Carbon-Based Transducers for Solid-Contact Calcium Ion-Selective Electrodes: Mesopore and Nitrogen-Doping Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Materials
2.3. Material Characterizations
2.4. Electrode Pretreatment
2.5. Electrochemical Measurements
3. Results
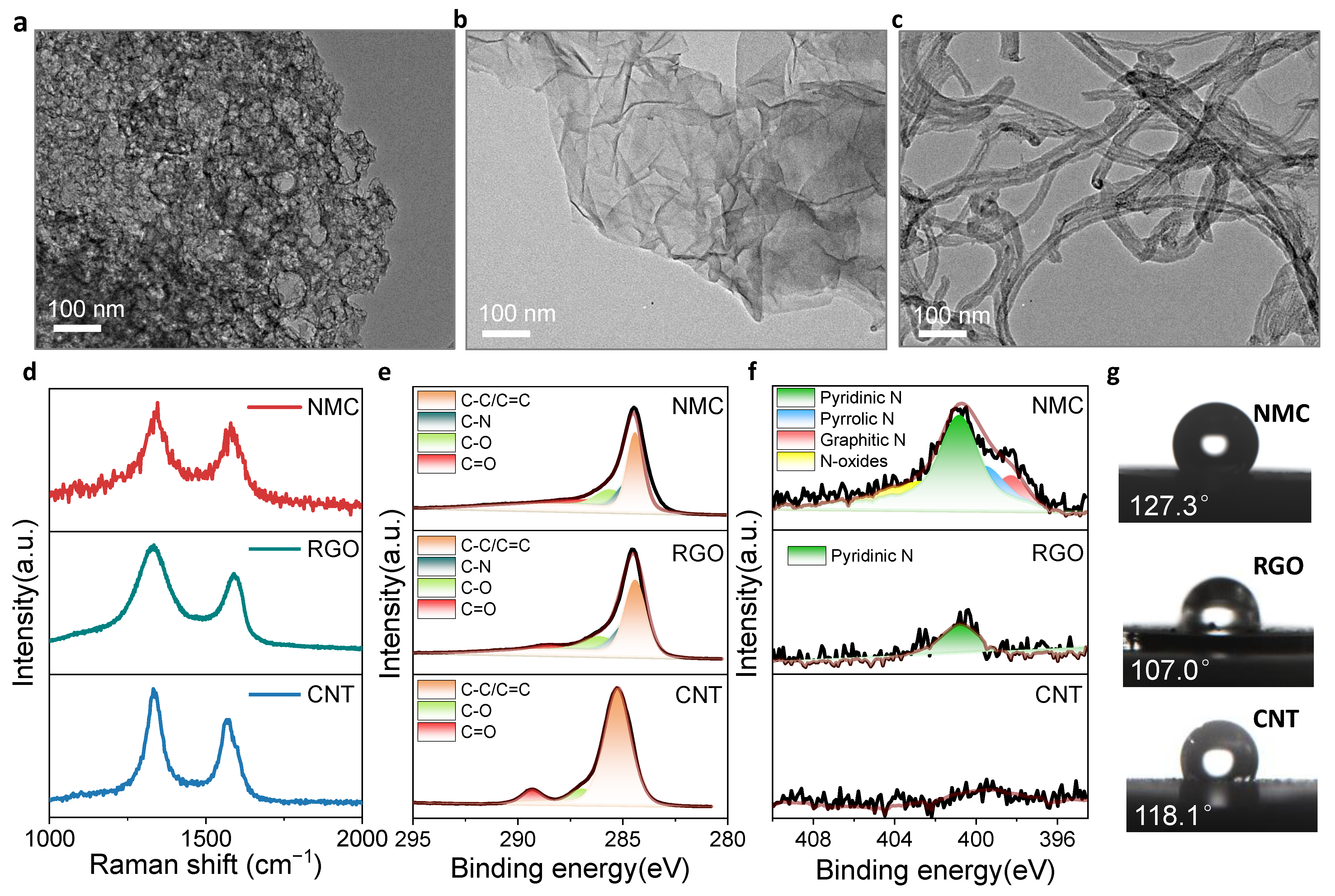
3.1. Structure Comparison for NMC, RGO and CNT
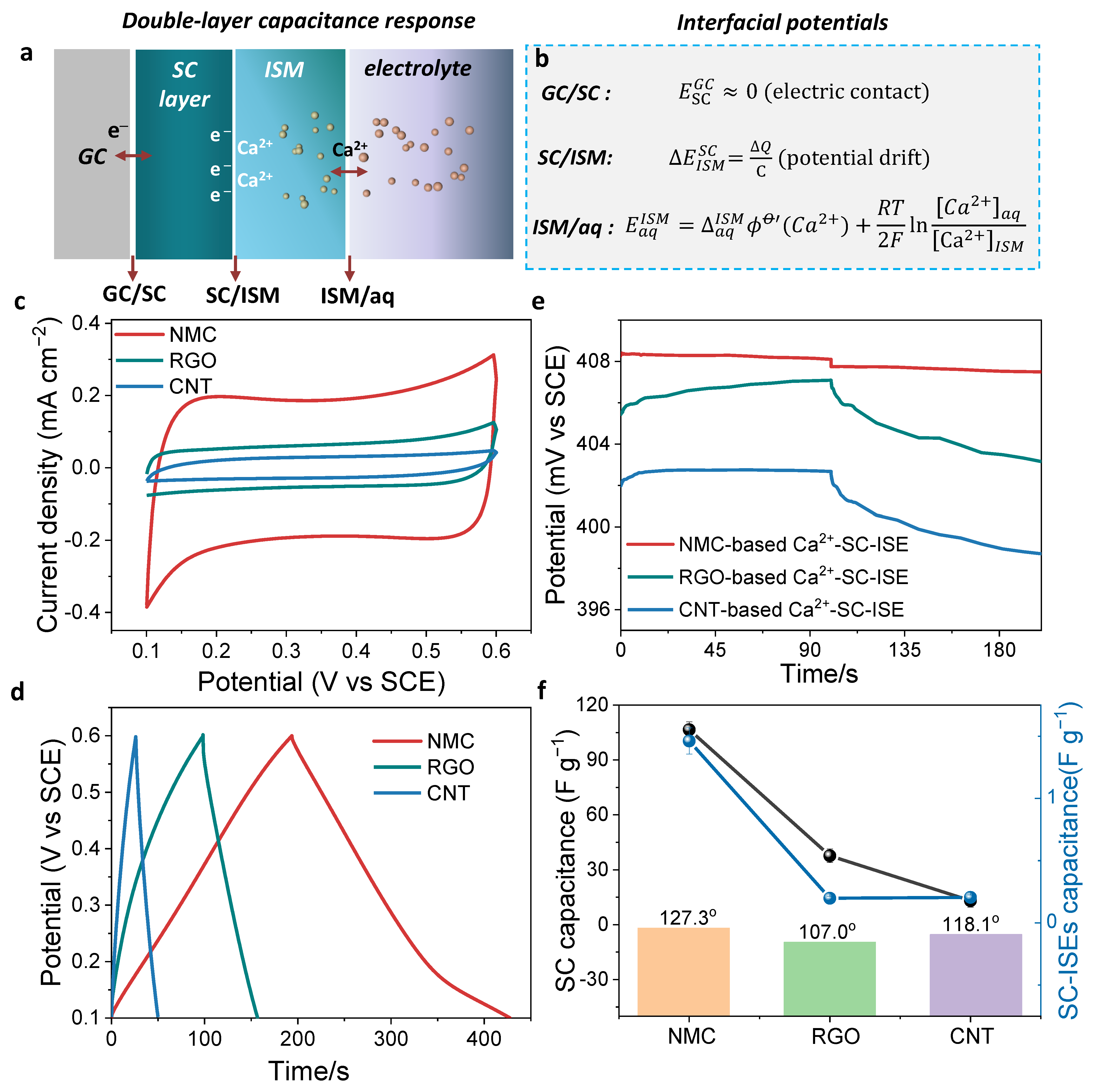
3.2. Capacitance Evaluation
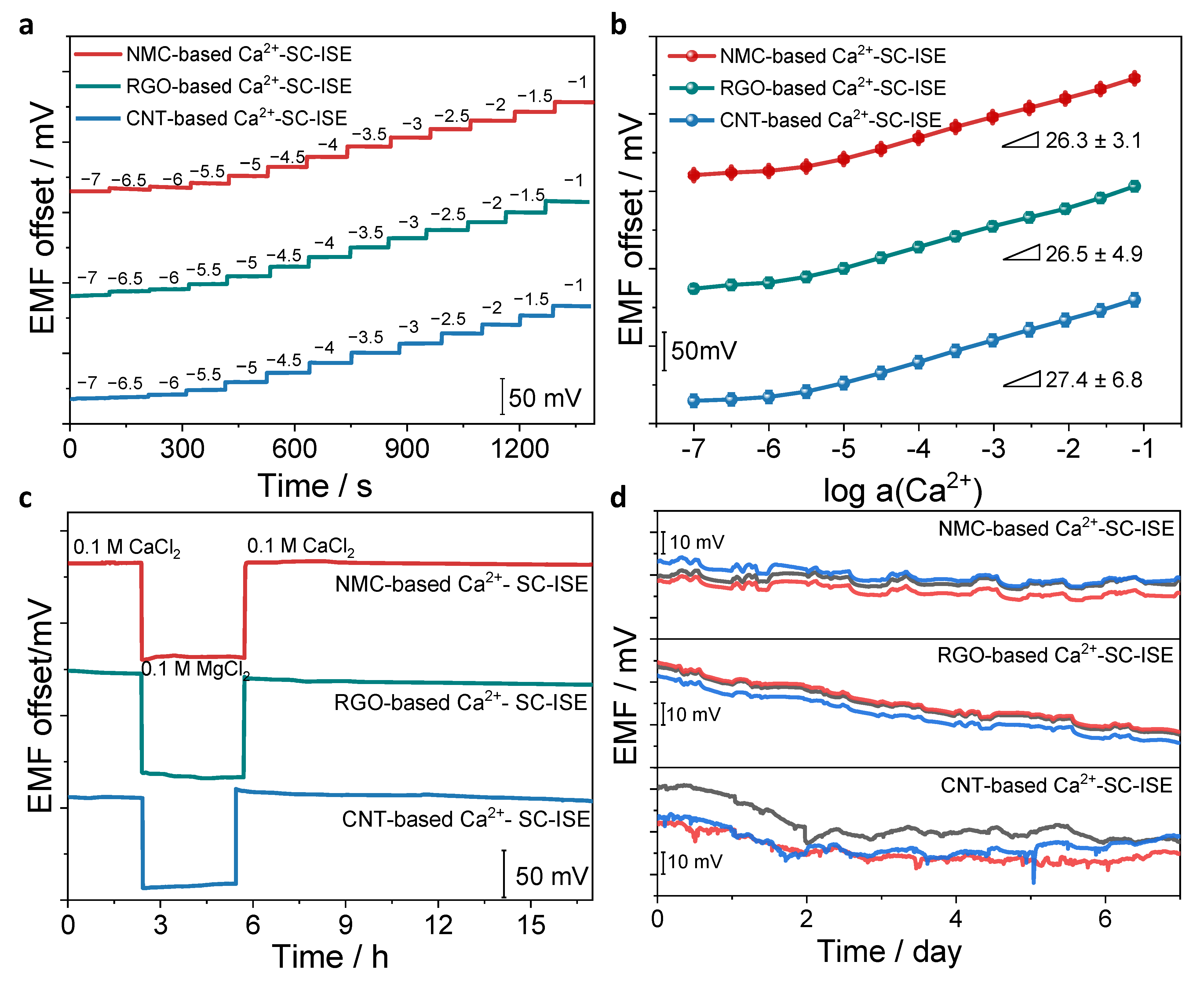
3.3. Analytical Performances
3.4. Calcium Detection in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2021, 93, 72–102. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Ge, L.; Lisak, G. Highly reproducible solid contact ion selective electrodes: Emerging opportunities for potentiometry-A review. Anal. Chim. Acta 2021, 1162, 338304. [Google Scholar] [CrossRef] [PubMed]
- Anastasova-Ivanova, S.; Mattinen, U.; Radu, A.; Bobacka, J.; Lewenstam, A.; Migdalski, J.; Danielewskic, M.; Diamond, D. Development of miniature all-solid-state potentiometric sensing system. Sens. Actuators B-Chem. 2010, 146, 199–205. [Google Scholar] [CrossRef]
- Jaramillo, E.A.; Noell, A.C. Development of Miniature Solid Contact Ion Selective Electrodes for in situ Instrumentation. Electroanalysis 2020, 32, 1896–1904. [Google Scholar] [CrossRef]
- Athavale, R.; Kokorite, I.; Dinkel, C.; Bakker, E.; Wehrli, B.; Crespo, G.A.; Brand, A. In Situ Ammonium Profiling Using Solid-Contact Ion-Selective Electrodes in Eutrophic Lakes. Anal. Chem. 2015, 87, 11990–11997. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.A. Recent Advances in Ion-selective membrane electrodes for in situ environmental water analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Qi, L.; Liang, R.; Jiang, T.; Qin, W. Anti-fouling polymeric membrane ion-selective electrodes. TrAC-Trends Anal. Chem. 2022, 150, 116572. [Google Scholar] [CrossRef]
