The Selection of Efficient Antiscalant for RO Facility, Control of Its Quality and Evaluation of the Economical Efficiency of Its Application
Abstract
1. Introduction
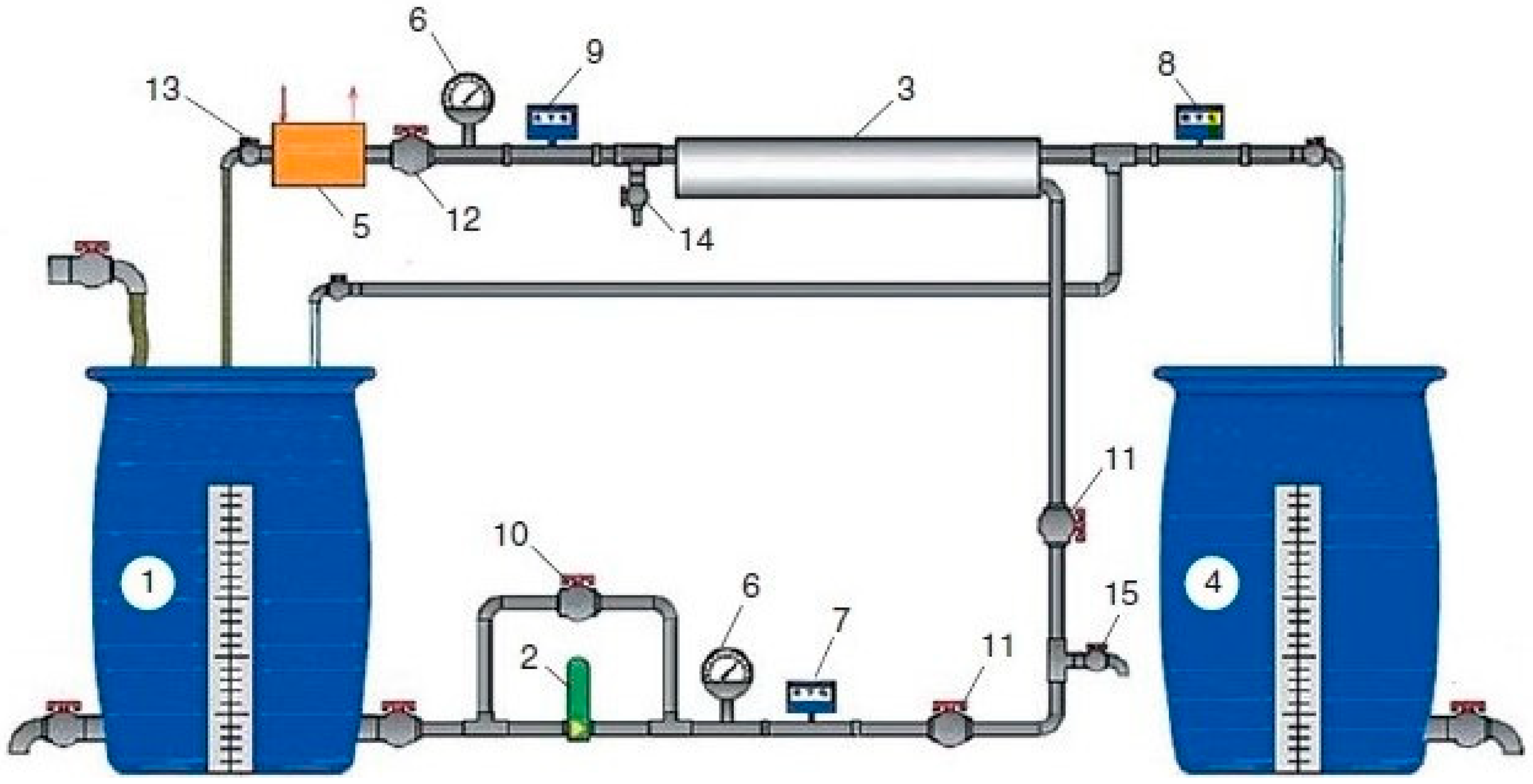
2. Experimental Part: Materials and Equipment
- Determination of calcium carbonate crystallization rates depending on antiscalant dose and membrane type.
