Micromechanism Study of Molecular Compatibility of PVDF/PEI Blend Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Blend Membrane
2.3. Membrane Characterization
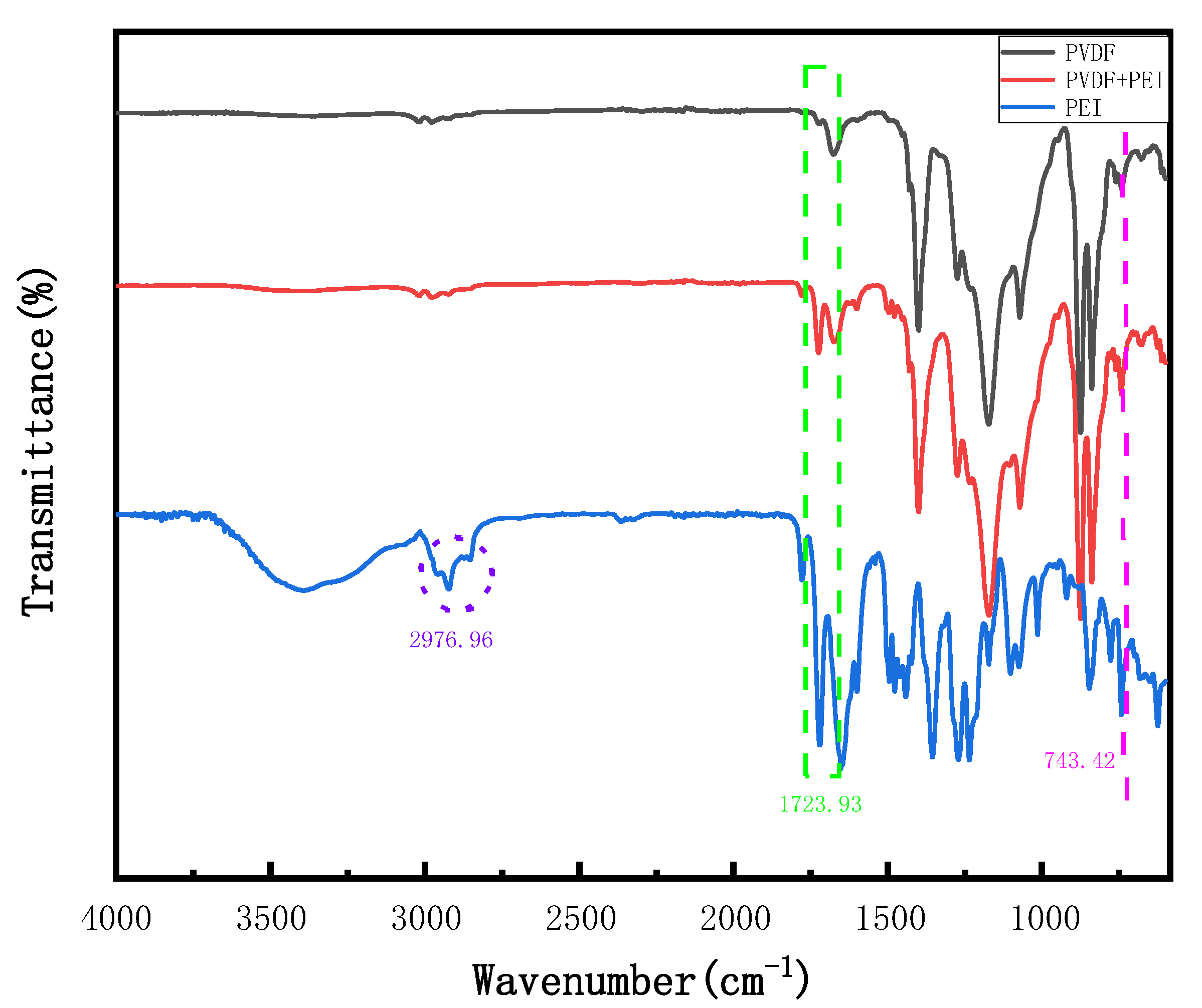
2.3.1. Basic Physicochemical Characterization
2.3.2. Crystalline Structure
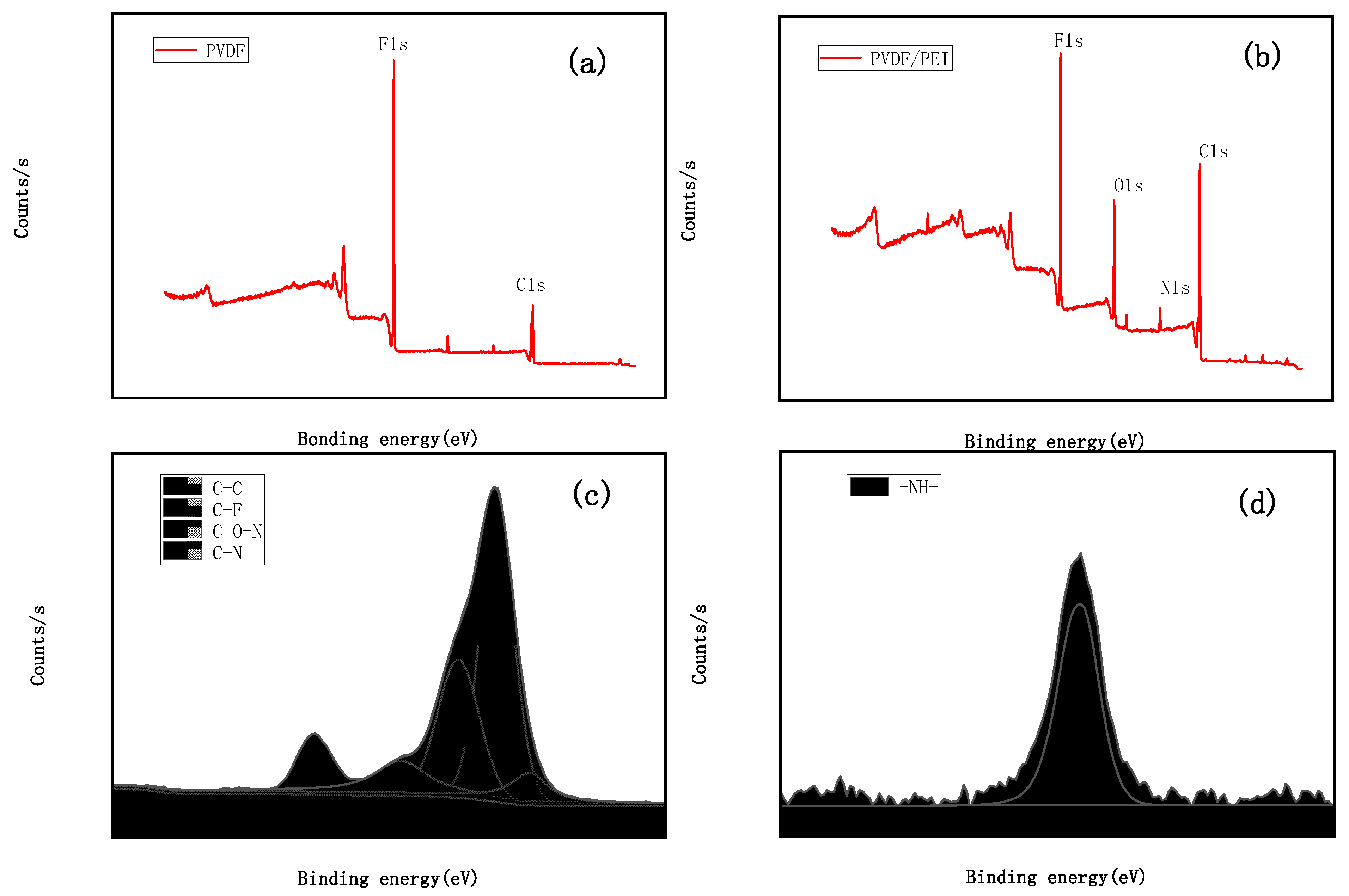
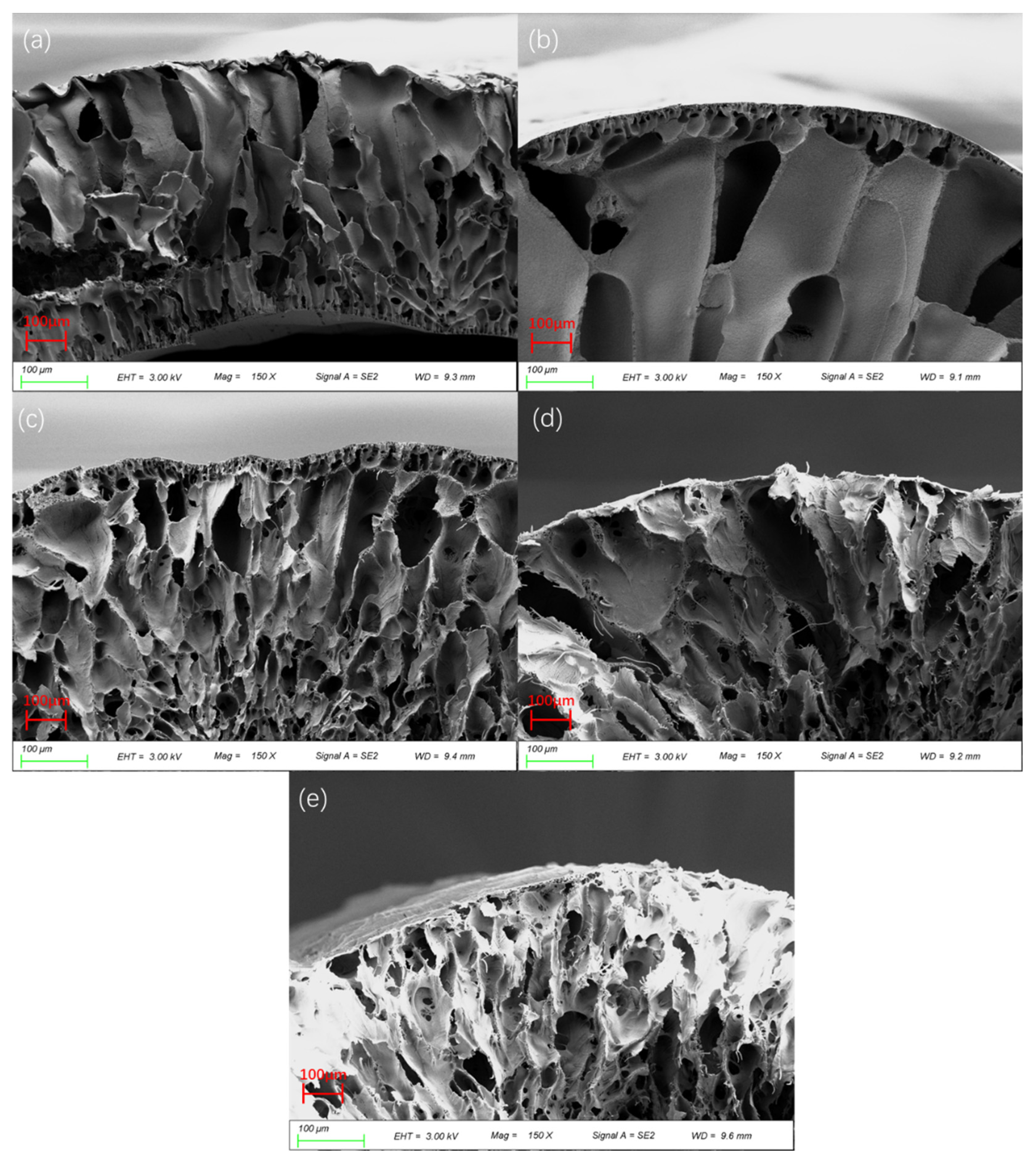
2.3.3. Membrane Morphology and Microstructure
2.3.4. Membrane Hydrophilicity
2.3.5. Membrane Porosity
2.3.6. Mechanical Properties of Membrane
2.3.7. The Permeability, Anti-Fouling Performance for the Membrane
3. Results and Discussion
3.1. Effect of PEI Content on Microstructure of Membrane
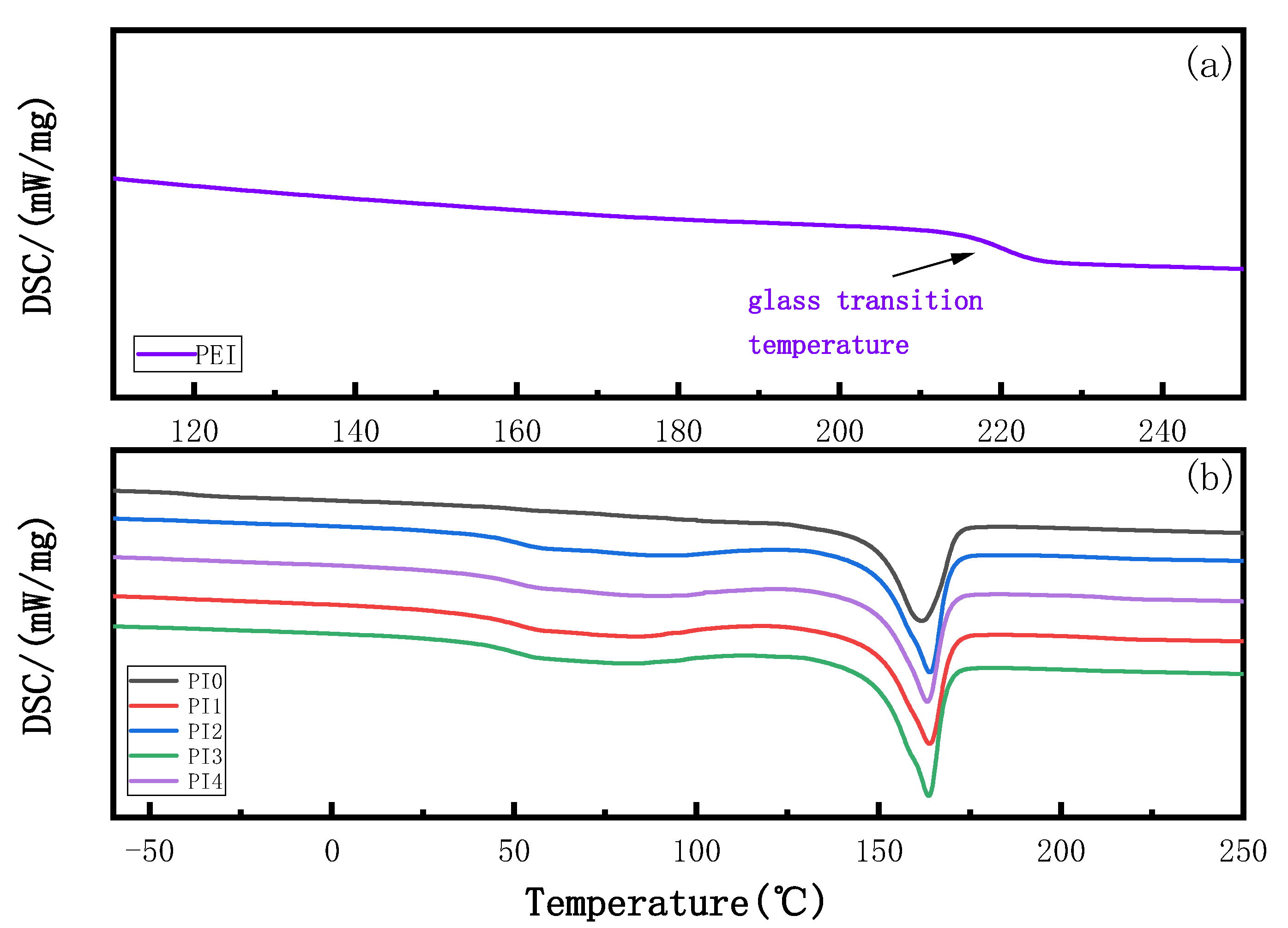
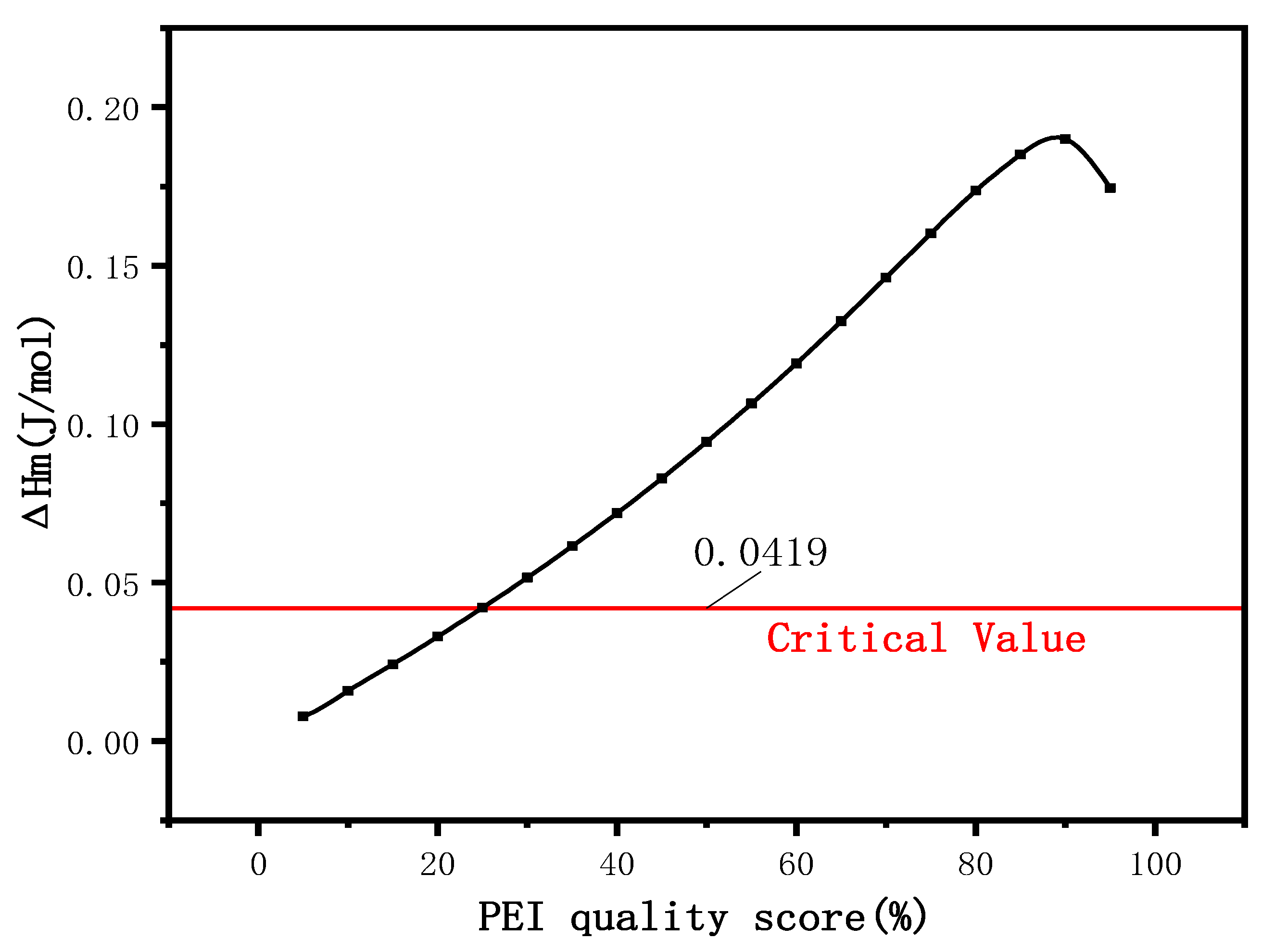
3.2. Effect of PEI Content on DSC of Membranes
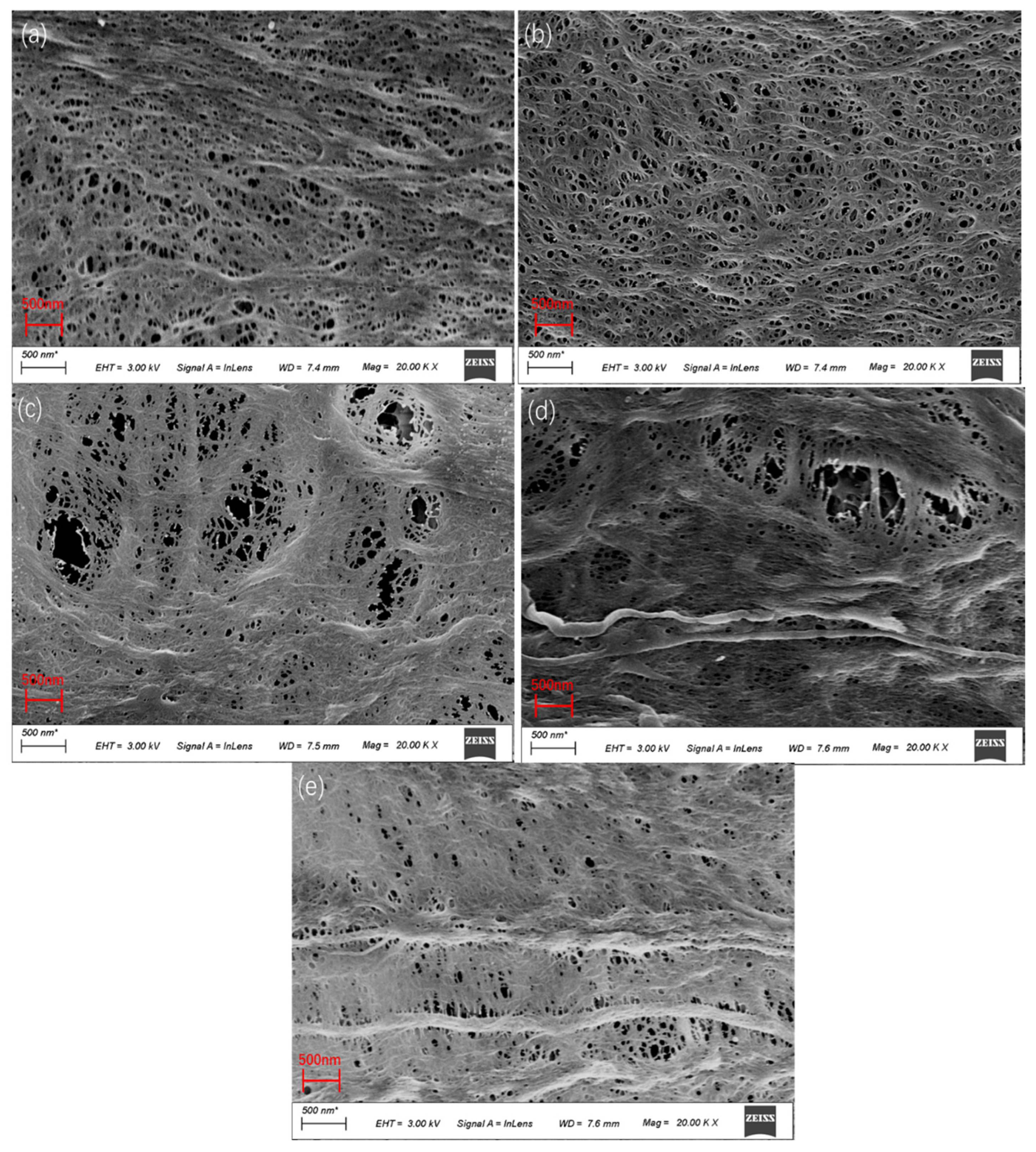
3.3. Effect of PEI Content on Membrane Morphology
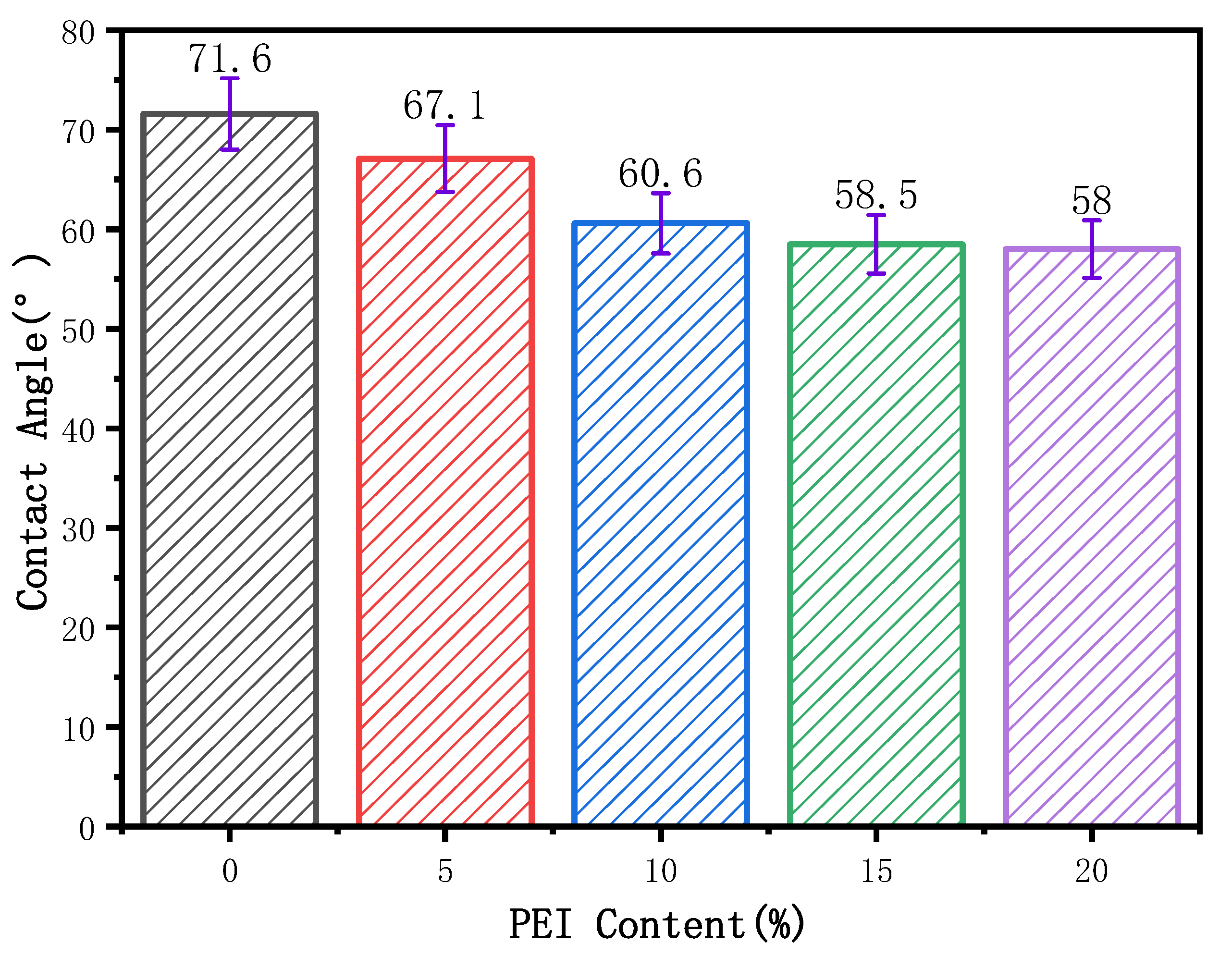
3.4. Effect of PEI Content on Membrane Water Contact Angle
3.5. Effect of PEI Content on Membrane Porosity
3.6. Effect of PEI Content on Mechanical Properties of Membrane
