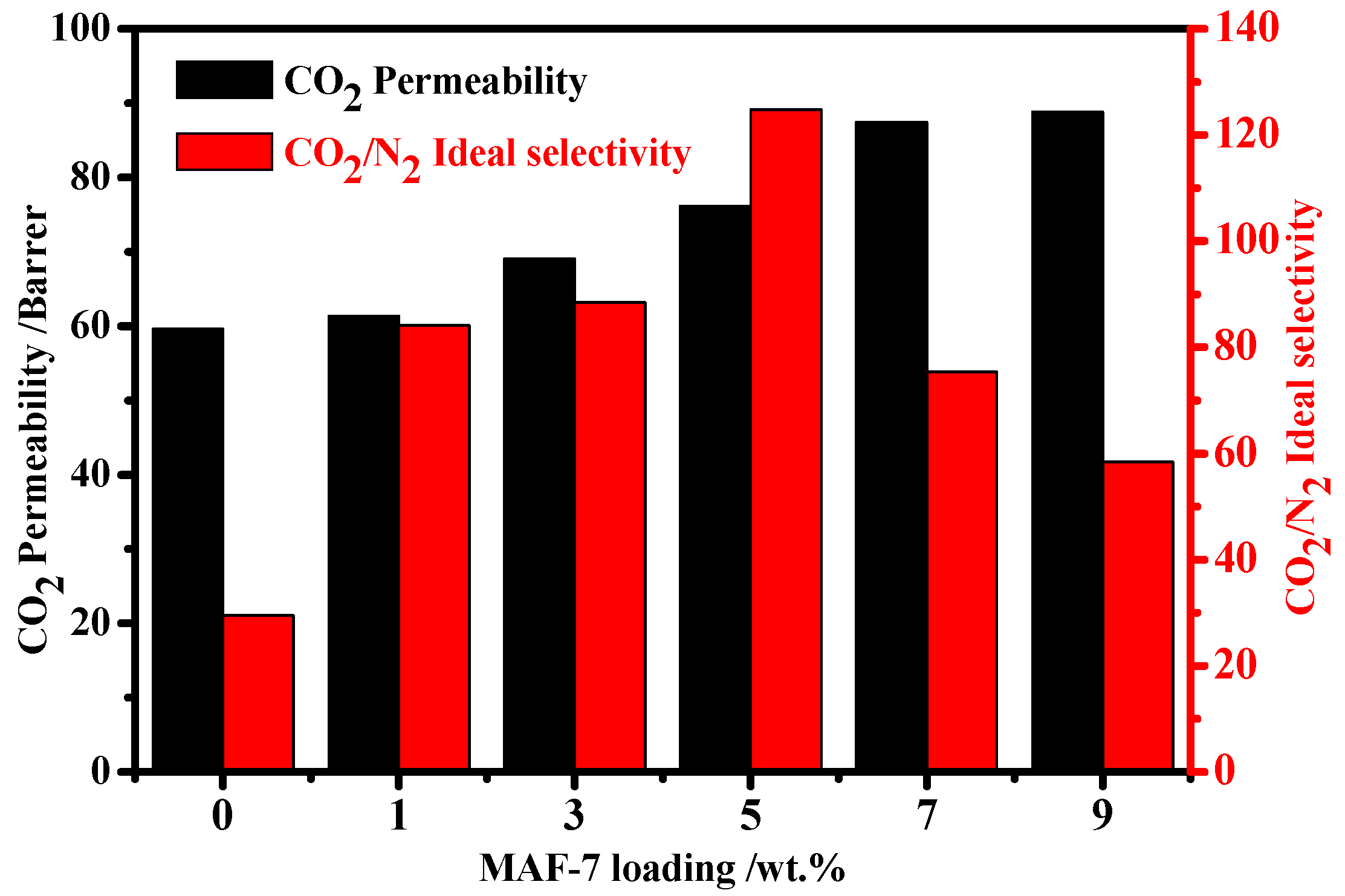
3.6.1. Effect of MAF-7 Loading in MMMs
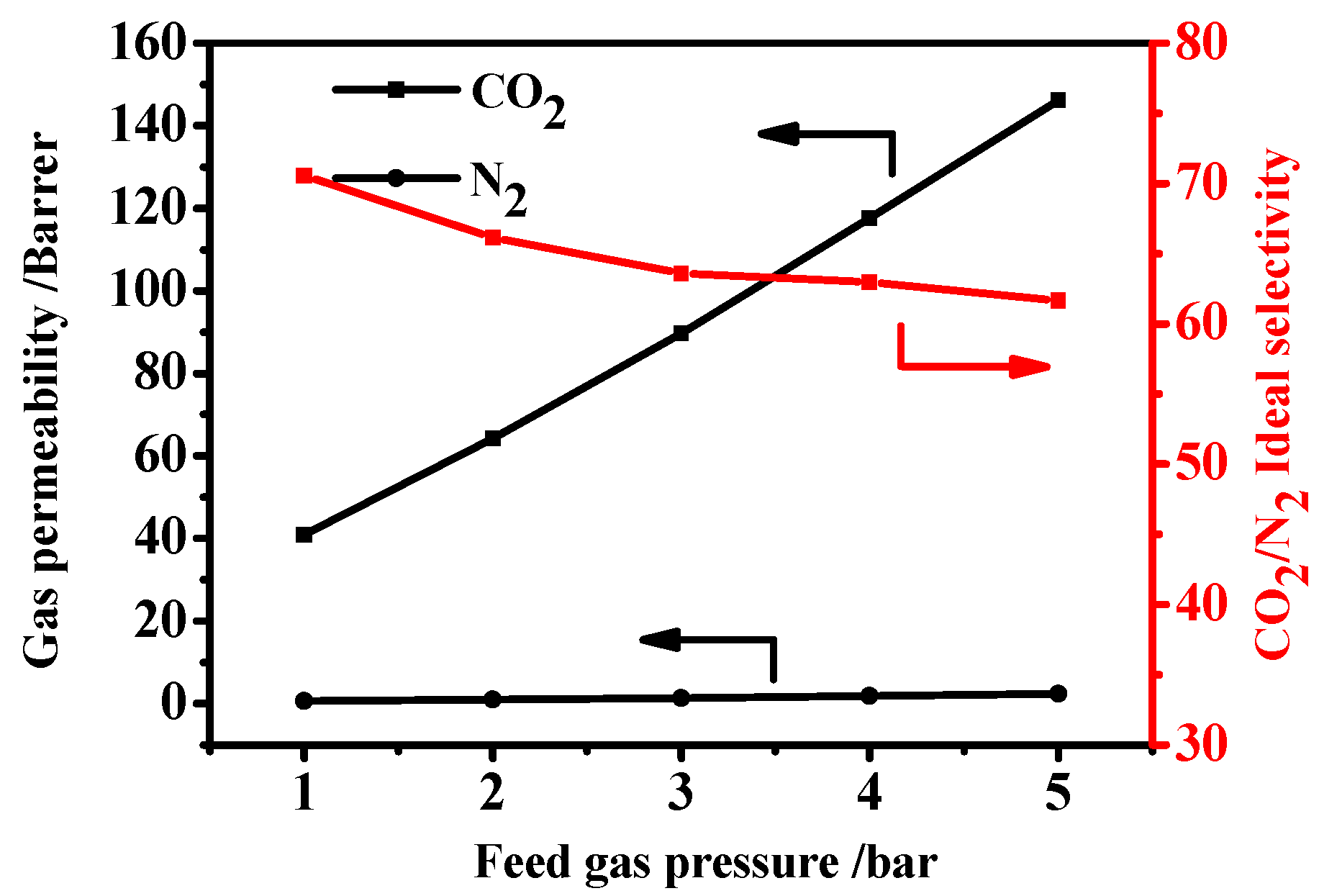
3.6.2. Effect of Feed Pressure
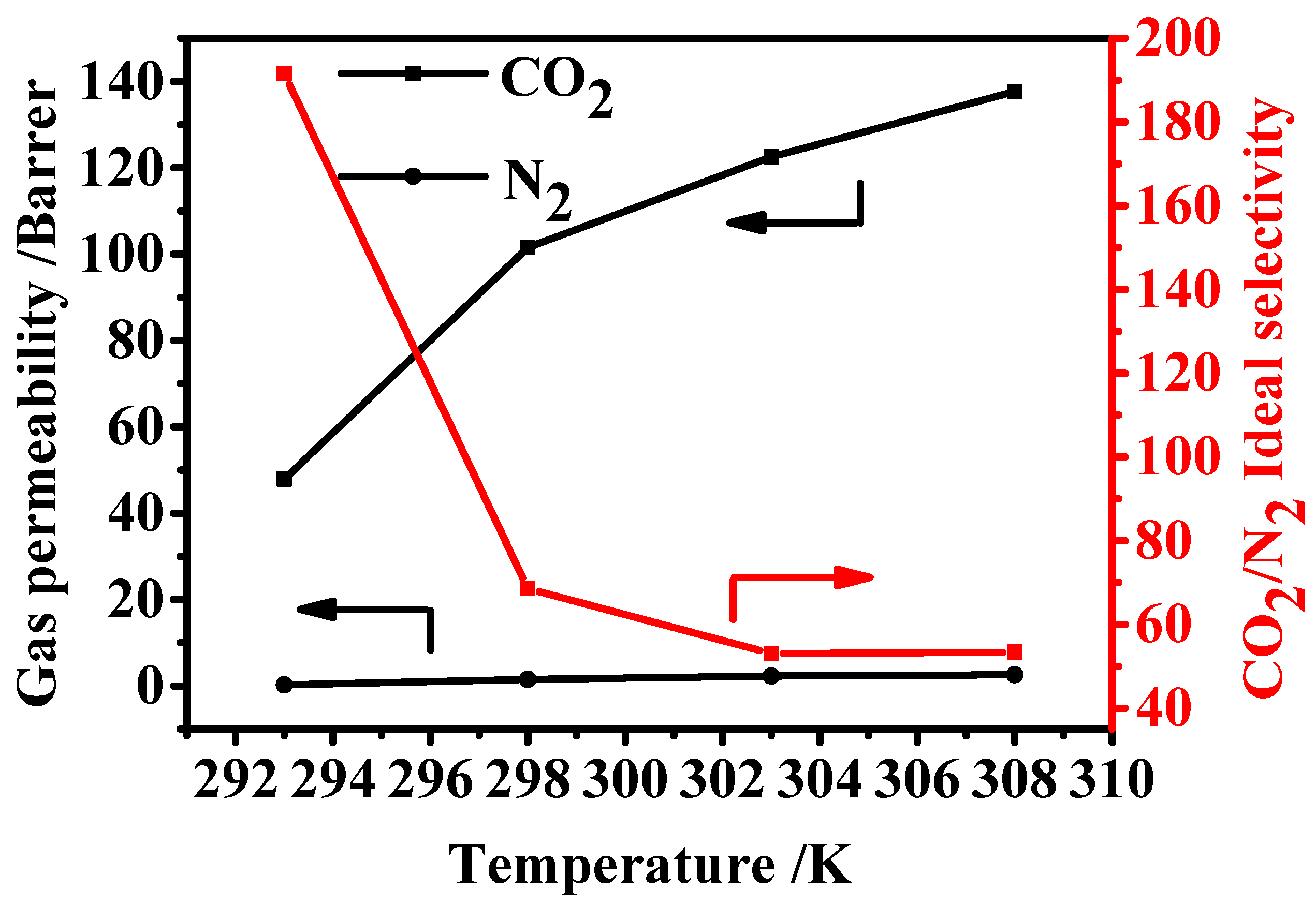
3.6.3. Effect of Testing Temperatures
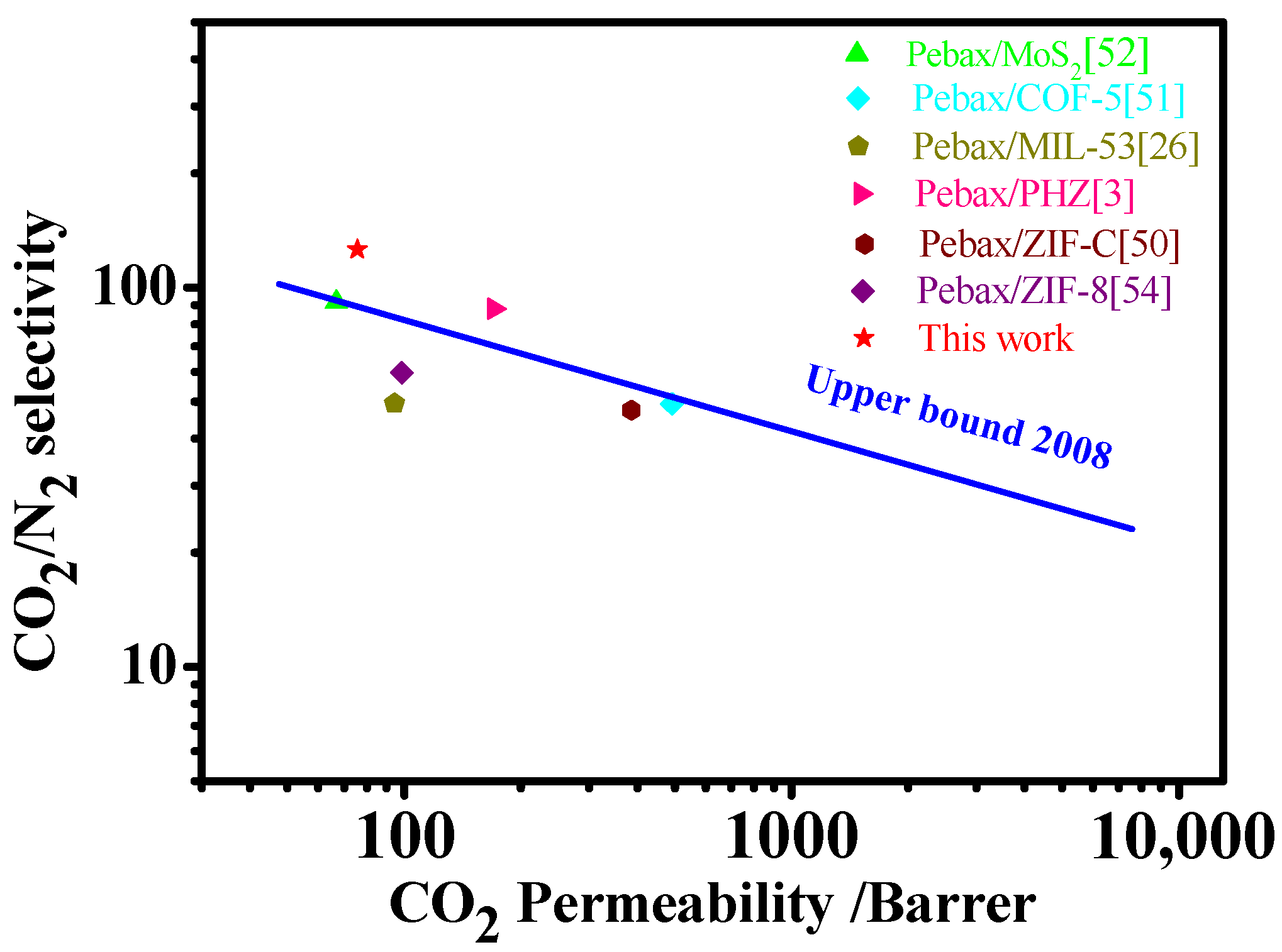
3.7. Comparison with Previous Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Ding, R.; Dai, Y.; Zheng, W.; Li, X.; Yan, X.; Liu, Y.; Ruan, X.; Li, S.; Yang, X.; Yang, K.; et al. Vesicles-shaped MOF-based mixed matrix membranes with intensified interfacial affinity and CO2 transport freeway. Chem. Eng. J. 2021, 414, 128807. [Google Scholar] [CrossRef]
- Fan, D.; Ozcan, A.; Ramsahye, N.A.; Maurin, G.; Semino, R. Putting Forward NUS-8-CO2H/PIM-1 as a Mixed Matrix Membrane for CO2 Capture. ACS Appl. Mater. Interfaces 2022, 14, 16820–16829. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Barooah, M.; Mandal, B. Synthesis, characterization and CO2 separation performance of novel PVA/PG/ZIF-8 mixed matrix membrane. J. Membr. Sci. 2019, 572, 198–209. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Wu, H.; Sun, S.; You, X.; Yuan, B.; Hou, J.; Duan, C.; Jiang, Z. Incorporating amino acids functionalized graphene oxide nanosheets into Pebax membranes for CO2 separation. Sep. Purif. Technol. 2022, 288, 120682. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, Z.; Liu, D.; Zhong, C. Mixed-matrix membranes for CO2 separation: Role of the third component. J. Mater. Chem. A 2019, 7, 24738–24759. [Google Scholar] [CrossRef]
