Study of Membranes with Nanotubes to Enhance Osmosis Desalination Efficiency by Using Machine Learning towards Sustainable Water Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Synthesis
2.2. Membrane Quality Check
2.3. Membrane Analysis and Test
3. Results and Discussion
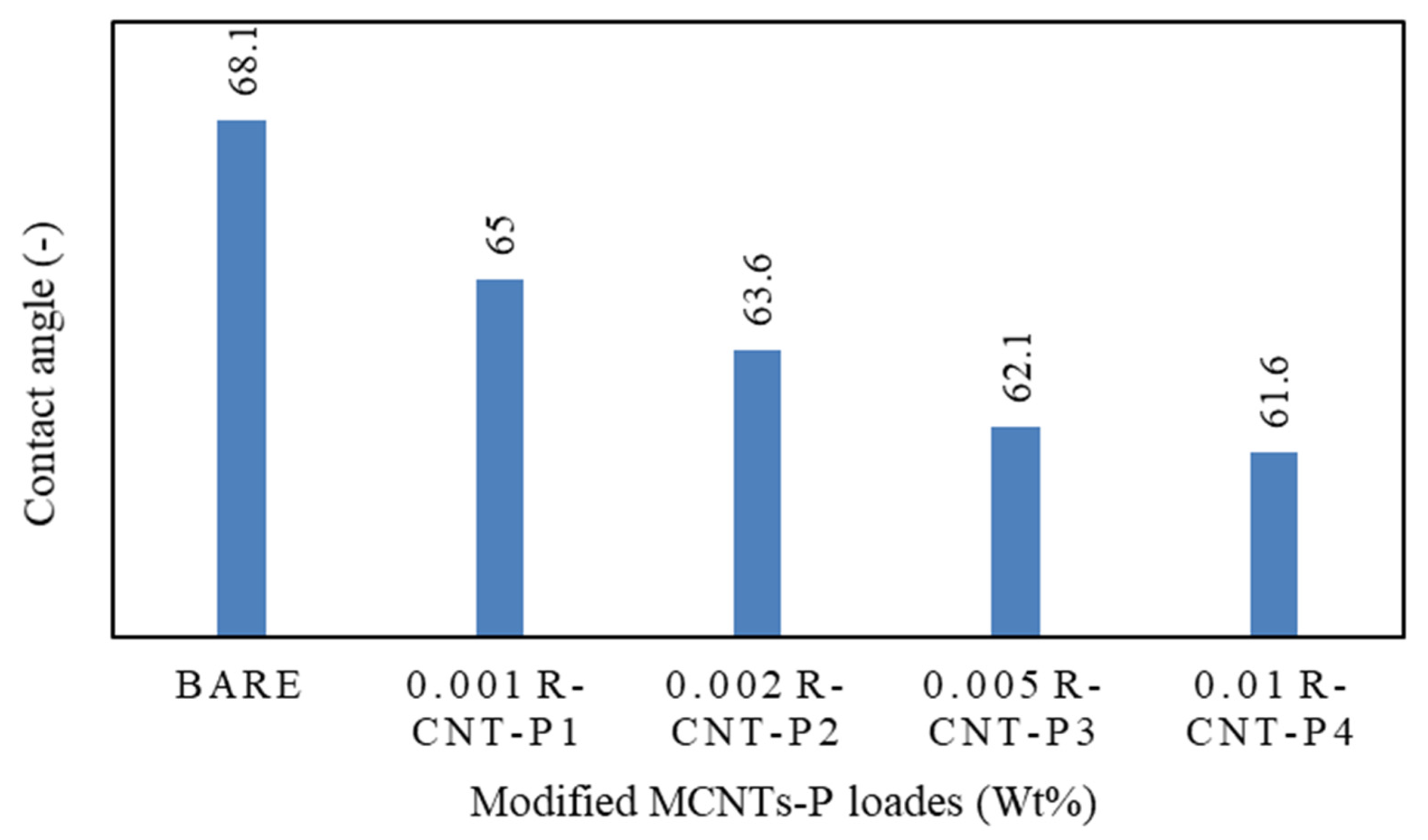
3.1. Investigation of Synthesized Membranes for Reverse Osmosis Process
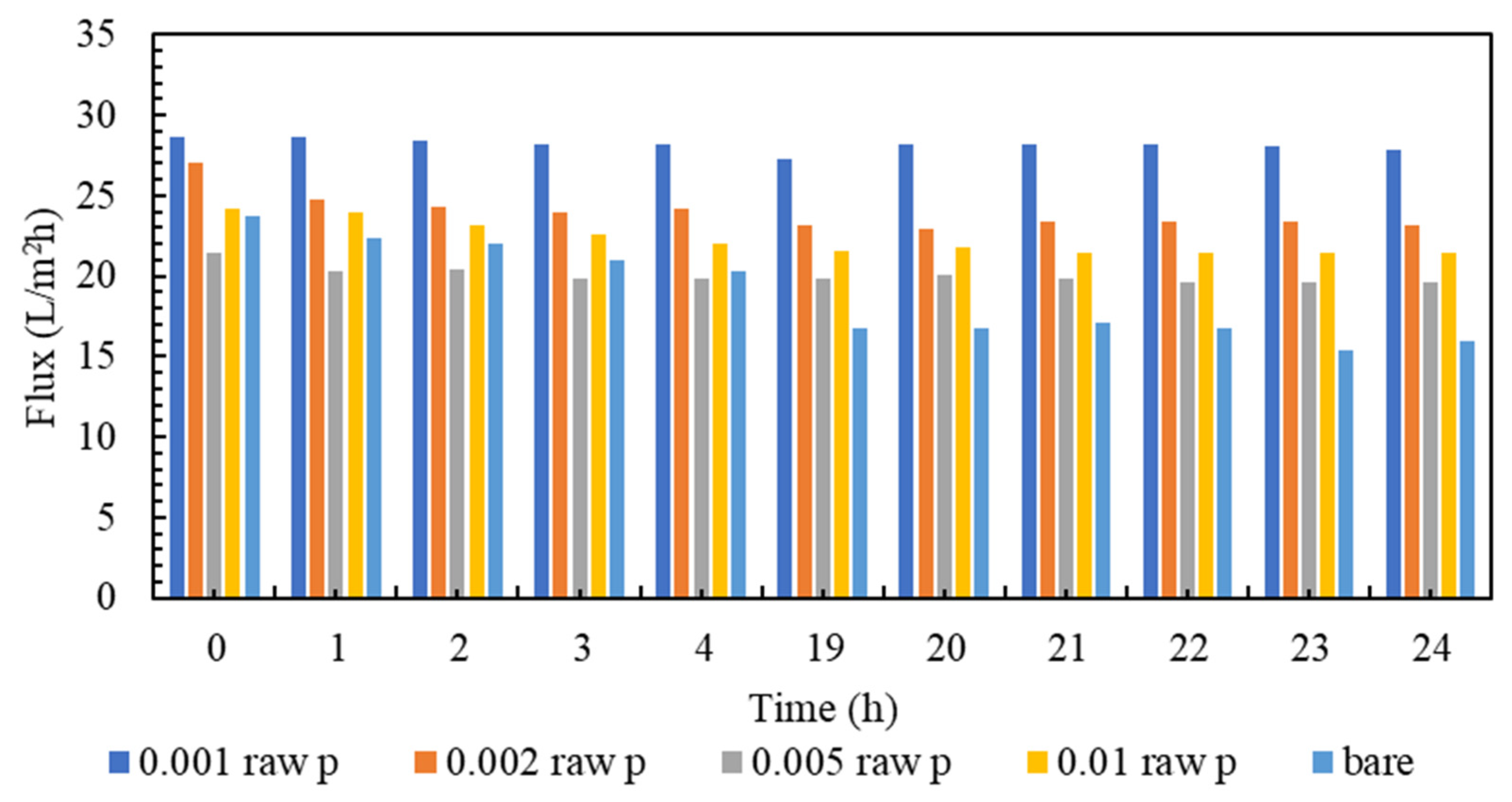
3.2. The Performance of Reverse Osmosis Membrane in Transmittance and Rejection
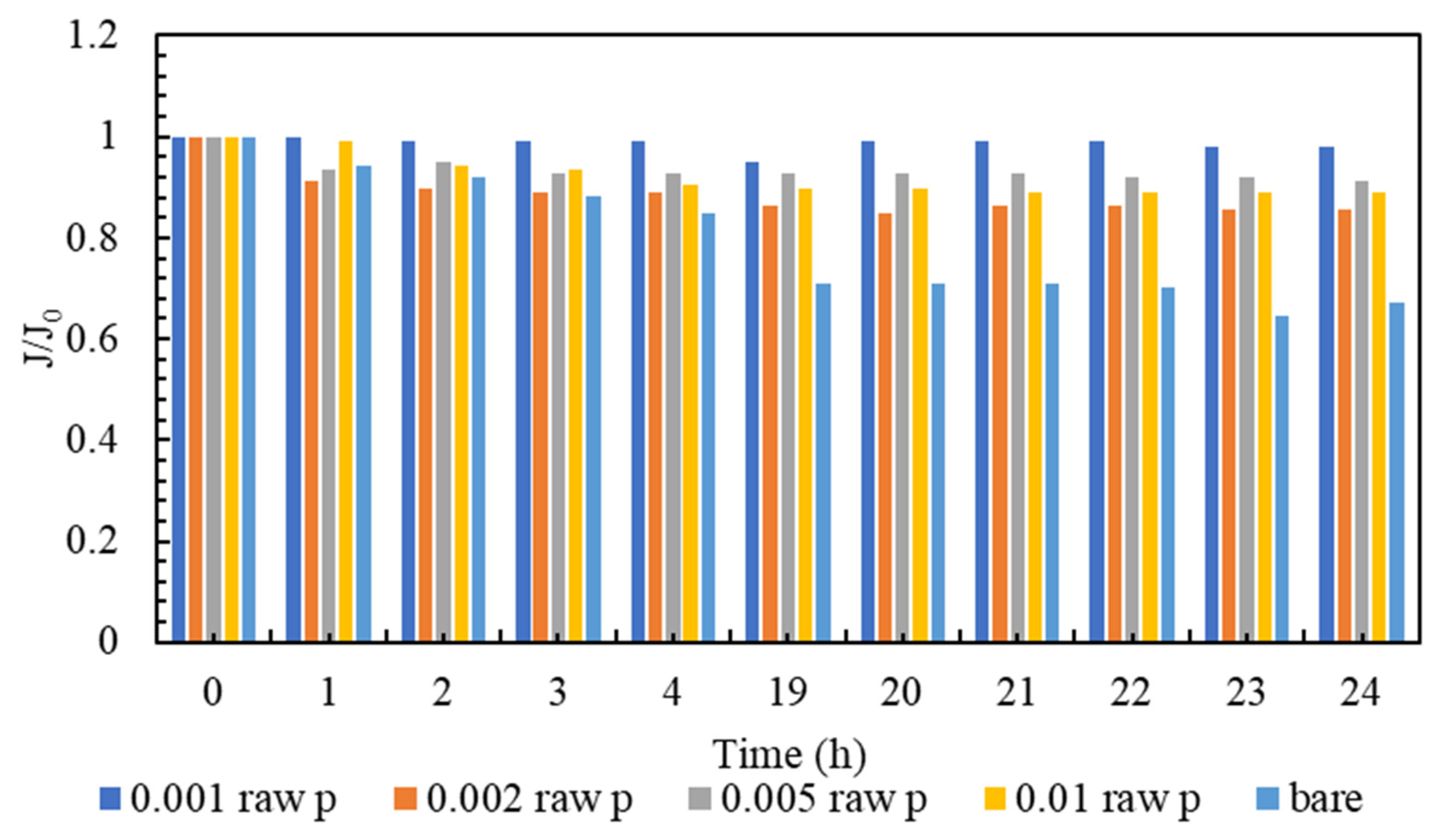
3.3. Membrane Clogging
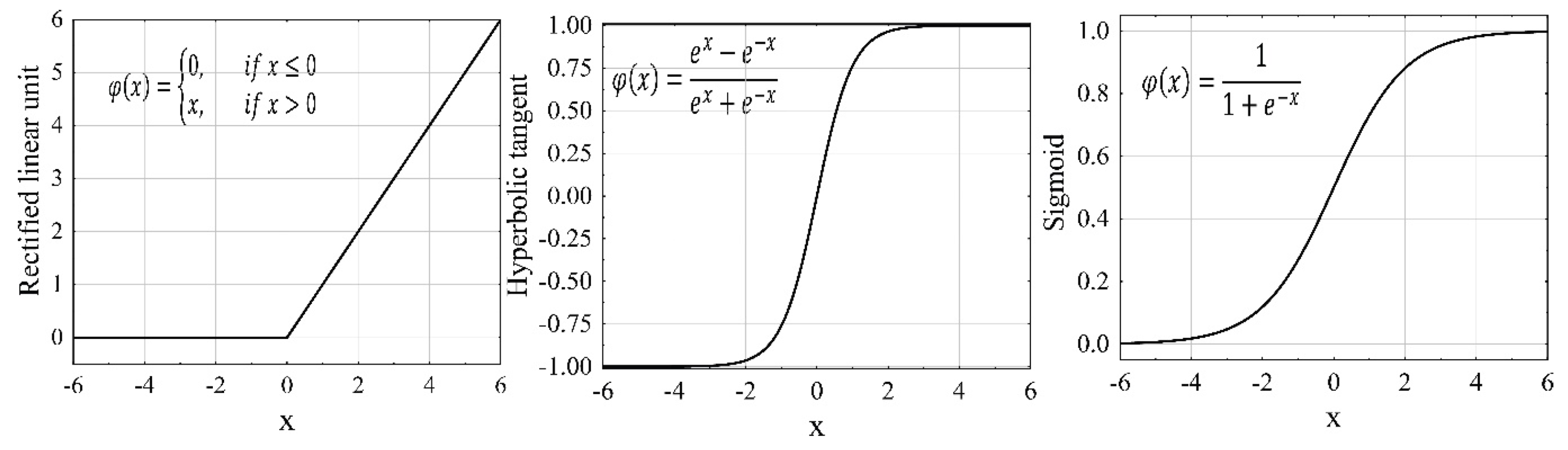
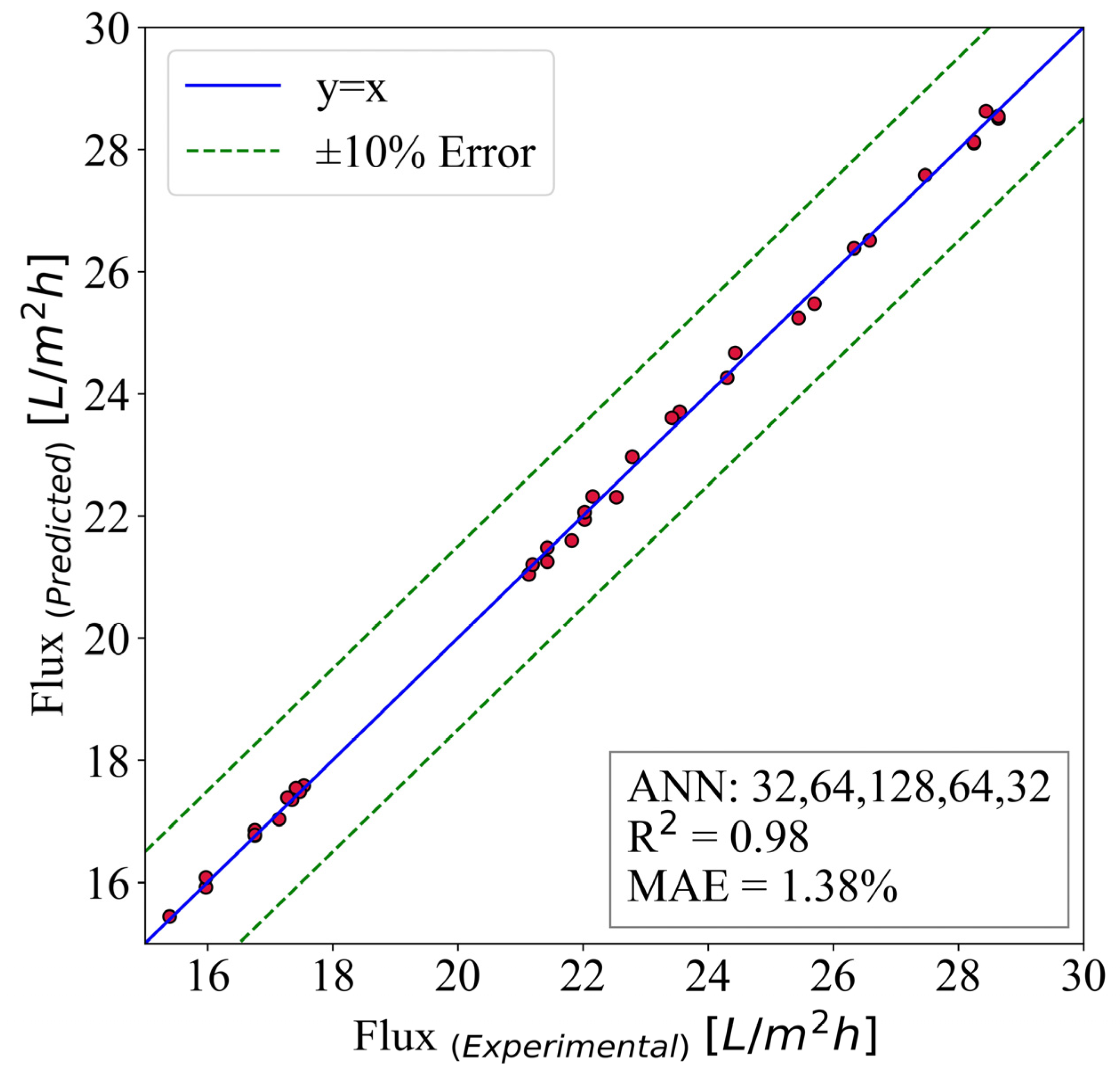
4. Artificial Neural Networks
4.1. The Hyperparameter Tuning Process
4.2. Predictive Model for Normalized Flux
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, R.; Tan, Y.; Meng, F.; Zhang, Y.; Huang, Y.X. PVDF/MAF-4 composite membrane for high flux and scaling-resistant membrane distillation. Desalination 2022, 540, 116013. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for low pressure membranes in water treatment: A review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef]
- Yin, L.; Wang, L.; Keim, B.D.; Konsoer, K.; Zheng, W. Wavelet analysis of dam injection and discharge in three gorges dam and reservoir with precipitation and river discharge. Water 2022, 14, 567. [Google Scholar] [CrossRef]
- Lin, X.; Lu, K.; Hardison, A.K.; Liu, Z.; Xu, X.; Gao, D.; Gong, J.; Gardner, W.S. Membrane inlet mass spectrometry method (REOX/MIMS) to measure 15N-nitrate in isotope-enrichment experiments. Ecol. Indic. 2021, 126, 107639. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Optimization, characterization and nanofiltration properties test of MWNTs/polyester thin film nanocomposite membrane. J. Membr. Sci. 2013, 428, 425–433. [Google Scholar] [CrossRef]
- Ge, D.; Yuan, H.; Xiao, J.; Zhu, N. Insight into the enhanced sludge dewaterability by tannic acid conditioning and pH regulation. Sci. Total Environ. 2019, 679, 298–306. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Yin, L.; Zheng, W. Statistical analysis of regional air temperature characteristics before and after dam construction. Urban Clim. 2022, 41, 101085. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.; Rao, D.; Chang, T.; Guo, Z. A nonlinear attachment-detachment model with adsorption hysteresis for suspension-colloidal transport in porous media. J. Hydrol. 2019, 578, 124080. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. 2008, 120, 5701–5704. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Bubble behavior and nucleation site density in subcooled flow boiling using a novel method for simulating the microstructure of surface roughness. Korean J. Chem. Eng. 2022, 39, 2945–2958. [Google Scholar] [CrossRef]