3.7. Filtration Test of Membrane
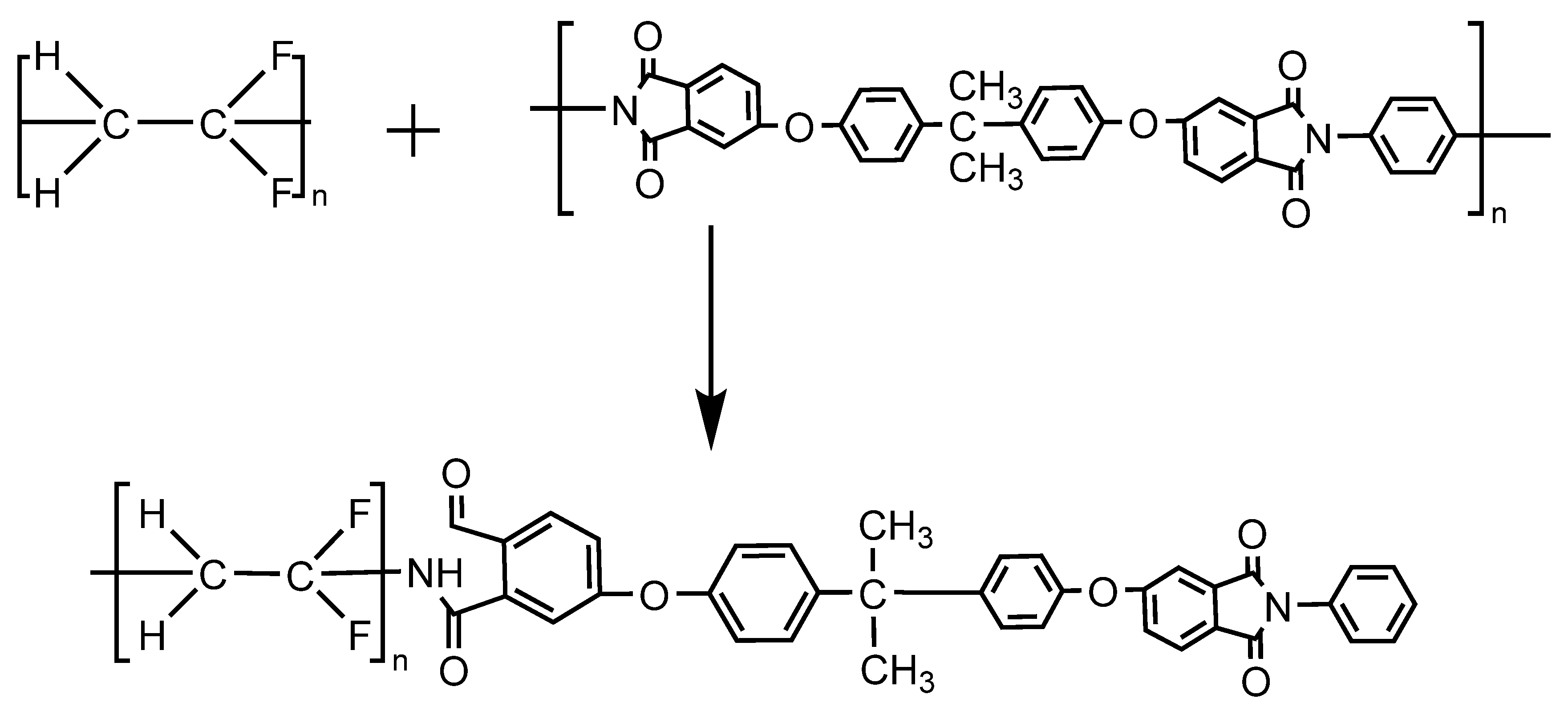
3.8. Compatibility Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javadi, O.; Zonouz, A.F.; Soltanieh, M.; Mousavi, S.A. PVDF/PU blend membrane separator for lithium-ion batteries via non-solvent-induced phase separation (NIPS). J. Solid State Electrochem. 2021, 25, 2385–2394. [Google Scholar] [CrossRef]
- Zhao, J.; Han, H.; Wang, Q.; Yan, C.; Li, D.; Yang, J.; Feng, X.; Yang, N.; Zhao, Y.; Chen, L. Hydrophilic and anti-fouling PVDF blend ultrafiltration membranes using polyacryloylmorpholine-based triblock copolymers as amphiphilic modifiers. React. Funct. Polym. 2019, 139, 92–101. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Xiao, K.; Sun, J.; Mo, Y.; Fang, Z.; Liang, P.; Huang, X.; Ma, J.; Ma, B. Effect of membrane pore morphology on microfiltration organic fouling: PTFE/PVDF blend membranes compared with PVDF membranes. Desalination 2014, 343, 217–225. [Google Scholar] [CrossRef]