- Li, E.; Chen, Z.; Duan, C.; Yuan, B.; Yan, S.; Luo, X.; Pan, F.; Jiang, Z. Enhanced CO2-capture performance of polyimide-based mixed matrix membranes by incorporating ZnO@MOF nanocomposites. Sep. Purif. Technol. 2022, 289, 120714. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed Matrix Membranes for Improved Gas Separation Performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, B.; Ding, R.; Li, D.; Jiang, X.; He, G.; Wu, X. A Novel Composite Material UiO-66@HNT/Pebax Mixed Matrix Membranes for Enhanced CO2/N2 Separation. Membranes 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Guo, H.; Liu, X.; Zhang, B. Mixed matrix membrane comprising glycine grafted CuBTC for enhanced CO2 separation performances with excellent stability under humid atmosphere. Sep. Purif. Technol. 2022, 295, 121287. [Google Scholar] [CrossRef]
- Habib, N.; Durak, O.; Zeeshan, M.; Uzun, A.; Keskin, S. A novel IL/MOF/polymer mixed matrix membrane having superior CO2/N2 selectivity. J. Membr. Sci. 2022, 658, 120712. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, C.; Zheng, Y.; Wu, Y.; Song, C.; Liu, Q.; Wang, Z. Modification of CO2-selective mixed matrix membranes by a binary composition of poly(ethylene glycol)/NaY zeolite. J. Membr. Sci. 2021, 627, 119239. [Google Scholar] [CrossRef]
- Gou, Y.; Xiao, L.; Yang, Y.; Guo, X.; Zhang, F.; Zhu, W.; Xiao, Q. Incorporation of open-pore MFI zeolite nanosheets in polydimethylsiloxane (PDMS) to isomer-selective mixed matrix membranes. Microporous Mesoporous Mater. 2021, 315, 110930. [Google Scholar] [CrossRef]
- Gong, H.; Lee, S.; Bae, T.H. Mixed-matrix membranes containing inorganically surface-modified 5A zeolite for enhanced CO2/CH4 separation. Microporous Mesoporous Mater. 2017, 237, 82–89. [Google Scholar] [CrossRef]
