Poly(ethylene oxide)-Based Copolymer-IL Composite Membranes for CO2 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
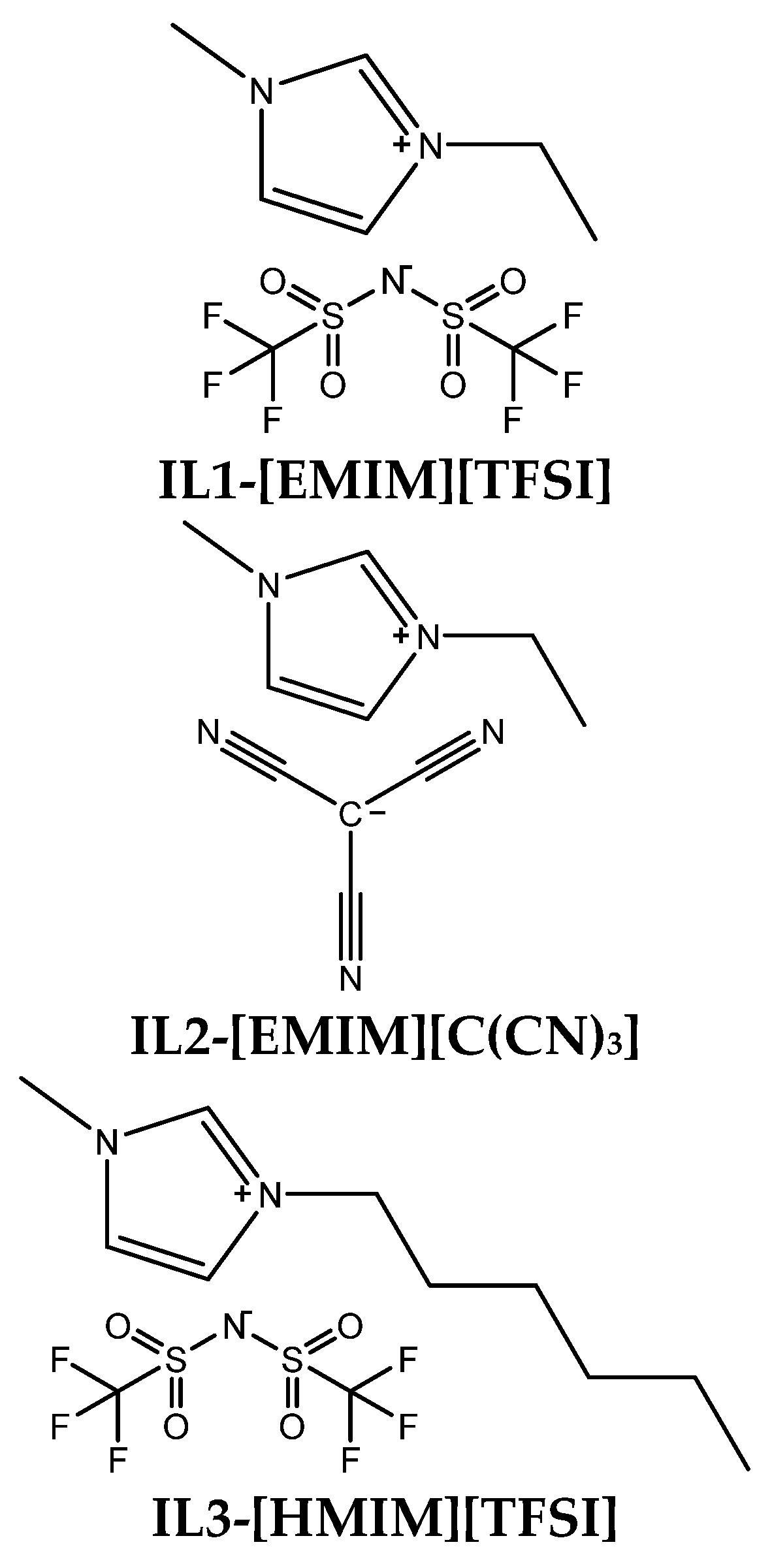
2.2. IL Preparation
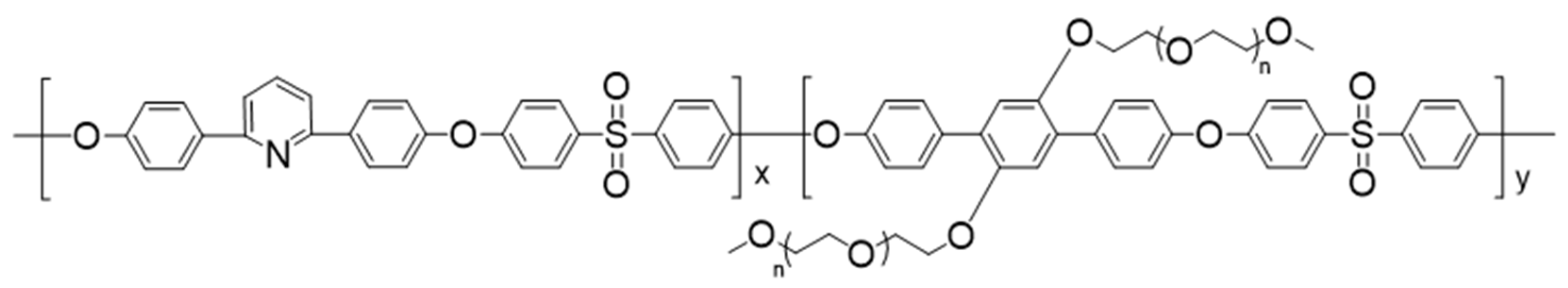
2.3. Synthesis of Precursor Copolymer (2,6Py-PEO350)
2.4. Membrane Preparation
2.5. Membrane Characterization
2.6. Single Gas and Gas/Water Vapor Permeation Properties
3. Results and Discussion
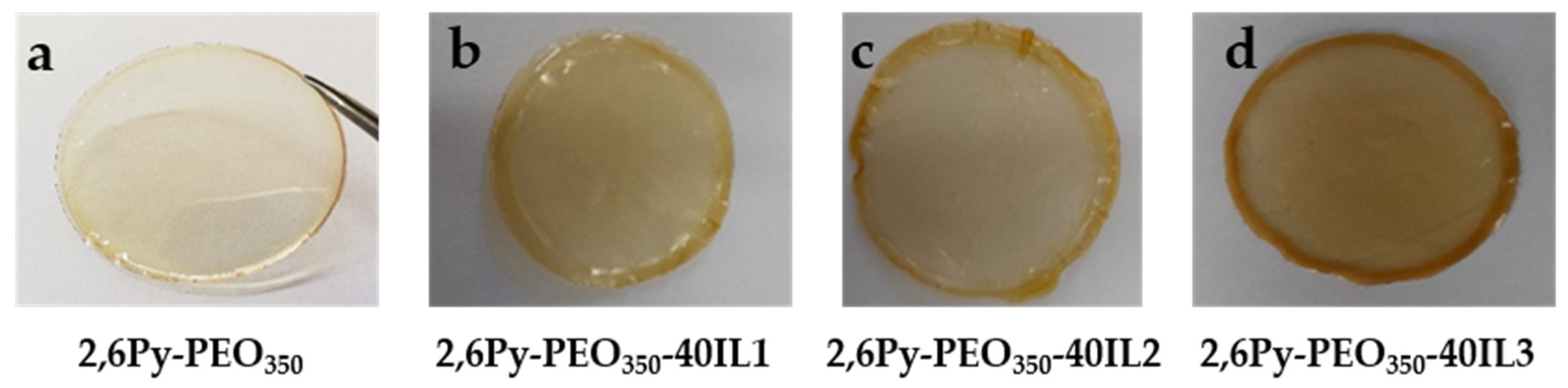
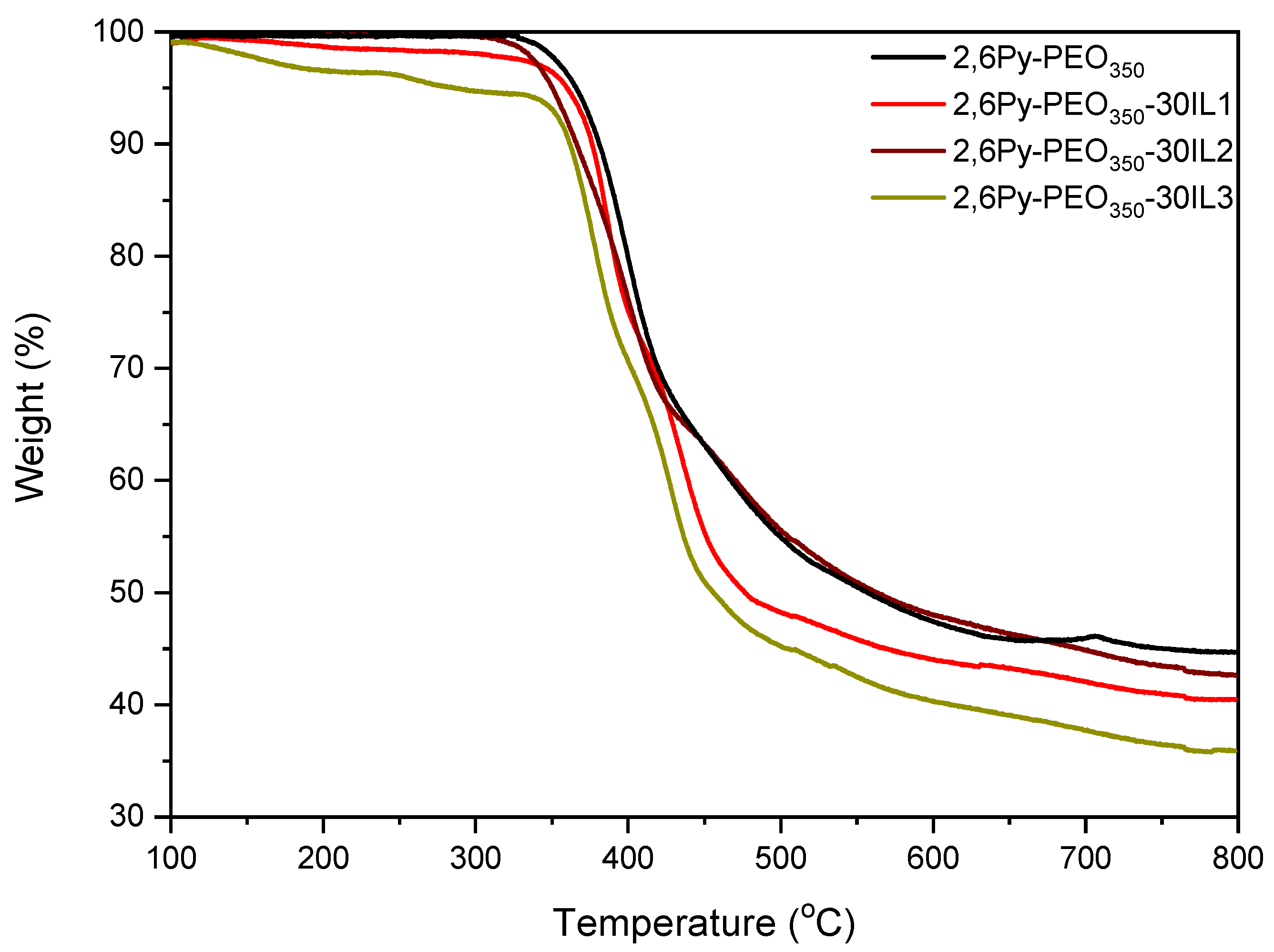
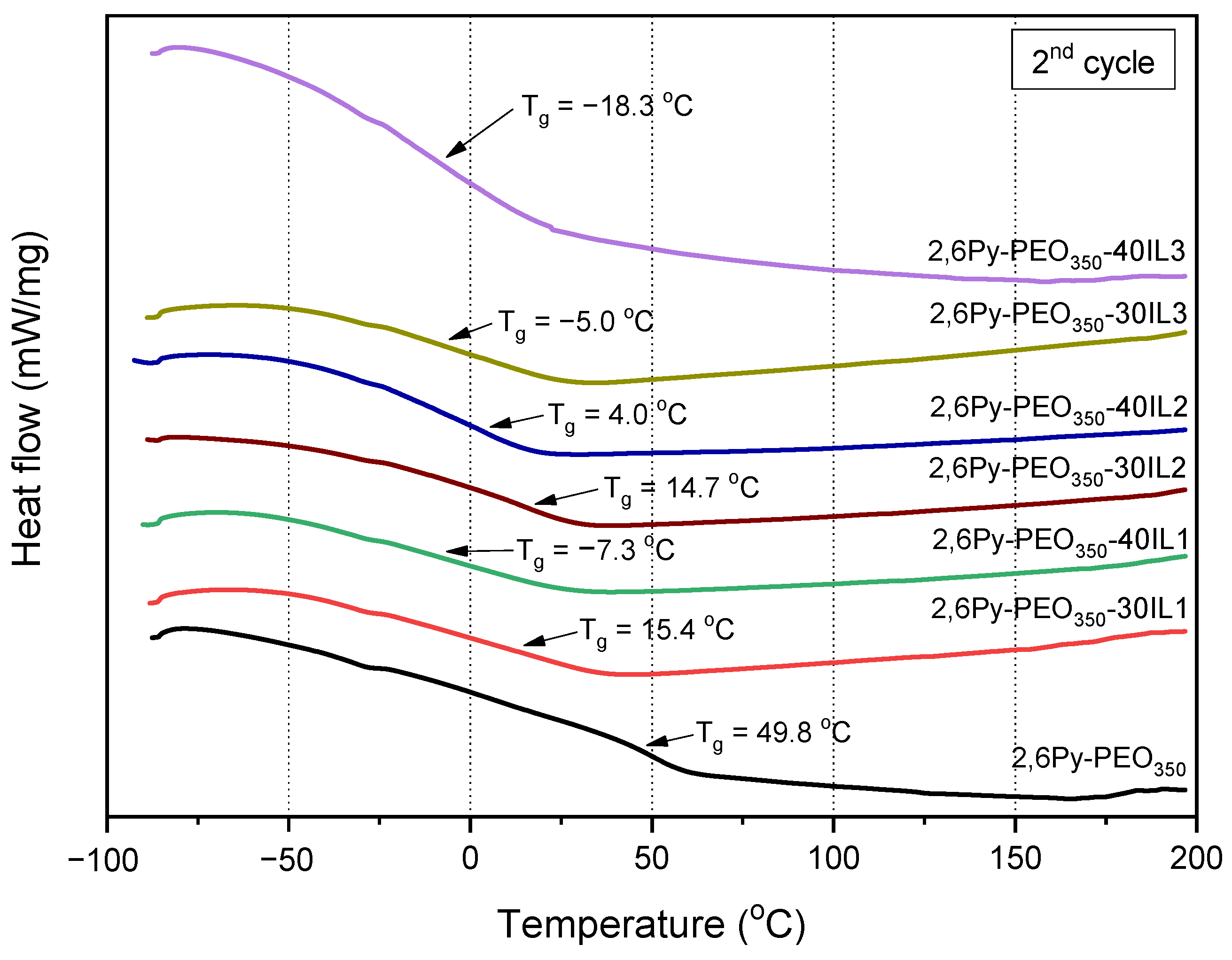
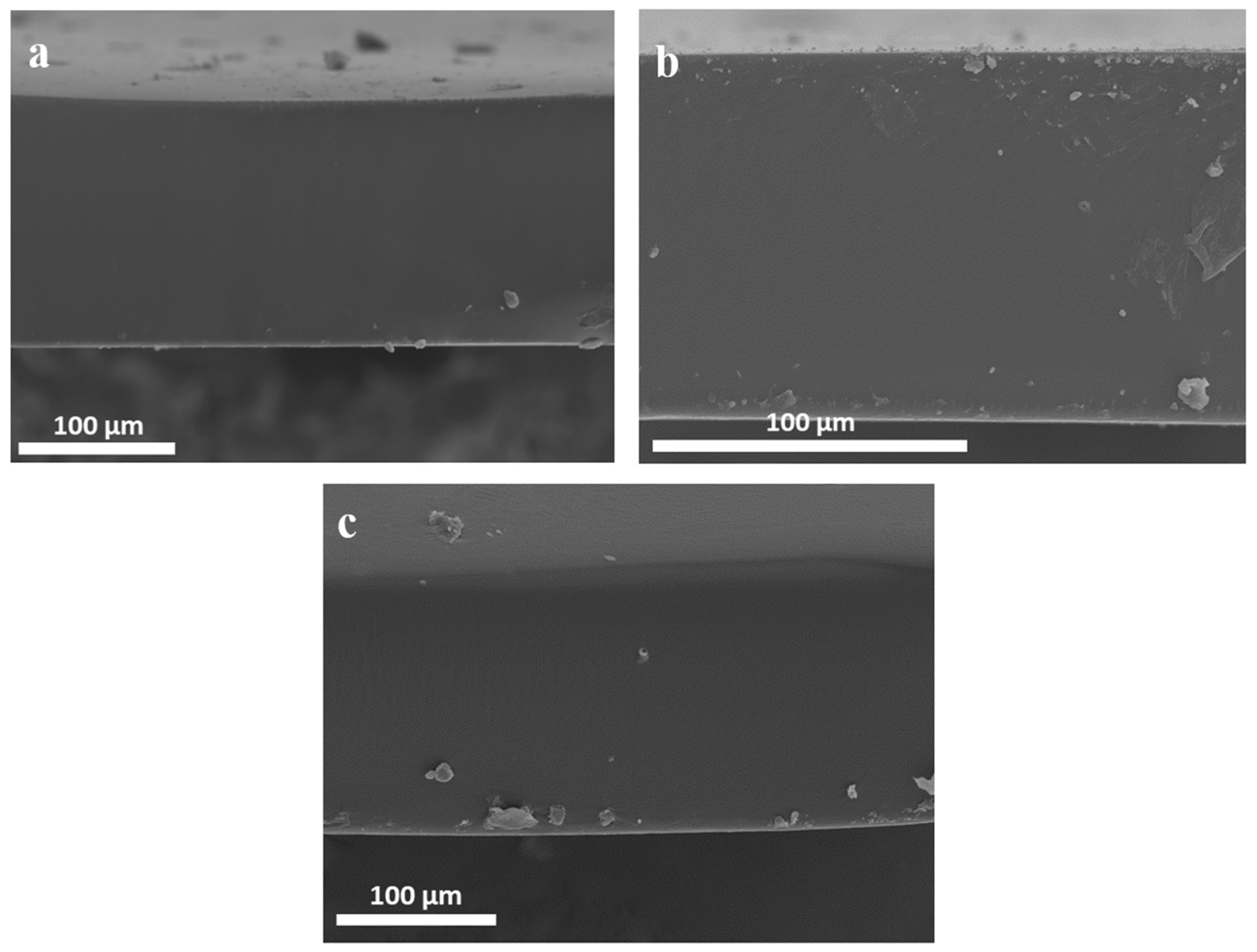
3.1. Preparation and Characterization of Polymer-IL Composite Membranes
3.2. Gas Separation Properties of Polymer-IL Composite Membranes
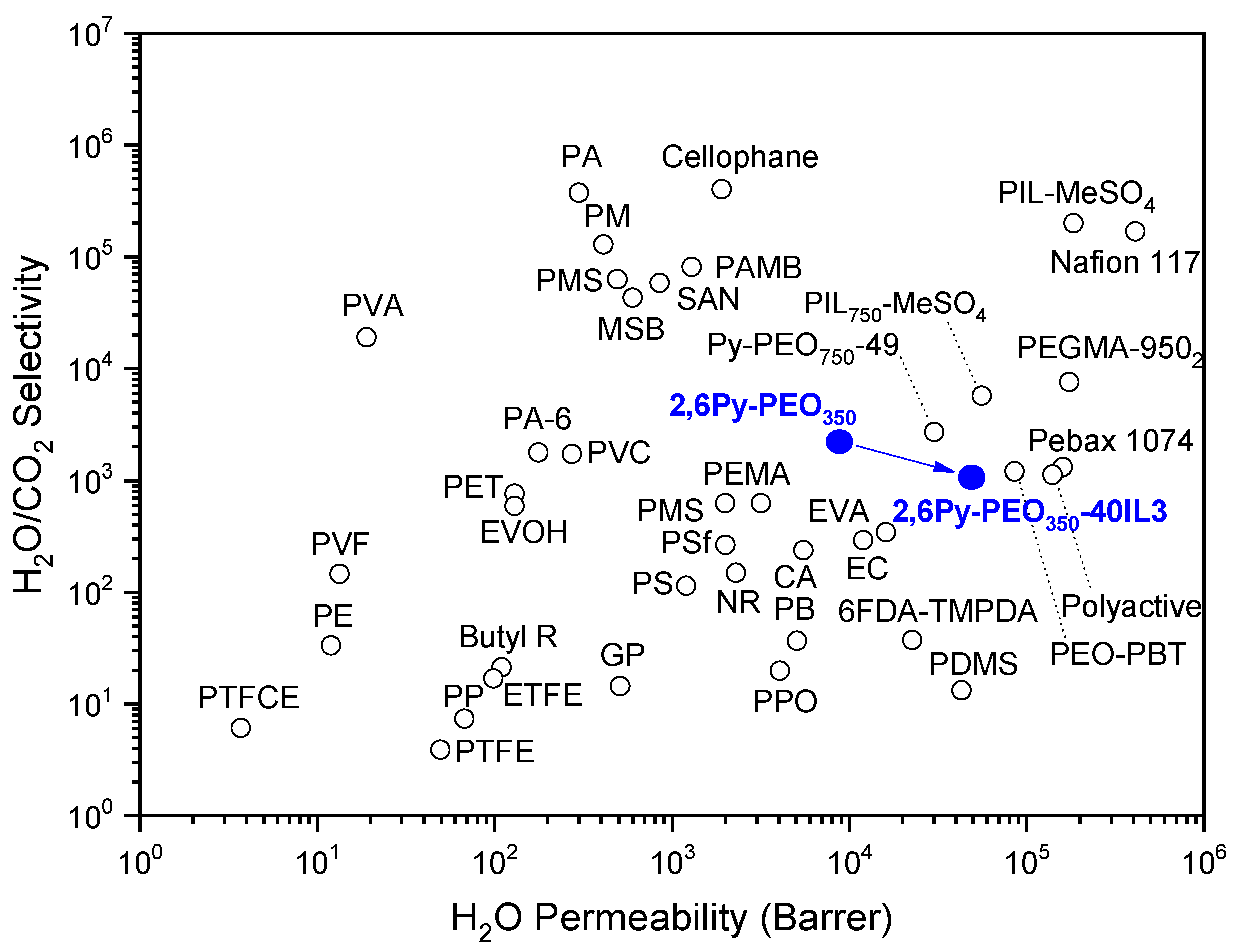
3.3. Water Vapor Separation Properties of Polymer-IL Composite Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobson, M.Z. Review of Solutions to Global Warming, Air Pollution, and Energy Security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 328. [Google Scholar]
- Ghosal, K.; Freeman, B.D. Gas separation using polymer membranes: An overview. Polym. Technol. 1994, 5, 673–697. [Google Scholar] [CrossRef]
- Metz, S.J.; Mulder, M.H.V.; Wessling, M. Gas-permeation properties of poly(ethylene oxide) poly(butylene terephthalate) block copolymers. Macromolecules 2004, 37, 4590–4597. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Kumar, M.; Vovusha, H.; Shevate, R.; Villalobos, L.F.; Schwingenschlögl, U.; Peinemann, K.-V. Scalable Synthesis of Amphiphilic Copolymers for CO2- and Water-Selective Membranes: Effect of Copolymer Composition and Chain Length. Macromolecules 2019, 52, 6213–6226. [Google Scholar] [CrossRef]
- Ioannidi, A.; Vroulias, D.; Kallitsis, J.; Ioannides, T.; Deimede, V. Synthesis and characterization of poly(ethylene oxide) based copolymer membranes for efficient gas/vapor separation: Effect of PEO content and chain length. J. Membr. Sci. 2021, 632, 119353. [Google Scholar] [CrossRef]
- Ioannidi, A.; Anastasopoulos, C.; Vroulias, D.; Kallitsis, J.; Ioannides, T.; Deimede, V. Synthesis and properties of Polymeric ionic liquids (PILs) bearing hydrophilic PEO groups: Evaluation of gas and water vapor separation performance. Sep. Purif. Technol. 2022, 280, 119790. [Google Scholar] [CrossRef]