- Salem, M.S.; El-Shazly, A.H.; Nady, N.; Elmarghany, M.R.; Sabry, M.N. PES/PVDF blend membrane and its composite with graphene nanoplates: Preparation, characterization, and water desalination via membrane distillation. Desalination Water Treat. 2019, 166, 9–23. [Google Scholar] [CrossRef]
- Ningrum, R.D.C.; Kusumawati, N. Development and Characterization of Polysulfone/Polyvinylidene Flouride Blend Membrane Induced by Delayed Liquid-Liquid Demixing. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 716–722. [Google Scholar] [CrossRef] [Green Version]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Grasso, G.; Galiano, F.; Yoo, M.J.; Mancuso, R.; Park, H.B.; Gabriele, B.; Figoli, A.; Drioli, E. Development of graphene-PVDF composite membranes for membrane distillation. J. Membr. Sci. 2020, 604, 118017. [Google Scholar] [CrossRef]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Wang, J.; Zhang, F.; Gao, S.; Wang, A.; Fang, W.; Jin, J. Zwitterionic Nanohydrogel Grafted PVDF Membranes with Comprehensive Antifouling Property and Superior Cycle Stability for Oil-in-Water Emulsion Separation. Adv. Funct. Mater. 2018, 28, 1804121. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, N.N.; Yang, N.; Hou, S.; Ma, J.; Zhang, L.; Sun, Y.; Jiang, B. Rapid and robust modification of PVDF ultrafiltration membranes with enhanced permselectivity, antifouling and antibacterial performance. Sep. Purif. Technol. 2021, 262, 118316. [Google Scholar] [CrossRef]
- Ashford, M.W.; Xu, C.; Molloy, J.J.; Carpenter-Warren, C.; Slawin, A.; Leach, A.G.; Watson, A. Catalytic Enantioselective Synthesis of Heterocyclic Vicinal Fluoroamines by Using Asymmetric Protonation: Method Development and Mechanistic Study. Chem. Eur. J. 2020, 26, 12249–12255. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, W.; Qian, X.; Wang, H.; Guo, C.; Li, N.; Xu, Z.; Teng, K.; Wang, Z. Positive charged PEI-TMC composite nanofiltration membrane for separation of Li+ and Mg2+ from brine with high Mg2+/Li+ ratio. Desalination 2019, 449, 57–68. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, X.; Da, X.; Qiu, M.; Chen, X.; Fan, Y. Fabrication of a charged PDA/PEI/Al2O3 composite nanofiltration membrane for desalination at high temperatures. Sep. Purif. Technol. 2021, 263, 118388. [Google Scholar] [CrossRef]