- Luque-Alled, J.M.; Tamaddondar, M.; Foster, A.B.; Budd, P.M.; Gorgojo, P. PIM-1/Holey Graphene Oxide Mixed Matrix Membranes for Gas Separation: Unveiling the Role of Holes. ACS Appl. Mater. Interfaces 2021, 13, 55517–55533. [Google Scholar] [CrossRef]
- Luque-Alled, J.M.; Ameen, A.W.; Alberto, M.; Tamaddondar, M.; Foster, A.B.; Budd, P.M.; Vijayaraghavan, A.; Gorgojo, P. Gas separation performance of MMMs containing (PIM-1)-functionalized GO derivatives. J. Membr. Sci. 2021, 623, 118902. [Google Scholar] [CrossRef]
- Zhao, Y.; Jung, B.; Ansaloni, L.; Ho, W.S.W. Multiwalled carbon nanotube mixed matrix membranes containing amines for high pressure CO2/H2 separation. J. Membr. Sci. 2014, 459, 233–243. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, R.; Hou, J.; Wei, Z.; Li, X. Mixed-Matrix Membranes Containing Carbon Nanotubes Composite with Hydrogel for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2016, 8, 29044–29051. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.S.; Sridhar, S.; Sankarshana, T.; Ravikumar, Y.V.L. Gas Permeation Behavior of Pebax-1657 Nanocomposite Membrane Incorporated with Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2010, 49, 6530–6538. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Wang, C.; Chang, H.; Guo, R. Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J. Membr. Sci. 2020, 598, 117794. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Li, Q.; Guan, K.; Li, Y.; Jin, W. UiO-66-polyether block amide mixed matrix membranes for CO2 separation. J. Membr. Sci. 2016, 513, 155–165. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 570, 343–354. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax (R) MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Fonseca, J.; Gong, T.; Jiao, L.; Jiang, H. Metal-organic frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J. Mater. Chem. A 2021, 9, 10562–10611. [Google Scholar] [CrossRef]
- Li, H.; Yin, Y.; Zhu, L.; Xiong, Y.; Li, X.; Guo, T.; Xing, W.; Xue, Q. A hierarchical structured steel mesh decorated with metal organic framework/graphene oxide for high-efficient oil/water separation. J. Hazard. Mater. 2019, 373, 725–732. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, J.; Li, R.; Zhu, H.; Duan, J.; Guo, Y.; Liu, G.; Jin, W. ZIF-301 MOF/6FDA-DAM polyimide mixed-matrix membranes for CO2/CH4 separation. Sep. Purif. Technol. 2021, 264, 118431. [Google Scholar] [CrossRef]
- Thur, R.; Van Velthoven, N.; Slootmaekers, S.; Didden, J.; Verbeke, R.; Smolders, S.; Dickmann, M.; Egger, W.; De Vos, D.; Vankelecom, I.F.J. Bipyridine-based UiO-67 as novel filler in mixed-matrix membranes for CO2-selective gas separation. J. Membr. Sci. 2019, 576, 78–87. [Google Scholar] [CrossRef]
- Loloei, M.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on NH2-MIL-53 (Al) and 6FDA-ODA polyimide for CO2 separation: Effect of the processing route on improving MOF-polymer interfacial interaction. Sep. Purif. Technol. 2021, 270, 118786. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Zornoza, B.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation. RSC Adv. 2015, 5, 102350–102361. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Misdan, N.; Nazri, N.A.M. Modified ZIF-8 mixed matrix membrane for CO2/CH4 separation. AIP Conf. Proc. 2017, 1891, 020091. [Google Scholar]