- van de Velde, L.; d’Angremont, E.; Olthuis, W. Solid contact potassium selective electrodes for biomedical applications—A review. Talanta 2016, 160, 56–65. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. TrAC-Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Duezguen, A.; Maroto, A.; Mairal, T.; O’Sullivan, C.; Rius, F.X. Solid-contact potentiometric aptasensor based on aptamer functionalized carbon nanotubes for the direct determination of proteins. Analyst 2010, 135, 1037–1041. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC-Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Lv, E.; Li, Y.; Ding, J.; Qin, W. Magnetic-Field-Driven Extraction of Bioreceptors into Polymeric Membranes for Label-Free Potentiometric Biosensing. Angew. Chem. Int. Ed. 2021, 60, 2609–2613. [Google Scholar] [CrossRef]
- Huang, M.-R.; Li, X.-G. Highly sensing and transducing materials for potentiometric ion sensors with versatile applicability. Prog. Mater. Sci. 2022, 125, 100885. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Buehlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC-Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, L.; Gan, S.; Tang, Y.; Qiu, S.; Lyu, Y.; Ma, Y.; Niu, L. Defective vs high-quality graphene for solid-contact ion-selective electrodes: Effects of capacitance and hydrophobicity. Electrochem. Commun. 2021, 129, 107091. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors based on conducting polymers. Electroanalysis 2003, 15, 366–374. [Google Scholar] [CrossRef]
- Bobacka, J. Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Vanamo, U.; Bobacka, J. Electrochemical control of the standard potential of solid-contact ion-selective electrodes having a conducting polymer as ion-to-electron transducer. Electrochim. Acta 2014, 122, 316–321. [Google Scholar] [CrossRef]
- Bahro, C.; Goswami, S.; Gernhart, S.; Koley, D. Calibration-free Solid-State Ion-Selective Electrode Based on a Polarized PEDOT/PEDOT-S-Doped Copolymer as Back Contact. Anal. Chem. 2022, 94, 8302–8308. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Qin, W. A solid-contact potassium-selective electrode with MoO2 microspheres as ion-to-electron transducer. Anal. Chim. Acta 2017, 982, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Qin, W. All-Solid-State Polymeric Membrane Ion-Selective Electrodes Based on NiCo(2)S(4)as a Solid Contact. Anal. Chem. 2022, 94, 3574–3580. [Google Scholar] [CrossRef]