- Liu, S.L.; Shao, L.; Chua, M.L.; Lau, C.H.; Wang, H.; Quan, S. Recent progress in the design of advanced PEO-containing membranes for CO2 removal. Prog. Polym. Sci. 2013, 38, 1089–1120. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, X.; He, S.; Yang, X.; Long, J.; Zhang, Y.; Shao, L. Rational design of poly (ethylene oxide) based membranes for sustainable CO2 capture. J. Mater. Chem. A 2020, 8, 24233–24252. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Chen, V. Improving CO2 separation performance of thin film composite hollow fiber with Pebax®1657/ionic liquid gel membranes. J. Membr. Sci. 2017, 537, 54–68. [Google Scholar] [CrossRef]
- Gao, H.; Bai, L.; Han, J.; Yang, B.; Zhang, S.; Zhang, X. Functionalized ionic liquid membranes for CO2 separation. Chem. Commun. 2018, 54, 12671–12685. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Lee, J.H.; Park, M.S.; Kim, J.-H.; Kim, J.H. Hybrid membranes based on ionic-liquid-functionalized poly(vinyl benzene chloride) beads for CO2 capture. J. Membr. Sci. 2019, 572, 365–373. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Loïs, S.; Sheridan, E.; et al. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Jansen, J.C.; Friess, K.; Clarizia, G.; Schauer, J.; Izák, P. High Ionic Liquid Content Polymeric Gel Membranes: Preparation and Performance. Macromolecules 2011, 44, 39–45. [Google Scholar] [CrossRef]
- Friess, K.; Jansen, J.C.; Bazzarelli, F.; Izák, P.; Jarmarová, V.; Kačírková, M.; Schauer, J.; Clarizia, G.; Bernardo, P. High ionic liquid content polymeric gel membranes: Correlation of membrane structure with gas and vapour transport properties. J. Membr. Sci. 2012, 415–416, 801–809. [Google Scholar] [CrossRef]
- Rabiee, H.; Ghadimi, A.; Mohammadi, T. Gas transport properties of reverse-selective poly(ether-b-amide6)/[Emim][BF4] gel membranes for CO2/light gases separation. J. Membr. Sci. 2015, 476, 286–302. [Google Scholar] [CrossRef]