- Zaboli, S.; Alimoradi, H.; Shams, M. Numerical investigation on improvement in pool boiling heat transfer characteristics using different nanofluid concentrations. J. Therm. Anal. Calorim. 2022, 147, 10659–10676. [Google Scholar] [CrossRef]
- Roodbari, M.; Alimoradi, H.; Shams, M.; Aghanajafi, C. An experimental investigation of microstructure surface roughness on pool boiling characteristics of TiO2 nanofluid. J. Therm. Anal. Calorim. 2022, 147, 3283–3298. [Google Scholar] [CrossRef]
- Alimoradi, H.; Zaboli, S.; Shams, M. Numerical simulation of surface vibration effects on improvement of pool boiling heat transfer characteristics of nanofluid. Korean J. Chem. Eng. 2022, 39, 69–85. [Google Scholar] [CrossRef]
- Wang, M.R.; Deng, L.; Liu, G.C.; Wen, L.; Wang, J.G.; Huang, K.B.; Tang, H.T.; Pan, Y.M. Porous organic polymer-derived nanopalladium catalysts for chemoselective synthesis of antitumor benzofuro [2,3-b] pyrazine from 2-bromophenol and isonitriles. Org. Lett. 2019, 21, 4929–4932. [Google Scholar] [CrossRef]
- Alimoradi, H.; Soltani, M.; Shahali, P.; Moradi Kashkooli, F.; Larizadeh, R.; Raahemifar, K.; Adibi, M.; Ghasemi, B. Experimental investigation on improvement of wet cooling tower efficiency with diverse packing compaction using ANN-PSO algorithm. Energies 2020, 14, 167. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Enhancement in the Pool Boiling Heat Transfer of Copper Surface by Applying Electrophoretic Deposited Graphene Oxide Coatings. Int. J. Multiph. Flow 2022, 159, 104350. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. A Collect. Rev. Nat. J. 2010, 452, 337–346. [Google Scholar]
- Dai, Z.; Ma, Z.; Zhang, X.; Chen, J.; Ershadnia, R.; Luan, X.; Soltanian, M.R. An integrated experimental design framework for optimizing solute transport monitoring locations in heterogeneous sedimentary media. J. Hydrol. 2022, 614, 128541. [Google Scholar] [CrossRef]
- Yang, Y.P.; Weng, H.H. An underground pollution of water purification processing equipment develop. In Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2013; Volume 807, pp. 1372–1375. [Google Scholar]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Qi, S.; Li, R.; Zhang, X.; Song, X.; Gao, C. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019, 580, 101–109. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, J.Y.; Hong, S.P.; Lee, J.M.; Roh, S.M.; Kim, S.H.; Park, H.B. ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition. J. Membr. Sci. 2019, 570, 23–33. [Google Scholar] [CrossRef]
