Layered Perovskites BaLnnInnO3n+1 (n = 1, 2) for Electrochemical Applications: A Mini Review
Abstract
:1. Introduction
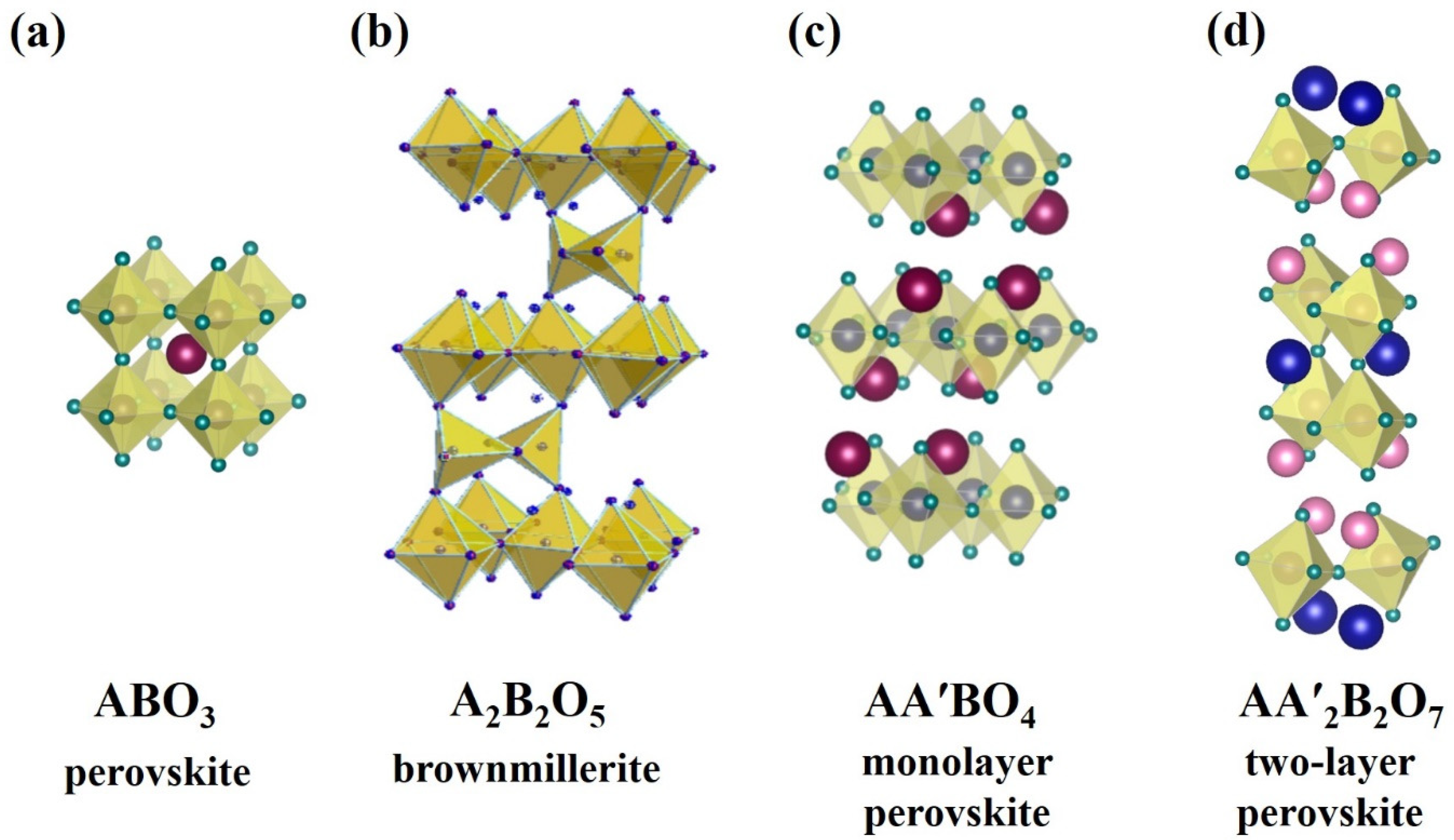
2. Structural Features of Layered Perovskite-Related Materials
3. Oxygen-Ionic Transport in Layered Perovskite-Related Materials
4. Proton Transport in Layered Perovskite-Related Materials
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Malerba, D. Poverty-energy-emissions pathways: Recent trends and future sustainable development goals. Int. J. Sustain. Energy Dev. 2019, 49, 109–124. [Google Scholar] [CrossRef]
- Buonomano, A.; Barone, G.; Forzano, C. Advanced energy technologies, methods, and policies to support the sustainable development of energy, water and environment systems. Energy Rep. 2022, 8, 4844–4853. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 48, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Mikulcic, H.; Kalogirou, S. Sustainable development using renewable energy technology. Renew. Energy 2020, 146, 2430–2437. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Corvalan, C.; Prats, E.V.; Sena, A.; Varangu, L.; Vinci, S. Towards climate resilient and environmentally sustainable health care facilities. Int. J. Environ. Res. Public Health 2020, 17, 8849. [Google Scholar] [CrossRef]
- Watts, N.; Amann, M.; Arnell, N.; Montgomery, H.; Costello, A. The 2020 report of the Lancet countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef]
- Kats, G.H. Slowing global warming and sustaining development: The promise of energy efficiency. Energy Policy 1990, 18, 25–33. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Cheok, Q.; Taweekun, J.; Azad, A.K. Solid oxide fuel cell (SOFC); A new approach of energy generation during the pandemic COVID-19. Int. J. Integr. Eng. 2020, 12, 245–256. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Cheok, Q.; Islam, S.N.; Abdalla, A.M.; Taweekun, J.; Azad, A.K.; Khalilpoor, N.; Issakhov, A. Advanced applications of fuel cells during the COVID-19 Pandemic. Int. J. Chem. Eng. 2021, 2021, 5539048. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Sustainability aspects of hydrogen and fuel cell systems. Int. J. Sustain. Energy Dev. 2011, 15, 137–146. [Google Scholar] [CrossRef]
- Branco, H.; Castro, R.; Lopes, A.S. Battery energy storage systems as a way to integrate renewable energy in small isolated power systems. Int. J. Sustain. Energy Dev. 2018, 43, 90–99. [Google Scholar] [CrossRef]
- International Energy Agency. The Future of Hydrogen: Seizing today’s opportunities. OECD 2019. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2019, 45, 3847–3869. [Google Scholar] [CrossRef]
- Easily, R.R.; Chi, Y.; Ibrahiem, D.M.; Chen, Y. Hydrogen strategy in decarbonization era: Egypt as a case study. Int. J. Hydrog. Energy 2022, 47, 18629–18647. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrog. Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Scovell, M.D. Explaining hydrogen energy technology acceptance: A critical review. Int. J. Hydrog. Energy 2022, 47, 10441–104591. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Kim, J.; Sengodan, S.; Kim, S.; Kwon, O.; Bu, Y.; Kim, G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2019, 109, 606–618. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef] [Green Version]