- Klepić, M.; Fuoco, A.; Monteleone, M.; Esposito, E.; Friess, K.; Petrusová, Z.; Izák, P.; Jansen, J.C. Tailoring the Thermal and Mechanical Properties of PolyActiveTM Poly(Ether-Ester) Multiblock Copolymers Via Blending with CO2-Phylic Ionic Liquid. Polymers 2020, 12, 890. [Google Scholar] [CrossRef]
- Bernardo, P.; Jansen, J.C.; Bazzarelli, F.; Tasselli, F.; Fuoco, A.; Friess, K.; Izák, P.; Jarmarová, V.; Kačírková, M.; Clarizia, G. Gas transport properties of Pebax®/room temperature ionic liquid gel membranes. Sep. Purif. Technol. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Li, X.; Ding, S.; Zhang, J.; Wei, Z. Optimizing microstructure of polymer composite membranes by tailoring different ionic liquids to accelerate CO2 transport. Int. J. Greenh. Gas Control. 2020, 101, 103136. [Google Scholar] [CrossRef]
- Uchytil, P.; Schauer, J.; Petrychkovych, R.; Setnickova, K.; Suen, S.Y. Ionic liquid membranes for carbon dioxide–methane separation. J. Membr. Sci. 2011, 383, 262–271. [Google Scholar] [CrossRef]
- Erdni-Goryaev, E.M.; Alentév, A.Y.; Belov, N.A.; Ponkratov, D.O.; Shaplov, A.S.; Lozinskaya, E.I.; Vygodskii, Y.S. Gas separation characteristics of new membrane materials based on poly(ethylene glycol)-crosslinked polymers and ionic liquids. Pet. Chem. 2012, 52, 494–498. [Google Scholar] [CrossRef]
- Chen, H.Z.; Li, P.; Chung, T.-S. PVDF/ionic liquid polymer blends with superior separation performance for removing CO2 from hydrogen and flue gas. Int. J. Hydrog. Energy 2012, 37, 11796–11804. [Google Scholar] [CrossRef]
- Jansen, J.C.; Clarizia, G.; Bernardo, P.; Bazzarelli, F.; Friess, K.; Randová, A.; Schauer, J.; Kubicka, D.; Kacirková, M.; Izák, P. Gas transport properties and pervaporation performance of fluoropolymer gel membranes based on pure and mixed ionic liquids. Sep. Purif. Technol. 2013, 109, 87–97. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Mukhtar, H.; Man, Z. The effect of incorporating ionic liquid into polyethersulfone-SAPO34 based mixed matrix membrane on CO2 gas separation performance. Sep. Purif. Technol. 2014, 135, 252–258. [Google Scholar] [CrossRef]