- Ding, W.; Meng, Q.; Dong, G.; Qi, N.; Zhao, H.; Shi, S. Metabolic engineering of threonine catabolism enables Saccharomyces cerevisiae to produce propionate under aerobic conditions. Biotechnol. J. 2022, 17, 2100579. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, Y.; Liu, S.; Tian, J.; Yang, B.; Yin, L. A deep fusion matching network semantic reasoning model. Appl. Sci. 2022, 12, 3416. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Boddu, V.; Kumar, M.; Shalaby, M.S.; Abdallah, H.; Chetty, R. Effect of ZnO morphology on GO-ZnO modified polyamide reverse osmosis membranes for desalination. Desalination 2019, 467, 245–256. [Google Scholar] [CrossRef]
- Zheng, W.; Tian, X.; Yang, B.; Liu, S.; Ding, Y.; Tian, J.; Yin, L. A few shot classification methods based on multiscale relational networks. Appl. Sci. 2022, 12, 4059. [Google Scholar] [CrossRef]
- Al Mayyahi, A. Thin-film composite (TFC) membrane modified by hybrid ZnO-graphene nanoparticles (ZnO-Gr NPs) for water desalination. J. Environ. Chem. Eng. 2018, 6, 1109–1117. [Google Scholar] [CrossRef]
- Zou, D.; Lee, Y.M. Design strategy of poly (vinylidene fluoride) membranes for water treatment. Prog. Polym. Sci. 2022, 128, 101535. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, Q.; Ng, T.C.A.; Zhang, J.; Zhang, X.; Zhang, L.; Zhang, X.; Wang, H.; Ng, H.Y.; Wang, J. Hierarchically porous interlayer for highly permeable and fouling-resistant ceramic membranes in water treatment. Sep. Purif. Technol. 2022, 293, 121092. [Google Scholar] [CrossRef]
- Pan, D.; Chen, H. Border pollution reduction in China: The role of livestock environmental regulations. China Econ. Rev. 2021, 69, 101681. [Google Scholar] [CrossRef]
- Beratto-Ramos, A.; Dagnino-Leone, J.; Martínez-Oyanedel, J.; Aranda, M.; Bórquez, R. Fabrication and filtration performance of aquaporin biomimetic membranes for water treatment. Sep. Purif. Rev. 2022, 51, 340–357. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ghosh, S. Ceramic Membranes in Water Treatment: Potential and Challenges for Technology Development. In Sustainable Water Treatment: Advances and Technological Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 325–381. [Google Scholar]