- Evaluation of antiscalant adsorption rates for different doses.
- Dependencies of adsorption rates on the antiscalant type and its dose.
3. Experimental Results
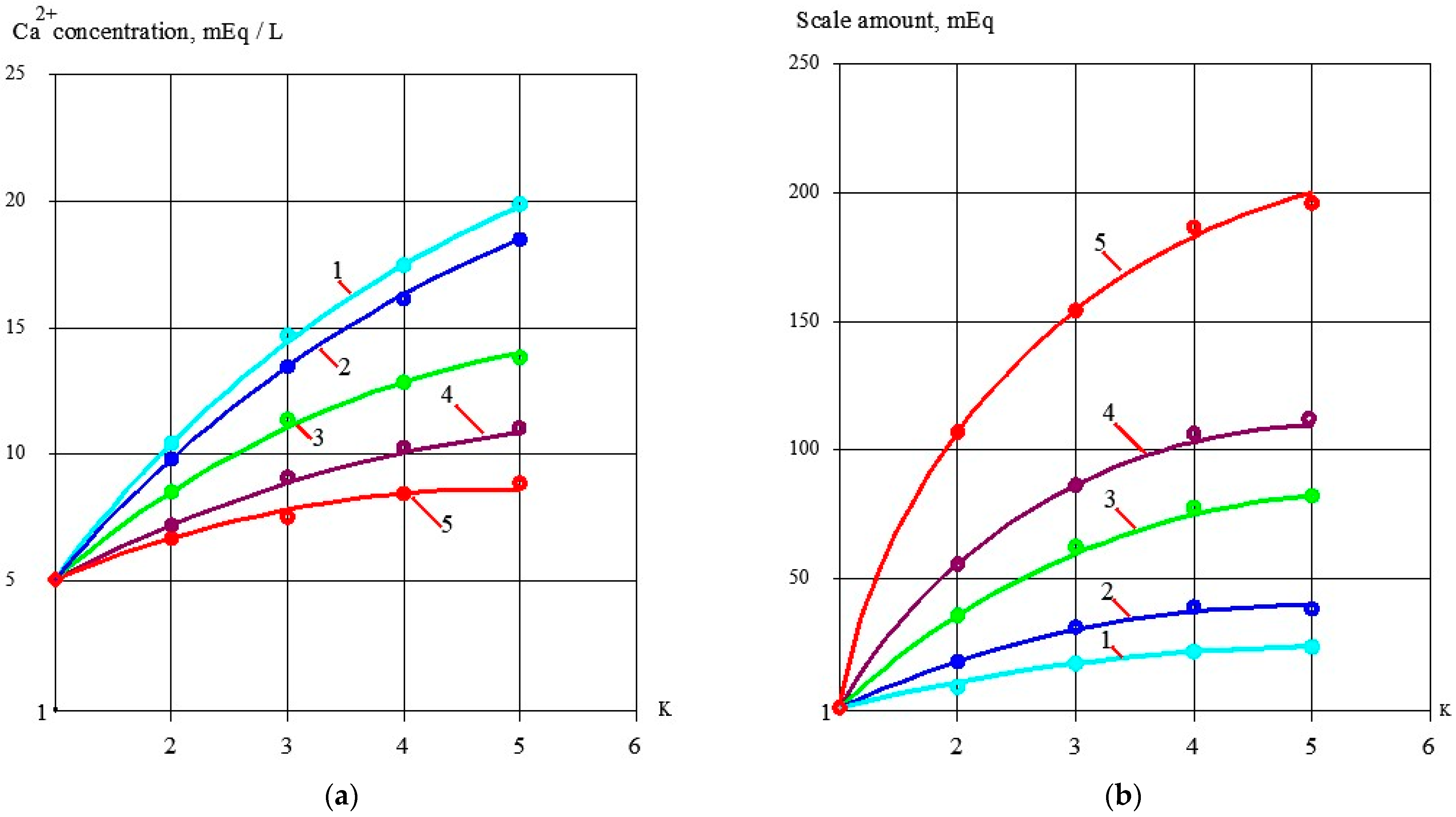