- Liang, L.; Gan, Q.; Nancarrow, P. Composite ionic liquid and polymer membranes for gas separation at elevated temperatures. J. Membr. Sci. 2014, 450, 407–417. [Google Scholar] [CrossRef]
- Hong, S.U.; Park, D.; Ko, Y.; Baek, I. Polymer-ionic liquid gels for enhanced gas transport. Chem. Commun. 2009, 7227–7229. [Google Scholar] [CrossRef]
- Klepić, M.; Setničkova, K.; Lanč, M.; Žák, M.; Izák, P.; Dendisová, M.; Fuoco, A.; Jansen, J.C.; Friess, K. Permeation and sorption properties of CO2-selective blend membranes based on polyvinyl alcohol (PVA) and 1-ethyl-3-methylimidazolium dicyanamide ([EMIM][DCA]) ionic liquid for effective CO2/H2 separation. J. Membr. Sci. 2020, 97, 117623. [Google Scholar] [CrossRef]
- Vöge, A.; Deimede, V.; Paloukis, F.; Neophytides, S.G.; Kallitsis, J.K. Synthesis and properties of aromatic polyethers containing poly(ethylene oxide) side chains as polymer electrolytes for lithium ion batteries. Mater. Chem. Phys. 2014, 148, 57–66. [Google Scholar] [CrossRef]
- Deimede, V.; Vroulias, D.; Kallitsis, J.K.; Ioannides, T. Pyridinium based PIL membranes with exceptional high water vapor permeability and selectivity. Sep. Pur. Technol. 2020, 251, 117412. [Google Scholar] [CrossRef]
- Deimede, V.; Voege, A.; Lainioti, G.; Elmasides, C.; Kallitsis, J.K. Large-Scale Separators Based on Blends of Aromatic Polyethers with PEO for Li-Ion batteries: Improving Thermal shrinkage and wettability behavior. Energy Technol. 2014, 2, 275–283. [Google Scholar] [CrossRef]
- Chouliaras, T.; Vollas, A.; Ioannides, T.; Deimede, V.; Kallitsis, J. Synthesis of imidazolium based PILs and investigation of their blend membranes for gas separation. Membranes 2019, 9, 164. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Malcaitè, E.; Lozinskaya, E.I.; Shaplov, A.S.; Tomé, L.C.; Marrucho, I.M. Poly(ionic liquid)–Ionic Liquid Membranes with Fluorosulfonyl-Derived Anions: Characterization and Biohydrogen Separation. ACS Sustain. Chem. Eng. 2020, 8, 7087–7096. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Shi, Y.; Xue, Z.; Mu, T. Quantitative Research on the Vaporization and Decomposition by Thermogravimetric Analysis-Mass Spectroscopy. Ind. Eng. Chem. Res. 2012, 51, 7418–7427. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mohshim, D.F.; Mukhtar, H.; Murugesan, T.; Man, Z.; Bustam, M.A. Synthesis, characterization, and CO2 separation performance of polyether sulfone/[EMIM][Tf2N] ionic liquid-polymeric membranes (ILPMs). J. Ind. Eng. Chem. 2017, 54, 98–106. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. Extension of the Ye and Shreeve group contribution method for density estimation of ionic liquids in a wide range of temperatures and pressures. Fluid Phase Equilibria 2008, 263, 26–32. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Shaplov, A.S.; Lozinskaya, E.I.; Buzin, M.I.; Vygodskii, Y.S. A New Volume-Based Approach for Predicting Thermophysical Behavior of Ionic Liquids and Ionic Liquid Crystals. J. Am. Chem. Soc. 2016, 138, 10076–10079. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Peinemann, K.-V. Nanostructured membrane material designed for carbon dioxide separation. J. Membr. Sci. 2010, 350, 124–129. [Google Scholar] [CrossRef]
- Feng, S.; Ren, J.; Li, Z.; Li, H.; Hua, K.; Li, X.; Deng, M. Poly(amide-12-b-ethylene oxide)/glycerol triacetate blend membranes for CO2 separation. Int. J. Greenh. Gas Control. 2013, 19, 41–48. [Google Scholar] [CrossRef]
- Feng, S.; Ren, J.; Hua, K.; Li, H.; Ren, X.; Deng, M. Poly(amide-12-b-ethylene oxide)/polyethylene glycol blend membranes for carbon dioxide separation. Sep. Purif. Technol. 2013, 116, 25–34. [Google Scholar] [CrossRef]
- Ghadimi, A.; Amirilargani, M.; Mohammadi, T.; Kasiri, N.; Sadatnia, B. Preparation of alloyed poly(ether block amide)/poly(ethylene glycol diacrylate) membranes for separation of CO2/H2 (syngas application). J. Membr. Sci. 2014, 458, 14–26. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Muldoon, J.; Aki, S.N.V.K.; Anderson, J.L.; Dixon, J.K.; Brennecke, J.F. Improving Carbon Dioxide Solubility in Ionic Liquids. J. Phys. Chem. B 2007, 111, 9001–9009. [Google Scholar] [CrossRef]
- Almantariotis, D.; Gefflaut, T.; Pádua, A.A.H.; Coxam, J.Y.; Costa Gomes, M.F.J. Effect of Fluorination and Size of the Alkyl Side-Chain on the Solubility of Carbon Dioxide in 1-Alkyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)amide Ionic Liquids. J. Phys. Chem. B. 2010, 114, 3608–3617. [Google Scholar] [CrossRef]