- Shi, G.; Yang, T.; Chung, T. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415, 577–586. [Google Scholar] [CrossRef]
- Dai, Y.; Johnson, J.R.; Karvan, O.; Sholl, D.S.; Koros, W.J. Ultem®/ZIF-8 mixed matrix hollow fiber membranes for CO2/N2 separations. J. Membr. Sci. 2012, 401–402, 76–82. [Google Scholar] [CrossRef]
- Song, S.; Jiang, H.; Wu, H.; Zhao, M.; Guo, Z.; Li, B.; Ren, Y.; Wang, Y.; Ye, C.; Guiver, M.D.; et al. Weakly pressure-dependent molecular sieving of propylene/propane mixtures through mixed matrix membrane with ZIF-8 direct-through channels. J. Membr. Sci. 2022, 648, 120366. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, A.; Lin, R.; Qi, X.; Chen, X. Pore Surface Tailored SOD-Type Metal-Organic Zeolites. Adv. Mater. 2011, 23, 1268–1271. [Google Scholar] [CrossRef]
- Du, N.Y.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef]
- Tena, A.; Shishatskiy, S.; Filiz, V. Poly(ether-amide) vs. poly(ether-imide) copolymers for post-combustion membrane separation processes. RSC Adv. 2015, 5, 22310–22318. [Google Scholar] [CrossRef]
- Ghadimi, A.; Amirilargani, M.; Mohammadi, T.; Kasiri, N.; Sadatnia, B. Preparation of alloyed poly(ether block amide)/poly(ethylene glycol diacrylate) membranes for separation of CO2/H2 (syngas application). J. Membr. Sci. 2014, 458, 14–26. [Google Scholar] [CrossRef]
- Lin, R.; Chen, D.; Lin, Y.; Zhang, J.; Chen, X. A Zeolite-Like Zinc Triazolate Framework with High Gas Adsorption and Separation Performance. Inorg. Chem. 2012, 51, 9950–9955. [Google Scholar] [CrossRef]