- Bandehali, S.; Moghadassi, A.; Parvizian, F.; Zhang, Y.; Hosseini, S.M.; Shen, J. New mixed matrix PEI nanofiltration membrane decorated by glycidyl-POSS functionalized graphene oxide nanoplates with enhanced separation and antifouling behaviour: Heavy metal ions removal. Sep. Purif. Technol. 2020, 242, 116745. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Cheng, Z.; Liu, M.; Ji, J.; Chang, X.; Xu, Q.-C.; Liu, Y.; Liu, X.; Qin, J. Mechanically strong and tough polyimide aerogels cross-linked with amine functionalized carbon nanotubes synthesized by fluorine displacement reaction. Compos. Sci. Technol. 2020, 195, 108204. [Google Scholar] [CrossRef]
- Yousefi, A.A. Influence of Polymer Blending on Crystalline Structure of Polyvinylidene Fluoride. Iran Polym. J. 2011, 20, 109–121. [Google Scholar]
- Ozekmekci, M.; Unlu, D.; Çopur, M. Removal of boron from industrial wastewater using PVP/PVDF blend membrane and GO/PVP/PVDF hybrid membrane by pervaporation. Korean J. Chem. Eng. 2021, 38, 1859–1869. [Google Scholar] [CrossRef]
- Kusumawati, N.; Santoso, A.B.; Wibawa, S.C.; Setiarso, P.; Muslim, S. Development of a New Polymer Membrane: Polyvinylidene fluoride/Polyetherimide Blend Membrane. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 2547. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P.; Gaur, M.S. Thermal analysis of polymer blends and double layer by DSC. Polym. Polym. Compos. 2020, 29, S11–S18. [Google Scholar] [CrossRef]
- Alvaredo-Atienza, A.; Chen, L.; San-Miguel, V.; Ridruejo, A.; Fernandez-Blazquez, J.P. Fabrication and Characterization of PEEK/PEI Multilayer Composites. Polymers 2020, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, N.; Setiarso, P.; Budi Santoso, A.; Chendra Wibawa, S.; Muslim, S. The development of PVDF/PEI blended membrane: Effect of stirring time on membrane characteristics and performance. Rasayan J. Chem. 2019, 12, 975–986. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, B.; Ji, L. Preparation of PVDF/PEI double-layer composite hollow fiber membranes for enhancing tensile strength of PVDF membranes. Membr. Water Treat. 2014, 5, 109–122. [Google Scholar] [CrossRef]