- Tomé, L.C.; Florindo, C.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Playing with ionic liquid mixtures to design engineered CO2 separation membranes. Phys. Chem. Chem. Phys. 2014, 16, 17172–17182. [Google Scholar] [CrossRef]
- Mahurin, S.M.; Yeary, J.S.; Baker, S.N.; Jiang, D.-e.; Dai, S.; Baker, G.A. Ring-opened heterocycles: Promising ionic liquids for gas separation and capture. J. Membr. Sci. 2012, 401–402, 61–67. [Google Scholar] [CrossRef]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of gases and vapors in glassy and rubbery polymers. In Materials Science of Membranes for Gas and Vapor Separation, 1st ed.; Yampolskii, Y., Pinnau, I., Freeman, B., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 1–47. [Google Scholar]
- Comesaña-Gándara, B.; Chen, J.; Grazia Bezzu, C.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Lin, H.; van Wagner, E.; Freeman, B.D.; Toy, L.G.; Gupta, R.P. Plasticization enhanced hydrogen purification using polymeric membranes. Science 2006, 311, 639–642. [Google Scholar] [CrossRef]
- Rowe, B.W.; Robeson, L.M.; Freeman, B.D.; Paul, D.R. Influence of temperature on the upper bound: Theoretical considerations and comparison with experimental results. J. Membr. Sci. 2010, 360, 58–69. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Luo, S.; Stevens, K.A.; Park, J.S.; Moon, J.D.; Liu, Q.; Freeman, B.D.; Guo, R. Highly CO2-selective gas separation membranes based on segmented copolymers of poly(ethylene oxide) reinforced with pentiptycene-containing polyimide hard segments. ACS Appl. Mater. Interfaces 2016, 8, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, H.; Ghadimi, A.; Abbasi, S. CO2 separation performance of poly (ether-b-amide6)/PTMEG blended membranes: Permeation and sorption properties. Chem. Eng. Res. Des. 2015, 98, 96–106. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Shao, L. Pushing CO2-philic Membranes performance to the limit by designing semi-interpenetrating networks (SIPN) for sustainable CO2 separations. Energy Environ. Sci. 2017, 10, 1339–1344. [Google Scholar] [CrossRef]