- Adam, M.R.; Othman, M.H.D.; Kurniawan, T.A.; Puteh, M.H.; Ismail, A.F.; Khongnakorn, W.; Rahman, M.A.; Jaafar, J. Advances in adsorptive membrane technology for water treatment and resource recovery applications: A critical review. J. Environ. Chem. Eng. 2022, 10, 107633. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Yogarathinam, L.T.; Raji, Y.O.; El-badawy, T.H. Recent development in modification of polysulfone membrane for water treatment application. J. Water Process Eng. 2021, 40, 101835. [Google Scholar]
- Tong, X.; Liu, S.; Qu, D.; Gao, H.; Yan, L.; Chen, Y.; Crittenden, J. Tannic acid-metal complex modified MXene membrane for contaminants removal from water. J. Membr. Sci. 2021, 622, 119042. [Google Scholar] [CrossRef]
- Chan, W.F.; Chen, H.Y.; Marand, E.; Johnson, J.K. Functionalized carbon nanotube nanocomposite membranes for water desalination: Experimental study. In Proceedings of the 2013 AIChE Annual Meeting, San Francisco, CA, USA, 3–8 November 2013. [Google Scholar]
- Shao, Z.; Zhai, Q.; Han, Z.; Guan, X. A linear AC unit commitment formulation: An application of data-driven linear power flow model. Int. J. Electr. Power Energy Syst. 2023, 145, 108673. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z. Lead sorption from aqueous solutions on chitosan nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 183–190. [Google Scholar] [CrossRef]
- Bhaumik, M.; Agarwal, S.; Gupta, V.K.; Maity, A. Enhanced removal of Cr (VI) from aqueous solutions using polypyrrole wrapped oxidized MWCNTs nanocomposites adsorbent. J. Colloid Interface Sci. 2016, 470, 257–267. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, D.; Lei, Y.; Cheng, R. Nano-TiO2 membrane adsorption reactor (MAR) for virus removal in drinking water. Chem. Eng. J. 2013, 230, 180–187. [Google Scholar] [CrossRef]
- Yang, R.; Hou, E.; Cheng, W.; Yan, X.; Zhang, T.; Li, S.; Yao, H.; Liu, J.; Guo, Y. Membrane-Targeting Neolignan-Antimicrobial Peptide Mimic Conjugates to Combat Methicillin-Resistant Staphylococcus aureus (MRSA) Infections. J. Med. Chem. 2022, 65, 16879–16892. [Google Scholar] [CrossRef]
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.H.; Bae, S.S.; Park, J.; Yoon, J. Carbon nanotube-based membranes: Fabrication and application to desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559. [Google Scholar] [CrossRef]
- Kim, E.S.; Hwang, G.; El-Din, M.G.; Liu, Y. Development of nanosilver and multiwalled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394, 37–48. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, K.; Baek, Y.; Kim, D.G.; Shim, J.; Yoon, J.; Lee, J.C. High-performance reverse osmosis CNT/polyamide nanocomposite membrane by controlled interfacial interactions. ACS Appl. Mater. Interfaces 2014, 6, 2819–2829. [Google Scholar] [CrossRef]