- Buckley, D.J. Aspects of polymer compatibility. Ann. N. Y. Acad. Sci. 1967, 29, 735–747. [Google Scholar] [CrossRef]



| Blend Membrane | PEI/PVDF (17 wt.%) | PVP (6 wt.%) | DMAC (77 wt.%) |
|---|---|---|---|
| PI0 | 0/100 | 6 | 77 |
| PI1 | 5/95 | 6 | 77 |
| PI2 | 10/90 | 6 | 77 |
| PI3 | 15/85 | 6 | 77 |
| PI4 | 20/80 | 6 | 77 |
| Element Type | Element Content (%) | |
|---|---|---|
| PVDF | PVDF/PEI | |
| F | 42.99 | 18.00 |
| N | 3.13 | 4.10 |
| O | 4.34 | 14.43 |
| C | 49.55 | 63.47 |
| PVDF Blends | F (β) (%) |
|---|---|
| PVDF | 90.73 |
| PVDF/PEI | 93.62 |
| Blend Membrane | Glass Transition Temperature (Tg) | Melting Temperature (Tm) | Enthalpy (J/g) | ||
|---|---|---|---|---|---|
| Onset | Inflect Point | Endset | |||
| PI0 | −46.82 °C | −40.65 °C | −35.06 °C | 161.82 °C | 38.66 |
| PI1 | 41.39 °C | 47.57 °C | 54.02 °C | 163.76 °C | 42.77 |
| PI2 | 42.22 °C | 48.88 °C | 55.70 °C | 163.93 °C | 43.54 |
| PI3 | 42.99 °C | 50.08 °C | 57.18 °C | 163.95 °C | 46.25 |
| PI4 | 42.04 °C | 48.08 °C | 55.36 °C | 163.36 °C | 37.90 |