- Zhou, M.; Gan, S.; Cai, B.; Li, F.; Ma, W.; Han, D.; Niu, L. Effective Solid Contact for Ion-Selective Electrodes: Tetrakis(4-chlorophenyl)borate (TB-) Anions Doped Nanocluster Films. Anal. Chem. 2012, 84, 3480–3483. [Google Scholar] [CrossRef]
- Zou, X.U.; Cheong, J.H.; Taitt, B.J.; Buehlmann, P. Solid Contact Ion-Selective Electrodes with a Well-Controlled Co(II)/Co(III) Redox Buffer Layer. Anal. Chem. 2013, 85, 9350–9355. [Google Scholar] [CrossRef]
- Zou, X.U.; Zhen, X.V.; Cheong, J.H.; Buehlmann, P. Calibration-Free Ionophore-Based Ion-Selective Electrodes With a Co(II)/Co(III) Redox Couple-Based Solid Contact. Anal. Chem. 2014, 86, 8687–8692. [Google Scholar] [CrossRef]
- Jaworska, E.; Naitana, M.L.; Stelmach, E.; Pomarico, G.; Wojciechowski, M.; Bulska, E.; Maksymiuk, K.; Paolesse, R.; Michalska, A. Introducing Cobalt(II) Porphyrin/Cobalt(III) Corrole Containing Transducers for Improved Potential Reproducibility and Performance of All-Solid-State Ion-Selective Electrodes. Anal. Chem. 2017, 89, 7107–7114. [Google Scholar] [CrossRef]
- Zhen, X.V.; Rousseau, C.R.; Buhlmann, P. Redox Buffer Capacity of Ion-Selective Electrode Solid Contacts Doped with Organometallic Complexes. Anal. Chem. 2018, 90, 11000–11007. [Google Scholar] [CrossRef]
- Jaworska, E.; Wojcik, M.; Kisiel, A.; Mieczkowski, J.; Michalska, A. Gold nanoparticles solid contact for ion-selective electrodes of highly stable potential readings. Talanta 2011, 85, 1986–1989. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupien, K. Potentiometric Sensors with Carbon Black Supporting Platinum Nanoparticles. Anal. Chem. 2013, 85, 10255–10261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, T.; Qin, W. An effective solid contact for an all-solid-state polymeric membrane Cd2+-selective electrode: Three-dimensional porous graphene-mesoporous platinum nanoparticle composite. Sens. Actuators B-Chem. 2017, 239, 438–446. [Google Scholar] [CrossRef]