- Hossain, I.; Kim, D.; Al Munsur, A.Z.; Roh, J.M.; Park, H.B.; Kim, T.-H. PEG/PPG-PDMS-based Crosslinked Copolymer Membranes Prepared by ROMP and in situ Membrane Casting for CO2 Separation: An approach to endow rubbery materials with properties of rigid polymers. ACS Appl. Mater. Interfaces 2020, 12, 27286–27299. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Clark, K.; Lin, H. Maximizing ether oxygen content in polymers for membrane CO2 removal from natural gas. ACS Appl. Mater. Interfaces 2019, 11, 10933–10940. [Google Scholar] [CrossRef]
- Jiang, X.; He, S.; Li, S.; Bai, Y.; Shao, L. Penetrating chains mimicking plant root branching to build mechanically robust, ultra-stable CO2-philic membranes for superior carbon capture. J. Mater. Chem. A 2019, 7, 16704–16711. [Google Scholar] [CrossRef]
- Xia, J.; Liu, S.; Chung, T.-S. Effect of end groups and grafting on the CO2 separation performance of poly(ethylene glycol) based membranes. Macromolecules 2011, 44, 7727–7736. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. PEG modified poly(amide-b-ethylene oxide) membranes for CO2 separation. J. Membr. Sci. 2008, 307, 88–95. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Khan, M.M.; Neumann, S.; Abetz, V. Influence of poly(ethylene glycol) segment length on CO2 permeation and stability of poly active membranes and their nanocomposites with PEG POSS. ACS Appl. Mater. Interfaces 2015, 7, 12289–12298. [Google Scholar] [CrossRef] [PubMed]
- Kammakakam, I.; Bara, J.E.; Jacson, E.M.; Lertxundi, J.; Mecerreyes, D.; Tomé, L.C. Tailored CO2-philic anionic poly(ionic liquid) composite membranes: Synthesis, characterization, and gas separation properties. ACS Sustain. Chem. Eng. 2020, 8, 5954–5965. [Google Scholar] [CrossRef]
- Dai, Z.; Bai, L.; Hval, K.N.; Zhang, X.; Zhang, S.; Deng, L. Pebax®/TSIL blend thin film composite membranes for CO2 separation. Sci. China Chem. 2016, 59, 538–546. [Google Scholar] [CrossRef]


| Polymer-IL Membranes | Membrane Thickness (μm) | CO2 Permeability (Barrer) | CH4 Permeability(Barrer) | H2 Permeability (Barrer) | CO2/CH4 Selectivity | CO2/H2 Selectivity |