| PEI | 212.64 °C | 220.07 °C | 227.11 °C | --- | --- |
| PVDF/PEI Membrane with Different Components | PI0 | PI1 | PI2 | PI3 | PI4 |
|---|---|---|---|---|---|
| Porosity (%) | 7.35 | 13.47 | 16.41 | 17.33 | 6.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Zhu, Y.; Yan, J.; Wu, W.; Wang, B. Micromechanism Study of Molecular Compatibility of PVDF/PEI Blend Membrane. Membranes 2022, 12, 809. https://doi.org/10.3390/membranes12080809
Gao M, Zhu Y, Yan J, Wu W, Wang B. Micromechanism Study of Molecular Compatibility of PVDF/PEI Blend Membrane. Membranes. 2022; 12(8):809. https://doi.org/10.3390/membranes12080809
Chicago/Turabian StyleGao, Ming, Yuanlu Zhu, Jiangyi Yan, Weixing Wu, and Beifu Wang. 2022. "Micromechanism Study of Molecular Compatibility of PVDF/PEI Blend Membrane" Membranes 12, no. 8: 809. https://doi.org/10.3390/membranes12080809
APA StyleGao, M., Zhu, Y., Yan, J., Wu, W., & Wang, B. (2022). Micromechanism Study of Molecular Compatibility of PVDF/PEI Blend Membrane. Membranes, 12(8), 809. https://doi.org/10.3390/membranes12080809