- Criscuolo, F.; Taurino, I.; Stradolini, F.; Carrara, S.; De Micheli, G. Highly-stable Li+ ion-selective electrodes based on noble metal nanostructured layers as solid-contacts. Anal. Chim. Acta 2018, 1027, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens. Actuators B-Chem. 2002, 82, 7–13. [Google Scholar] [CrossRef]
- Guzinski, M.; Jarvis, J.M.; Perez, F.; Pendley, B.D.; Lindner, E.; De Marco, R.; Crespo, G.A.; Acres, R.G.; Walker, R.; Bishop, J. PEDOT(PSS) as Solid Contact for Ion-Selective Electrodes: The Influence of the PEDOT(PSS) Film Thickness on the Equilibration Times. Anal. Chem. 2017, 89, 3508–3516. [Google Scholar] [CrossRef]
- Guzinski, M.; Jarvis, J.M.; D’Orazio, P.; Izadyar, A.; Pendley, B.D.; Lindner, E. Solid-Contact pH Sensor without CO2 Interference with a Superhydrophobic PEDOT-C-14 as Solid Contact: The Ultimate “Water Layer” Test. Anal. Chem. 2017, 89, 8468–8475. [Google Scholar] [CrossRef]
- He, N.; Papp, S.; Lindfors, T.; Hofler, L.; Latonen, R.-M.; Gyurcsanyi, R.E. Pre-Polarized Hydrophobic Conducting Polymer Solid-Contact Ion Selective Electrodes with Improved Potential Reproducibility. Anal. Chem. 2017, 89, 2598–2605. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Lai, C.-Z.; Fierke, M.A.; Stein, A.; Buehlmann, P. Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact. Anal. Chem. 2007, 79, 4621–4626. [Google Scholar] [CrossRef]
- Lai, C.-Z.; Joyer, M.M.; Fierke, M.A.; Petkovich, N.D.; Stein, A.; Buhlmann, P. Subnanomolar detection limit application of ion-selective electrodes with three-dimensionally ordered macroporous (3DOM) carbon solid contacts. J. Solid State Electrochem. 2009, 13, 123–128. [Google Scholar] [CrossRef]