- Medvedev, D. Trends in research and development of protonic ceramic electrolysis cells. Int. J. Hydrog. Energy 2019, 44, 26711–26740. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Zvonareva, I.; Fu, X.-Z.; Medvedev, D.; Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 2022, 15, 439–465. [Google Scholar] [CrossRef]
- Shim, J.H. Ceramics breakthrough. Nature Energy 2018, 3, 168–169. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; He, Q.; Cheng, C.; Dai, Y.; Chen, B.; Zhang, Y.; Ni, M. Materials development and prospective for protonic ceramic fuel cells. Int. J. Energy Res. 2021, 46, 2212–2240. [Google Scholar] [CrossRef]
- Chiara, A.; Giannici, F.; Pipitone, C.; Longo, A.; Aliotta, C.; Gambino, M.; Martorana, A. Solid-Solid Interfaces in Protonic Ceramic Devices: A Critical Review. ACS Appl. Mater. Interfaces 2020, 12, 55537–55553. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ji, Y.; Shao, Z. New Insights into the Proton-Conducting Solid Oxide Fuel Cells. J. Chin. Ceram. Soc. 2021, 49, 83–92. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; Zhao, S.; Li, Z.; Yu, N.; Ni, M. Scientometric review of proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 37406–37428. [Google Scholar] [CrossRef]
- Colomban, P. Proton conductors and their applications: A tentative historical overview of the early researches. Solid State Ionics 2019, 334, 125–144. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3–4, 359–363. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Maeda, N. High temperature fuel and steam electrolysis cells using proton conductive solid electrolytes. J. Power Sources 1982, 7, 293–301. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Tanaka, S. High temperature type proton conductors based on SrCeO3 and its application to solid electrolyte fuel cells. Solid State Ion. 1983, 9–10, 1021–1025. [Google Scholar] [CrossRef]
- Irvine, J.; Rupp, J.L.M.; Liu, G.; Xu, X.; Haile, S.; Qian, X.; Snyder, A.; Freer, R.; Ekren, D.; Skinner, S.; et al. Roadmap on inorganic perovskites for energy applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram. Int. 2021, 47, 23725–23748. [Google Scholar] [CrossRef]
- Tarasova, N.; Colomban, P.; Animitsa, I. The short-range structure and hydration process of fluorine-substituted double perovskites based on barium-calcium niobate Ba2CaNbO5.5. J. Phys. Chem. Solids 2018, 118, 32–39. [Google Scholar] [CrossRef]
- Cichy, K.; Skubida, W.; Świerczek, K. Structural transformations, water incorporation and transport properties of tin-substituted barium indate. J. Solid State Chem. 2018, 262, 58–67. [Google Scholar] [CrossRef]
- Domen, K.; Yoshimura, J.; Sekine, T.; Tanaka, A.; Onishi, T. A novel series of photocatalysts with an ion-exchangeable layered structure of niobate. Catal Lett. 1990, 4, 339–343. [Google Scholar] [CrossRef]
- Machida, M.; Yabunaka, J.; Kijima, T. Efficient photocatalytic decomposition of water with the novel layered tantalate RbNdTa2O7. Chem. Commun. 1999, 19, 1939–1940. [Google Scholar] [CrossRef]
- Machida, M.; Miyazaki, K.; Matsushima, S.; Arai, M. Photocatalytic properties of layered perovskite tantalates, MLnTa2O7 (M = Cs, Rb, Na, and H; Ln = La, Pr, Nd, and Sm). J. Mater. Chem. 2003, 13, 1433–1437. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Zvereva, I.A. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions. Russ. Chem. Rev. 2016, 85, 248–279. [Google Scholar] [CrossRef]