- Ordonez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid (R) mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Vogiatzis, K.D.; Mavrandonakis, A.; Klopper, W.; Froudakis, G.E. Ab initio Study of the Interactions between CO2 and N-Containing Organic Heterocycles. ChemPhysChem 2009, 10, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, D.; Ding, R.; Gao, J.; Ruan, X.; Jiang, X.; He, G.; Wu, X. Constructing MOF-doped two-dimensional composite material ZIF-90@C3N4 mixed matrix membranes for CO2/N2 separation. Sep. Purif. Technol. 2022, 280, 119803. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Zhang, X.; Zhang, Y. MoS2 Nanosheets Functionalized Composite Mixed Matrix Membrane for Enhanced CO2 Capture via Surface Drop-Coating Method. ACS Appl. Mater. Interfaces 2016, 8, 23371–23378. [Google Scholar] [CrossRef]
- Habibiannejad, S.A.; Aroujalian, A.; Raisi, A. Pebax-1657 mixed matrix membrane containing surface modified multi-walled carbon nanotubes for gas separation. RSC Adv. 2016, 6, 79563–79577. [Google Scholar] [CrossRef]
- Zhu, H.; Yuan, J.; Zhao, J.; Liu, G.; Jin, W. Enhanced CO2/N2 separation performance by using dopamine/polyethyleneimine-grafted TiO2 nanoparticles filled PEBA mixed-matrix membranes. Sep. Purif. Technol. 2019, 214, 78–86. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Li, K.; Ma, C.; Zhang, J.; Li, H.; Chang, X.; Zhu, L.; Xue, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep. Purif. Technol. 2020, 248, 117080. [Google Scholar] [CrossRef]