- Fierke, M.A.; Lai, C.-Z.; Buhlmann, P.; Stein, A. Effects of Architecture and Surface Chemistry of Three-Dimensionally Ordered Macroporous Carbon Solid Contacts on Performance of Ion-Selective Electrodes. Anal. Chem. 2010, 82, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, F.; Gan, S.; Jiang, Y.; An, Q.; Zhang, Q.; Niu, L. Using sp(2)-C dominant porous carbon sub-micrometer spheres as solid transducers in ion-selective electrodes. Electrochem. Commun. 2015, 50, 60–63. [Google Scholar] [CrossRef]
- Jiang, T.; Yin, B.; Liu, X.; Yang, L.; Pang, H.; Song, J.; Wu, S. Porous carbon-based robust, durable, and flexible electrochemical device for K+ detection in sweat. Analyst 2022, 147, 1144–1151. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an all-solid-state potassium ion-selective electrode using graphene as the solid-contact transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Hernandez, R.; Riu, J.; Bobacka, J.; Valles, C.; Jimenez, P.; Benito, A.M.; Maser, W.K.; Xavier Rius, F. Reduced Graphene Oxide Films as Solid Transducers in Potentiometric All-Solid-State Ion-Selective Electrodes. J. Phys. Chem. C 2012, 116, 22570–22578. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Zhou, M.; Gan, S.; Zhang, Q.; Han, D.; Niu, L. All-solid-state potassium-selective electrode using graphene as the solid contact. Analyst 2012, 137, 618–623. [Google Scholar] [PubMed]
- Ping, J.; Wang, Y.; Ying, Y.; Wu, J. Application of Electrochemically Reduced Graphene Oxide on Screen-Printed Ion-Selective Electrode. Anal. Chem. 2012, 84, 3473–3479. [Google Scholar]
- Yeung, K.K.; Li, J.; Huang, T.; Hosseini, I.I.; Al Mahdi, R.; Alam, M.M.; Sun, H.; Mahshid, S.; Yang, J.; Ye, T.T.; et al. Utilizing Gradient Porous Graphene Substrate as the Solid-Contact Layer To Enhance Wearable Electrochemical Sweat Sensor Sensitivity. Nano Lett. 2022, 22, 6647–6654. [Google Scholar]
- Crespo, G.A.; Macho, S.; Xavier Rius, F. Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal. Chem. 2008, 80, 1316–1322. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Bobacka, J.; Rius, F.X. Transduction Mechanism of Carbon Nanotubes in Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2009, 81, 676–681. [Google Scholar] [CrossRef]