- Baroña, G.N.B.; Lim, J.; Choi, M.; Jung, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.K.; Bindal, R.; Prabhakar, S.; Tewari, P.K. Composite polyamide reverse osmosis (RO) membranes–recent developments and future directions. Barc Newsl. 2011, 321, 43–51. [Google Scholar]
- Dumée, L.; Lee, J.; Sears, K.; Tardy, B.; Duke, M.; Gray, S. Fabrication of thin film composite poly (amide)-carbon-nanotube supported membranes for enhanced performance in osmotically driven desalination systems. J. Membr. Sci. 2013, 427, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Z. Subset simulation with adaptable intermediate failure probability for robust reliability analysis: An unsupervised learning-based approach. Struct. Multidiscip. Optim. 2022, 65, 1–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Joseph, A.J.J.M.; Zhang, Z.; Ma, C.; Gul, D.; Schellenberg, A.; Hu, N. Deterministic snap-through buckling and energy trapping in axially-loaded notched strips for compliant building blocks. Smart Mater. Struct. 2020, 29, 02LT03. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Williams, M.D.; Rahardianto, A.; Franklin, J.C.; Cohen, Y. High-recovery reverse osmosis desalination using intermediate chemical demineralization. J. Membr. Sci. 2007, 301, 131–141. [Google Scholar] [CrossRef]
- Yan, B.; Ma, C.; Zhao, Y.; Hu, N.; Guo, L. Geometrically Enabled Soft Electroactuators via Laser Cutting. Adv. Eng. Mater. 2019, 21, 1900664. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, H.; Bai, L.; Tang, M.; Chen, H.; Su, D. Fragility analyses of bridge structures using the logarithmic piecewise function-based probabilistic seismic demand model. Sustainability 2021, 13, 7814. [Google Scholar] [CrossRef]
- Yao, L.; Li, Y.; Cheng, Q.; Chen, Z.; Song, J. Modeling and optimization of metal-organic frameworks membranes for reverse osmosis with artificial neural networks. Desalination 2022, 532, 115729. [Google Scholar] [CrossRef]
- Foong, L.K.; Zhao, Y.; Bai, C.; Xu, C. Efficient metaheuristic-retrofitted techniques for concrete slump simulation. Smart Struct. Syst. Int. J. 2021, 27, 745–759. [Google Scholar]
- Wu, P.; Liu, A.; Fu, J.; Ye, X.; Zhao, Y. Autonomous surface crack identification of concrete structures based on an improved one-stage object detection algorithm. Eng. Struct. 2022, 272, 114962. [Google Scholar] [CrossRef]