|---|---|---|---|---|---|---|
| 2,6Py-PEO350 | 50 | 4.1 ± 0.1 | 0.122 ± 0.003 | 2.0 ± 0.1 | 33 ± 1 | 2.0 ± 0.1 |
| 2,6Py-PEO350-30IL1 | 136 | 17.3 ± 0.4 | 0.340 ± 0.008 | 2.8 ± 0.9 | 51 ± 2 | 6 ± 2 |
| 2,6Py-PEO350-40IL1 | 161 | 24.7 ± 0.6 | 0.48 ± 0.01 | 3.9 ± 0.1 | 51 ± 2 | 6.4 ± 0.2 |
| 2,6Py-PEO350-30IL2 | 47 | 4.2 ± 0.1 | 0.11 ± 0.02 | 1.1 ± 0.1 | 39 ± 7 | 3.8 ± 0.5 |
| 2,6Py-PEO350-40IL2 | 119 | 6.9 ± 0.2 | 0.14 ± 0.03 | 1.5 ± 0.1 | 49 ± 9 | 5 ± 2 |
| 2,6Py-PEO350-30IL3 | 78 | 14.0 ± 0.3 | 0.220 ± 0.006 | 2.6 ± 0.1 | 64 ± 2 | 5.4 ± 0.3 |
| 2,6Py-PEO350-40IL3 | 165 | 46.1 ± 0.8 | 1.2 ± 0.1 | 8.2 ± 0.4 | 39 ± 3 | 5.6 ± 0.3 |
| Polymer-IL | Membrane Thickness (μm) | CO2 Permeability (Barrer) | CH4 Permeability (Barrer) | H2 Permeability (Barrer) | H2O Permeability (×104 Barrer) | CO2/CH4 Selectivity | CO2/H2 Selectivity |
|---|---|---|---|---|---|---|---|
| 2,6Py-PEO350 Dry conditions | 50 | 4.1 ± 0.1 | 0.122 ± 0.003 | 2.0 ± 0.1 | 0.89 ± 0.02 | 33 ± 1 | 2.0 ± 0.1 |
| 2,6Py-PEO350-40IL3 Dry conditions | 165 | 46.1 ± 0.8 | 1.2 ± 0.1 | 8.2 ± 0.4 | 5.0 ± 0.1 | 39 ± 3 | 5.6 ± 0.3 |
| 2,6Py-PEO350-40IL3 Wet conditions | 166 | 52 ± 9 | 1.4 ± 0.1 | 11.0 ± 0.7 | 5.0 ± 0.1 | 37 ± 7 | 4.7 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vroulias, D.; Staurianou, E.; Ioannides, T.; Deimede, V. Poly(ethylene oxide)-Based Copolymer-IL Composite Membranes for CO2 Separation. Membranes 2023, 13, 26. https://doi.org/10.3390/membranes13010026
Vroulias D, Staurianou E, Ioannides T, Deimede V. Poly(ethylene oxide)-Based Copolymer-IL Composite Membranes for CO2 Separation. Membranes. 2023; 13(1):26. https://doi.org/10.3390/membranes13010026
Chicago/Turabian StyleVroulias, Dionysios, Eirini Staurianou, Theophilos Ioannides, and Valadoula Deimede. 2023. "Poly(ethylene oxide)-Based Copolymer-IL Composite Membranes for CO2 Separation" Membranes 13, no. 1: 26. https://doi.org/10.3390/membranes13010026
APA StyleVroulias, D., Staurianou, E., Ioannides, T., & Deimede, V. (2023). Poly(ethylene oxide)-Based Copolymer-IL Composite Membranes for CO2 Separation. Membranes, 13(1), 26. https://doi.org/10.3390/membranes13010026