- Deng, J.; Dai, Z.; Hou, J.; Deng, L. Morphologically Tunable MOF Nanosheets in Mixed Matrix Membranes for CO2 Separation. Chem. Mater. 2020, 32, 4174–4184. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Membr. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Lin, G.; Chen, C.; Wu, K.C.W.; Lin, C.; Tung, K. Characterization and molecular simulation of Pebax-1657-based mixed matrix membranes incorporating MoS2 nanosheets for carbon dioxide capture enhancement. J. Membr. Sci. 2019, 582, 358–366. [Google Scholar] [CrossRef]
- Aghaei, Z.; Naji, L.; Asl, V.H.; Khanbabaei, G.; Dezhagah, F. The influence of fumed silica content and particle size in poly (amide 6-b-ethylene oxide) mixed matrix membranes for gas separation. Sep. Purif. Technol. 2018, 199, 47–56. [Google Scholar] [CrossRef]
- Zheng, W.; Ding, R.; Yang, K.; Dai, Y.; Yan, X.; He, G. ZIF-8 nanoparticles with tunable size for enhanced CO2 capture of Pebax based MMMs. Sep. Purif. Technol. 2019, 214, 111–119. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, Y.; Ruan, X.; Zheng, W.; Yan, X.; Li, X.; He, G. ZIF-8 hollow nanotubes based mixed matrix membranes with high-speed gas transmission channel to promote CO2/N2 separation. J. Membr. Sci. 2021, 630, 119323. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.R.; Xu, N. Membranes with Fast and Selective Gas-Transport Channels of Laminar Graphene Oxide for Efficient CO2 Capture. Angew. Chem. 2015, 127, 588–592. [Google Scholar] [CrossRef]






| Membrane | Abbreviation |
|---|---|
| Pebax 1657 | P |
| Pebax 1657/MAF-7-1 | PM1 |
| Pebax 1657/MAF-7-3 | PM3 |
| Pebax 1657/MAF-7-5 | PM5 |
| Pebax 1657/MAF-7-7 | PM7 |
| Pebax 1657/MAF-7-9 | PM9 |
| Membrane | PCO2 | CO2/N2 | Refs. |
|---|---|---|---|
| (Barrer) | Selectivity | ||
| Pebax/MoS2 | 67 | 91 | [52] |