- Alimoradi, H.; Eskandari, E.; Pourbagian, M.; Shams, M. A parametric study of subcooled flow boiling of Al2O3/water nanofluid using numerical simulation and artificial neural networks. Nanoscale Microscale Thermophys. Eng. 2022, 26, 129–159. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Delnavaz, M.; Vatanpour, V. Simulation and characterization of novel reverse osmosis membrane prepared by blending polypyrrole coated multiwalled carbon nanotubes for brackish water desalination and antifouling properties using artificial neural networks. J. Membr. Sci. 2019, 581, 123–138. [Google Scholar] [CrossRef]
- Eskandari, E.; Alimoradi, H.; Pourbagian, M.; Shams, M. Numerical investigation and deep learning-based prediction of heat transfer characteristics and bubble dynamics of subcooled flow boiling in a vertical tube. Korean J. Chem. Eng. 2022, 39, 3227–3245. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, H.; Song, C.; Wang, Z. Predicting compressive strength of manufactured-sand concrete using conventional and metaheuristic-tuned artificial neural network. Measurement 2022, 194, 110993. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Q.; Yang, Z.; Yu, X.; Jia, B. A novel artificial bee colony algorithm for structural damage detection. Adv. Civ. Eng. 2020, 2020, 3743089. [Google Scholar] [CrossRef] [Green Version]
- Yeo, C.S.H.; Xie, Q.; Wang, X.; Zhang, S. Understanding and optimization of thin film nanocomposite membranes for reverse osmosis with machine learning. J. Membr. Sci. 2020, 606, 118135. [Google Scholar] [CrossRef]
- Zhao, Y.; Foong, L.K. Predicting Electrical Power Output of Combined Cycle Power Plants Using a Novel Artificial Neural Network Optimized by Electrostatic Discharge Algorithm. Measurement 2022, 198, 111405. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, X.; Foong, L.K. Predicting the splitting tensile strength of concrete using an equilibrium optimization model. Steel Compos. Struct. Int. J. 2021, 39, 81–93. [Google Scholar]
- Ruiz-García, A.; Feo-García, J. Operating and maintenance cost in seawater reverse osmosis desalination plants. Artificial neural network based model. Desalination Water Treat. 2017, 73, 73–79. [Google Scholar] [CrossRef]
- Joy, V.M.; Feroz, S.; Dutta, S. Artificial intelligence-based multiobjective optimization of reverse osmosis desalination pretreatment using a hybrid ZnO-immobilized/photo-Fenton process. J. Chemom. 2022, 36, e3434. [Google Scholar] [CrossRef]
- Salgado-Reyna, A.; Soto-Regalado, E.; Gómez-González, R.; Cerino-Córdova, F.J.; García-Reyes, R.B.; Garza-González, M.T.; Alcalá-Rodríguez, M.M. Artificial neural networks for modeling the reverse osmosis unit in a wastewater pilot treatment plant. Desalination Water Treat. 2015, 53, 1177–1187. [Google Scholar] [CrossRef]