- Xavier Rius-Ruiz, F.; Crespo, G.A.; Bejarano-Nosas, D.; Blondeau, P.; Riu, J.; Xavier Rius, F. Potentiometric Strip Cell Based on Carbon Nanotubes as Transducer Layer: Toward Low-Cost Decentralized Measurements. Anal. Chem. 2011, 83, 8810–8815. [Google Scholar] [CrossRef]
- Yuan, D.; Anthis, A.H.C.; Afshar, M.G.; Pankratova, N.; Cuartero, M.; Crespo, G.A.; Bakker, E. All-Solid-State Potentiometric Sensors with a Multiwalled Carbon Nanotube Inner Transducing Layer for Anion Detection in Environmental Samples. Anal. Chem. 2015, 87, 8640–8645. [Google Scholar] [CrossRef]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon Nanotube-Based Ion Selective Sensors for Wearable Applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Abidin, A.F.Z.; Loh, K.S.; Wong, W.Y.; Mohamad, A.B.; Puspasari, I. Effect of carbon precursor and initial pH on cobalt-doped carbon xerogel for oxygen reduction. Int. J. Hydrog. Energy 2018, 43, 11047–11055. [Google Scholar] [CrossRef]
- Cha, J.H.; Shin, G.J.; Kang, M.J.; Lee, H.I.; Rhee, K.Y.; Park, S.J. A study on the effect of electron acceptor-donor interactions on the mechanical and interfacial properties of carbon black/natural rubber composites. Compos. Pt. B-Eng. 2018, 136, 143–148. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Lau, A.; Ma, T.; Lin, H.; Jia, B. Hybridized Graphene for Supercapacitors: Beyond the Limitation of Pure Graphene. Small 2021, 17, 2007311. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yoon, H.; Abu Zahed, M.; Zhang, S.; Yoon, S.; Kim, D.; Kim, D.; Park, J. A Highly Stable and Flexible Ca2+ Ion-Selective Sensor Based on Treated PEDOT:PSS Transducing Laser. IEEE Sens. J. 2022, 22, 11213–11221. [Google Scholar] [CrossRef]
- Hernandez, R.; Riu, J.; Rius, F.X. Determination of calcium ion in sap using carbon nanotube-based ion-selective electrodes. Analyst 2010, 135, 1979–1985. [Google Scholar] [CrossRef]