- Krasheninnikova, O.V.; Syrov, E.V.; Smirnov, S.M.; Suleimanov, E.V.; Fukina, D.G.; Knyazev, A.V.; Titaev, D.N. Synthesis, crystal structure and photocatalytic activity of new Dion-Jacobson type titanoniobates. J. Solid State Chem. 2022, 315, 123445. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Ekthammathat, N.; Dumrongrojthanath, P.; Thingtem, S.; Thongtem, T. Hydrothermal synthesis, structure, and optical properties of pure and silver-doped Bi2MoO6 nanoplates. Russ. J. Phys. Chem. 2015, 89, 2443–2448. [Google Scholar] [CrossRef]
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent advancements in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M = W, Mo, Cr) for environmental remediation and clean energy production. Ind. Eng. Chem. Res. 2021, 95, 1–15. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrog. Energy 2020, 45, 19078–19111. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Deng, Y.; Qian, Y.; Jia, X.; Ma, M.; Yang, C.; Liu, K.; Wang, Z.; Qu, S.; et al. The application of perovskite materials in solar water splitting. J. Semicond. 2020, 41, 011701. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T.S. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef] [PubMed]
- Benedek, N.A.; Rondinelli, J.M.; Djani, H.; Ghosez, P.; Lightfoot, P. Understanding ferroelectricity in layered perovskites: New ideas and insights from theory and experiments. Dalton Trans. 2015, 44, 10543–10558. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, W.C.; Rodrigues, G.L.C.; Araújo, B.S.; de Aguiar, F.A.A.; de Abreu Silva, A.N.A.; Fechine, P.B.A.; de Araujo Paschoal, C.W.; Ayala, A.P. Pressure-induced structural phase transitions in the multiferroic four-layer Aurivillius ceramic Bi5FeTi3O15. Ceramics 2020, 46, 18056–180621. [Google Scholar] [CrossRef]
- Zulhadjri; Wendari, T.P.; Ikhram, M.; Putri, Y.E.; Septiani, U.; Imelda. Enhanced dielectric and ferroelectric responses in La3+/Ti4+ co-substituted SrBi2Ta2O9 Aurivillius phase. Ceram. Int. 2022, 48, 10328–103321. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, S.; Wang, F.; Liu, J.; Shi, J.; Xing, J.; Chen, Q.; Zhu, J.; Wang, Q. Bismuth titanate based piezoceramics: Structural evolutions and electrical behaviors at different sintering temperatures. J. Alloys Compd. 2021, 882, 160637. [Google Scholar] [CrossRef]
- Chen, C.; Ning, H.; Lepadatu, S.; Cain, M.; Yan, H.; Reece, M.J. Ferroelectricity in Dion-Jacobson ABiNb2O7 (A = Rb, Cs) compounds. J. Mater. Chem. C 2015, 3, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Kudo, A.; Kaneko, E. Photoluminescence of layered perovskite oxides with triple-octahedra slabs containing titanium and niobium. J. Mater. Sci. Lett. 1997, 16, 224–226. [Google Scholar] [CrossRef]
- Pavani, K.; Graça, M.P.F.; Kumar, J.S.; Neves, A.J. Photoluminescence varied by selective excitation in BiGdWO6:Eu3+ phosphor. Opt. Mater. 2017, 74, 120–127. [Google Scholar] [CrossRef]
- Mamidi, S.; Gundeboina, R.; Kurra, S.; Velchuri, R.; Muga, V. Aurivillius family of layered perovskites, BiREWO6 (RE = La, Pr, Gd, and Dy): Synthesis, characterization, and photocatalytic studies. C. R. Chim. 2018, 21, 547–552. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, X.; Zhao, J.; Molokeev, M.; Lin, Z.; Liu, Q.; Xia, Z. Two-dimensional-layered perovskite ALaTa2O7:Bi3+ (A = K and Na) phosphors with versatile structures and tunable photoluminescence. ACS Appl. Mater. Interfaces 2018, 10, 24648–24655. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.P.; Singh, A.K.; Kundu, T.K.; Sundaresan, A. Visible-light excited polar Dion-Jacobson Rb(Bi1-xEux)2Ti2NbO10 perovskites: Photoluminescence properties and in vitro bioimaging. J. Mater. Chem. B 2022, 10, 935–944. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. New compounds of the K2NiF4 type. Acta Cryst. 1957, 10, 538–539. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. The compound Sr3Ti2O7 and its structure. Acta Cryst. 1958, 11, 54–55. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Rao, C.N.R. Crystal Chemistry and Magnetic Properties of Layered Metal Oxides Possessing the K2NiF4 or Related Structures. J. Solid State Chem. 1984, 53, 193–216. [Google Scholar] [CrossRef]
- Ganculi, D. Cationic radius ratio and formation of K2NiF4-type compounds. J. Solid State Chem. 1979, 30, 353–356. [Google Scholar] [CrossRef]
- Tyitov, Y.O.; Slobodyanik, M.S.; Polubinskii, V.V. Criteria of existence of one- and two-layer compounds (MeIILn)n+1BnO3n+1. Dopov. Natsyional’noyi Akad. Nauk. Ukrayini 2018, 2018, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Tyitov, Y.O.; Byilyavina, N.M.; Markyiv, V.Y.; Slobodyanik, M.S.; Krajevs’ka, Y.A. Synthesis and crystal structure of BaLaInO4 and SrLnInO4 (Ln−La, Pr). Dopov. Natsyional’noyi Akad. Nauk. Ukrayini 2009, 10, 160–166. [Google Scholar]
- Fijii, K.; Yashima, M. Discovery and development of BaNdInO4 -A brief review. J. Ceram. Soc. Jpn. 2018, 126, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Chroneos, A.; Yildiz, B.; Tarancón, A.; Parfitt, D.; Kilner, J.A. Oxygen diffusion in solid oxide fuel cell cathode and electrolyte materials: Mechanistic insights from atomistic simulations. Energy Environ. Sci. 2011, 4, 2774–2789. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor Doping and Oxygen Vacancy Migration in Layered Perovskite NdBaInO4-Based Mixed Conductors. J. Phys. Chem. C 2016, 120, 6416–6426. [Google Scholar] [CrossRef]
- Fujii, K.; Esaki, Y.; Omoto, K.; Yashima, M.; Hoshikawa, A.; Ishigaki, T.; Hester, J.R. New Perovskite-Related Structure Family of Oxide-Ion Conducting Materials NdBaInO4. Chem. Mater. 2014, 26, 2488–2491. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Kreimesh, H.; Fedorova, I. Protonic transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper structure. Appl. Sci. 2022, 12, 4082. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Belova, K.; Egorova, A.; Abakumova, E.; Medvedev, D. Layered Perovskites BaM2In2O7 (M = La, Nd): From the Structure to the Ionic (O2–, H+) Conductivity. Materials 2022, 15, 3488. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Electrical properties of new protonic conductors Ba1+xLa1–xInO4–0.5x with Ruddlesden-Popper structure. J Solid State Electrochem. 2020, 24, 1497–1508. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4. Solid State Comm. 2021, 323, 114093. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping. Ionics 2020, 26, 5075–5088. [Google Scholar] [CrossRef]
- Tarasova, N.A.; Galisheva, A.O.; Animitsa, I.E.; Lebedeva, E.L. Oxygen-Ion and Proton Transport in Sc-Doped Layered Perovskite BaLaInO4. Russ. J. Electrochem. 2021, 57, 1008–1014. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Anokhina, I.; Gilev, A.; Cheremisina, P. Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4. Ceram. Int. 2022, 48, 15677–15685. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I. Proton transport in the gadolinium-doped layered perovskite BaLaInO4. Materials 2022, 15, 7351. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Bedarkova, A. Advanced proton-conducting ceramics based on layered perovskite BaLaInO4 for energy conversion technologies and devices. Materials 2022, 15, 6841. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Korona, D. Incorporation and Conduction of Protons in Ca, Sr, Ba-Doped BaLaInO4 with Ruddlesden-Popper Structure. Materials 2019, 12, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, K.; Shiraiwa, M.; Esaki, Y.; Yashima, M.; Kim, S.J.; Lee, S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985–11990. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Yan, Y.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016, 288, 262–265. [Google Scholar] [CrossRef]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef]
- Proton Transport in Alkali-Earth Doped Layered Perovskites Based on BaLa2In2O7. Inorganics 2022, 10, 161. [CrossRef]
- Tarasova, N.A. Local structure and ionic transport in acceptor-doped layered perovskite BaLa2In2O7. Chim. Techno Acta 2022, 9, 20229415. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Abakumova, E.; Belova, K.; Kreimesh, H. Novel high conductive ceramic materials based on two-layer perovskite BaLa2In2O7. Int. J. Mol. Sci. 2022, 23, 12813. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Verinkina, E. Synthesis, Hydration Processes and Ionic Conductivity of Novel Gadolinium-Doped Ceramic Materials Based on Layered Perovskite BaLa2In2O7 for Electrochemical Purposes. Processes 2022, 10, 2536. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Abakumova, E. Cation and oxyanion doping of layered perovskite BaNd2In2O7: Oxygen-ion and proton transport. Int. J. Hydrog. Energy 2022, in press. [CrossRef]







| Composition | Values of Protonic Conductivity at 450 °C, S/cm | Ref. |
|---|---|---|
| BaLaInO4 | 4 × 10−7 | [83] |
| Ba1.1La0.9InO3.95 | 0.8 × 10−5 | [83] |
| BaLa0.9Sr0.1InO3.95 | 1.4 × 10−5 | [83] |
| BaLa0.9Ca0.1InO3.95 | 1.9 × 10−5 | [83] |
| BaLaIn0.9Ti0.1O4.05 | 0.2 × 10−5 | [78] |
| BaLaIn0.9Zr0.1O4.05 | 0.2 × 10−5 | [78] |
| BaLa0.9Nd0.1InO4 | 1.1 × 10−5 | [82] |
| BaLa0.9Gd0.1InO4 | 1.0 × 10−5 | [81] |
| BaLaIn0.1Y0.9O4 | 3.3 × 10−5 | [80] |
| BaLaIn0.1Sc0.9O4 | 1.2 × 10−5 | [79] |
| BaLa2In2O7 | 1.7 × 10−6 | [75] |
| BaLa0.9Ca0.1InO3.95 | 2.3 × 10−6 | [74] |
| Ba1.05La1.95In2O6.975 | 5.4 × 10−6 | [87] |
| Ba1.1La1.9In2O6.95 | 10 × 10−6 | [87] |
| Ba1.2La1.8In2O6.9 | 63 × 10−6 | [87] |
| Ba1.25La1.75In2O6.875 | 64 × 10−6 | [87] |
| BaLa1.9Sr0.1In2O6.95 | 9.4 × 10−6 | [87] |
| BaLa1.85Sr0.15In2O6.925 | 14 × 10−6 | [87] |
| BaLa1.8Sr0.2In2O6.9 | 17 × 10−6 | [87] |
| BaLa1.95Gd0.05In2O7 | 1.8 × 10−6 | [90] |
| BaLa1.9Gd0.1In2O7 | 2.3 × 10−6 | [90] |
| BaLa1.85Gd0.15In2O7 | 2.7 × 10−6 | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, N. Layered Perovskites BaLnnInnO3n+1 (n = 1, 2) for Electrochemical Applications: A Mini Review. Membranes 2023, 13, 34. https://doi.org/10.3390/membranes13010034
Tarasova N. Layered Perovskites BaLnnInnO3n+1 (n = 1, 2) for Electrochemical Applications: A Mini Review. Membranes. 2023; 13(1):34. https://doi.org/10.3390/membranes13010034
Chicago/Turabian StyleTarasova, Nataliia. 2023. "Layered Perovskites BaLnnInnO3n+1 (n = 1, 2) for Electrochemical Applications: A Mini Review" Membranes 13, no. 1: 34. https://doi.org/10.3390/membranes13010034
APA StyleTarasova, N. (2023). Layered Perovskites BaLnnInnO3n+1 (n = 1, 2) for Electrochemical Applications: A Mini Review. Membranes, 13(1), 34. https://doi.org/10.3390/membranes13010034