- Zarrabi, H.; Yekavalangi, M.E.; Vatanpour, V.; Shockravi, A.; Safarpour, M. Improvement in desalination performance of thin film nanocomposite nanofiltration membrane using amine-functionalized multiwalled carbon nanotube. Desalination 2016, 394, 83–90. [Google Scholar] [CrossRef]
- Hollas, J.M. Modern Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Nunes, S.P.; Peinemann, K.V. Membrane Technology; Wiley-VCH: Hoboken, NJ, USA, 2001. [Google Scholar]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep. Purif. Technol. 2012, 90, 69–82. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2014. [Google Scholar]
- Werder, T.; Walther, J.H.; Jaffe, R.L.; Halicioglu, T.; Noca, F.; Koumoutsakos, P. Molecular dynamics simulation of contact angles of water droplets in carbon nanotubes. Nano Lett. 2001, 1, 697–702. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multiwalled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Son, M.; Choi, H.G.; Liu, L.; Celik, E.; Park, H.; Choi, H. Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin-film composite membrane for desalination. Chem. Eng. J. 2015, 266, 376–384. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G.Z.; Qiu, S.; Cheng, L.H.; Chen, H.L. Preparation of high-flux thin film nanocomposite reverse osmosis membranes by incorporating functionalized multiwalled carbon nanotubes. Desalination Water Treat. 2011, 34, 19–24. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multiwalled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Vatanpour, V.; Zoqi, N. Surface modification of commercial seawater reverse osmosis membranes by grafting of hydrophilic monomer blended with carboxylated multiwalled carbon nanotubes. Appl. Surf. Sci. 2017, 396, 1478–1489. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Moayedi, H.; Bahiraei, M.; Foong, L.K. Employing TLBO and SCE for optimal prediction of the compressive strength of concrete. Smart Struct. Syst. 2020, 26, 753–763. [Google Scholar]
- Lejarazu-Larrañaga, A.; Landaburu-Aguirre, J.; Senán-Salinas, J.; Ortiz, J.M.; Molina, S. Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy. Membranes 2022, 12, 864. [Google Scholar] [CrossRef]






| Membrane | Roughness Parameter | ||
|---|---|---|---|
| (nm) | |||
| Bare PA RO | 54.6 | 68.8 | 431 |
| 0.001 R-CNT-P1 | 16.39 | 16.39 | 206.8 |
| 0.002 R-CNT-P2 | 32.11 | 42 | 304.2 |
| 0.005 R-CNT-P3 | 54.6 | 67.5 | 449.4 |
| 0.01 R-CNT-P4 | 28.71 | 34.98 | 210.1 |
| Number | Input | Hidden Layers | MAE (%) | |
|---|---|---|---|---|
| 1 | (32) | 3.98% | 0.95 | |
| 2 | (32,64) | 2.67% | 0.96 | |