- Shao, Y.Z.; Yao, Y.; Jiang, C.M.; Zhao, F.N.; Liu, X.X.; Ying, Y.B.; Ping, J.F. Two-dimensional MXene nanosheets (types Ti3C2Tx and Ti2CTx) as new ion-to-electron transducers in solid-contact calcium ion-selective electrodes. Microchim. Acta 2019, 186, 750. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.M.; Yao, Y.; Cai, Y.L.; Ping, J.F. All-solid-state potentiometric sensor using single-walled carbon nanohorns as transducer. Sens. Actuator B-Chem. 2019, 283, 284–289. [Google Scholar] [CrossRef]
- Zeng, X.Z.; Qin, W. A solid-contact Ca2+-selective electrode based on an inorganic redox buffer of Ag@AgCl/1-tetradecyl-3-methylimidazolium chloride as ion-to-electron transducer. Talanta 2020, 209, 120570. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.J.; Fu, M.L.; Liang, R.N. Solid-contact Ca2+-selective electrodes based on two-dimensional black phosphorus as ion-to-electron transducers. RSC Adv. 2017, 7, 43905–43908. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tang, Y.; Liang, R.; Zhong, L.; Xu, J.; Lu, H.; Xu, X.; Han, T.; Bao, Y.; Ma, Y.; et al. Carbon-Based Transducers for Solid-Contact Calcium Ion-Selective Electrodes: Mesopore and Nitrogen-Doping Effects. Membranes 2022, 12, 903. https://doi.org/10.3390/membranes12090903
Zhang Y, Tang Y, Liang R, Zhong L, Xu J, Lu H, Xu X, Han T, Bao Y, Ma Y, et al. Carbon-Based Transducers for Solid-Contact Calcium Ion-Selective Electrodes: Mesopore and Nitrogen-Doping Effects. Membranes. 2022; 12(9):903. https://doi.org/10.3390/membranes12090903
Chicago/Turabian StyleZhang, Yirong, Yitian Tang, Rongfeng Liang, Lijie Zhong, Jiexian Xu, Huici Lu, Xiaofeng Xu, Tingting Han, Yu Bao, Yingming Ma, and et al. 2022. "Carbon-Based Transducers for Solid-Contact Calcium Ion-Selective Electrodes: Mesopore and Nitrogen-Doping Effects" Membranes 12, no. 9: 903. https://doi.org/10.3390/membranes12090903
APA StyleZhang, Y., Tang, Y., Liang, R., Zhong, L., Xu, J., Lu, H., Xu, X., Han, T., Bao, Y., Ma, Y., Gan, S., & Niu, L. (2022). Carbon-Based Transducers for Solid-Contact Calcium Ion-Selective Electrodes: Mesopore and Nitrogen-Doping Effects. Membranes, 12(9), 903. https://doi.org/10.3390/membranes12090903