| Pebax/NIPAM-CNTs | 87 | 53 | [21] |
| Pebax/FS7 | 60.15 | 91.14 | [53] |
| Pebax/COF-5 | 493 | 49.3 | [51] |
| Pebax/MIL-53 | 95.7 | 49.9 | [26] |
| Pebax/ZIF-8 | 99.7 | 59.6 | [54] |
| Pebax/PHZ | 172.4 | 87.9 | [3] |
| Pebax/ZIF-C | 387.2 | 47.1 | [50] |
| Pebax/ZCN | 110.51 | 84.35 | [45] |
| Pebax/ZnO@ZIF-8 HNTs | 140 | 67 | [55] |
| Pebax/GO | 100 | 91 | [56] |
| Pebax 1657/MAF-7 | 76.15 | 124.84 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Y.; Chen, X.; Wang, Y.; He, M.; Shan, Y.; Li, Y.; Zhang, F.; Chen, X.; Kita, H. Preparation of Pebax 1657/MAF-7 Mixed Matrix Membranes with Enhanced CO2/N2 Separation by Active Site of Triazole Ligand. Membranes 2022, 12, 786. https://doi.org/10.3390/membranes12080786
Wang X, Zhang Y, Chen X, Wang Y, He M, Shan Y, Li Y, Zhang F, Chen X, Kita H. Preparation of Pebax 1657/MAF-7 Mixed Matrix Membranes with Enhanced CO2/N2 Separation by Active Site of Triazole Ligand. Membranes. 2022; 12(8):786. https://doi.org/10.3390/membranes12080786
Chicago/Turabian StyleWang, Xingqian, Yuping Zhang, Xinwei Chen, Yifei Wang, Mingliang He, Yongjiang Shan, Yuqin Li, Fei Zhang, Xiangshu Chen, and Hidetoshi Kita. 2022. "Preparation of Pebax 1657/MAF-7 Mixed Matrix Membranes with Enhanced CO2/N2 Separation by Active Site of Triazole Ligand" Membranes 12, no. 8: 786. https://doi.org/10.3390/membranes12080786
APA StyleWang, X., Zhang, Y., Chen, X., Wang, Y., He, M., Shan, Y., Li, Y., Zhang, F., Chen, X., & Kita, H. (2022). Preparation of Pebax 1657/MAF-7 Mixed Matrix Membranes with Enhanced CO2/N2 Separation by Active Site of Triazole Ligand. Membranes, 12(8), 786. https://doi.org/10.3390/membranes12080786