| 3 | (32,64,32) | 2.61% | 0.95 | |
| 4 | (32,64,64,32) | 2.48% | 0.96 | |
| 5 * | (32,64,128,64,32) | 2.06% | 0.98 | |
| 6 | (32,64,128,128,64,32) | 2.39% | 0.98 | |
| 7 | (32,64,128,256,128,64,32) | 2.30% | 0.97 | |
| 8 | (32,64,128,256,256,128,64,32) | 2.41% | 0.97 | |
| 9 | (32,64,128,256,512,256,128,64,32) | 2.40% | 0.97 |
| Number | Hidden Layers | Output Activation Function | MAE (%) | |
|---|---|---|---|---|
| 1 | (32,64,128,64,32) | Linear | 1.48% | 0.98 |
| 2 | ReLU | 2.06% | 0.98 | |
| 3 * | Sigmoid | 1.45% | 0.99 |
| Number | Hidden Layers | Batch Size | MAE (%) | |
|---|---|---|---|---|
| 1 | (32,64,128,64,32) | 2 | 1.84% | 0.97 |
| 2 | 4 | 1.46% | 0.97 | |
| 3 | 8 | 1.45% | 0.97 | |
| 4 | 16 | 1.45% | 0.98 | |
| 5 * | 32 | 1.39% | 0.98 | |
| 6 | 64 | 2.47% | 0.96 |
| Number | Hidden Layers | Epochs | MAE (%) | |
|---|---|---|---|---|
| 1 | (32,64,128,64,32) | 1000 | 3.81% | 0.94 |
| 2 | 2000 | 2.67% | 0.96 | |
| 3 | 5000 | 2.01% | 0.97 | |
| 4 | 10,000 | 1.95% | 0.98 | |
| 5 * | 20,000 | 1.36% | 0.98 | |
| 6 | 30,000 | 1.39% | 0.98 | |
| 7 | 40,000 | 1.38% | 0.98 | |
| 8 | 50,000 | 1.38% | 0.98 |
| Objective Function | Inputs | Hidden Layers | Epochs | Batch Size | Activation Function |
|---|---|---|---|---|---|
| Flux | (32,64,128,64,32) | 20,000 | 32 | Sigmoid | |
| Normalized flux (J/J0) | (64,64,32,32) | 30,000 | 16 | ReLU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amari, A.; Ali, M.H.; Jaber, M.M.; Spalevic, V.; Novicevic, R. Study of Membranes with Nanotubes to Enhance Osmosis Desalination Efficiency by Using Machine Learning towards Sustainable Water Management. Membranes 2023, 13, 31. https://doi.org/10.3390/membranes13010031
Amari A, Ali MH, Jaber MM, Spalevic V, Novicevic R. Study of Membranes with Nanotubes to Enhance Osmosis Desalination Efficiency by Using Machine Learning towards Sustainable Water Management. Membranes. 2023; 13(1):31. https://doi.org/10.3390/membranes13010031
Chicago/Turabian StyleAmari, Abdelfattah, Mohammed Hasan Ali, Mustafa Musa Jaber, Velibor Spalevic, and Rajko Novicevic. 2023. "Study of Membranes with Nanotubes to Enhance Osmosis Desalination Efficiency by Using Machine Learning towards Sustainable Water Management" Membranes 13, no. 1: 31. https://doi.org/10.3390/membranes13010031
APA StyleAmari, A., Ali, M. H., Jaber, M. M., Spalevic, V., & Novicevic, R. (2023). Study of Membranes with Nanotubes to Enhance Osmosis Desalination Efficiency by Using Machine Learning towards Sustainable Water Management. Membranes, 13(1), 31. https://doi.org/10.3390/membranes13010031