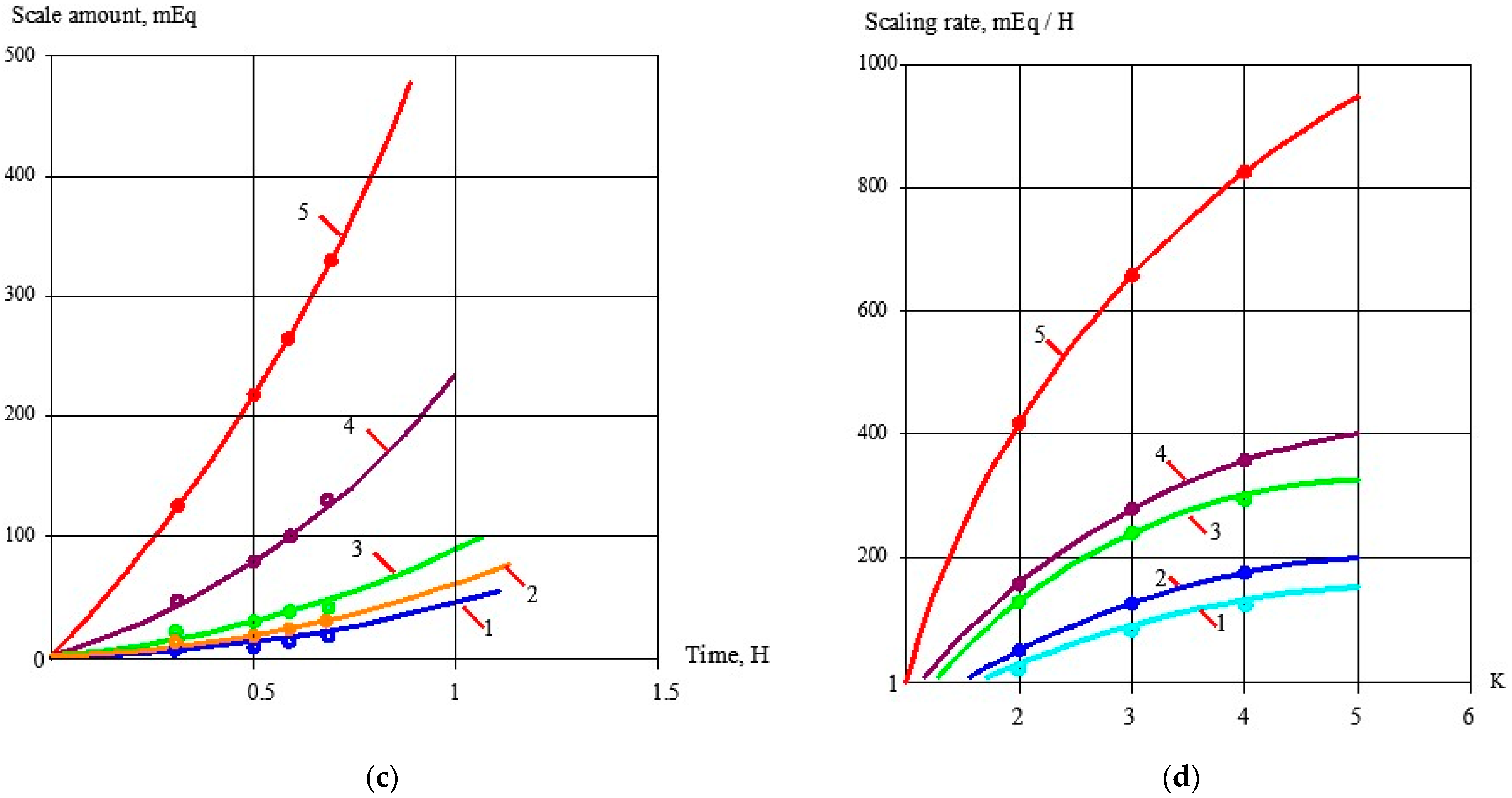
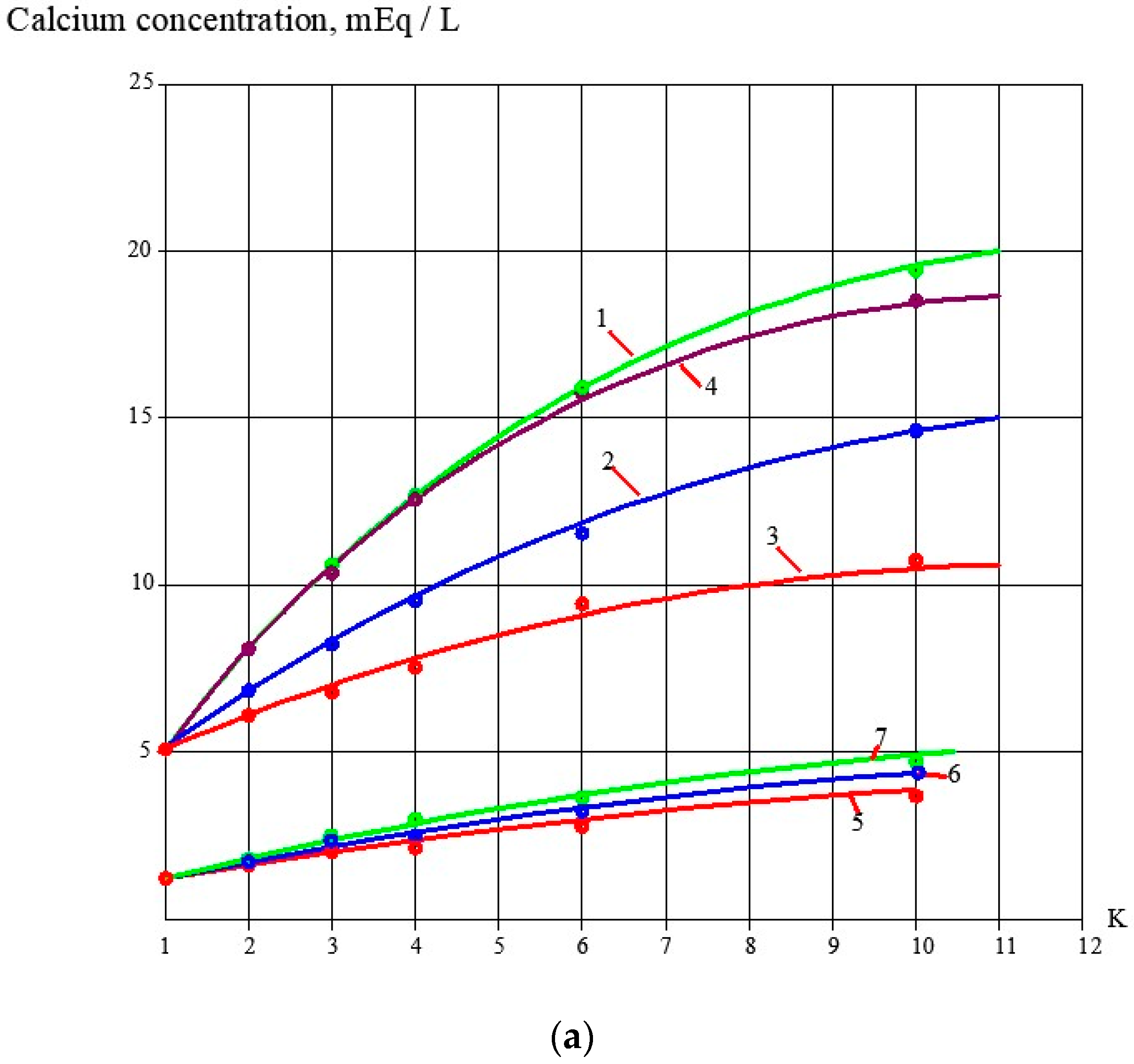
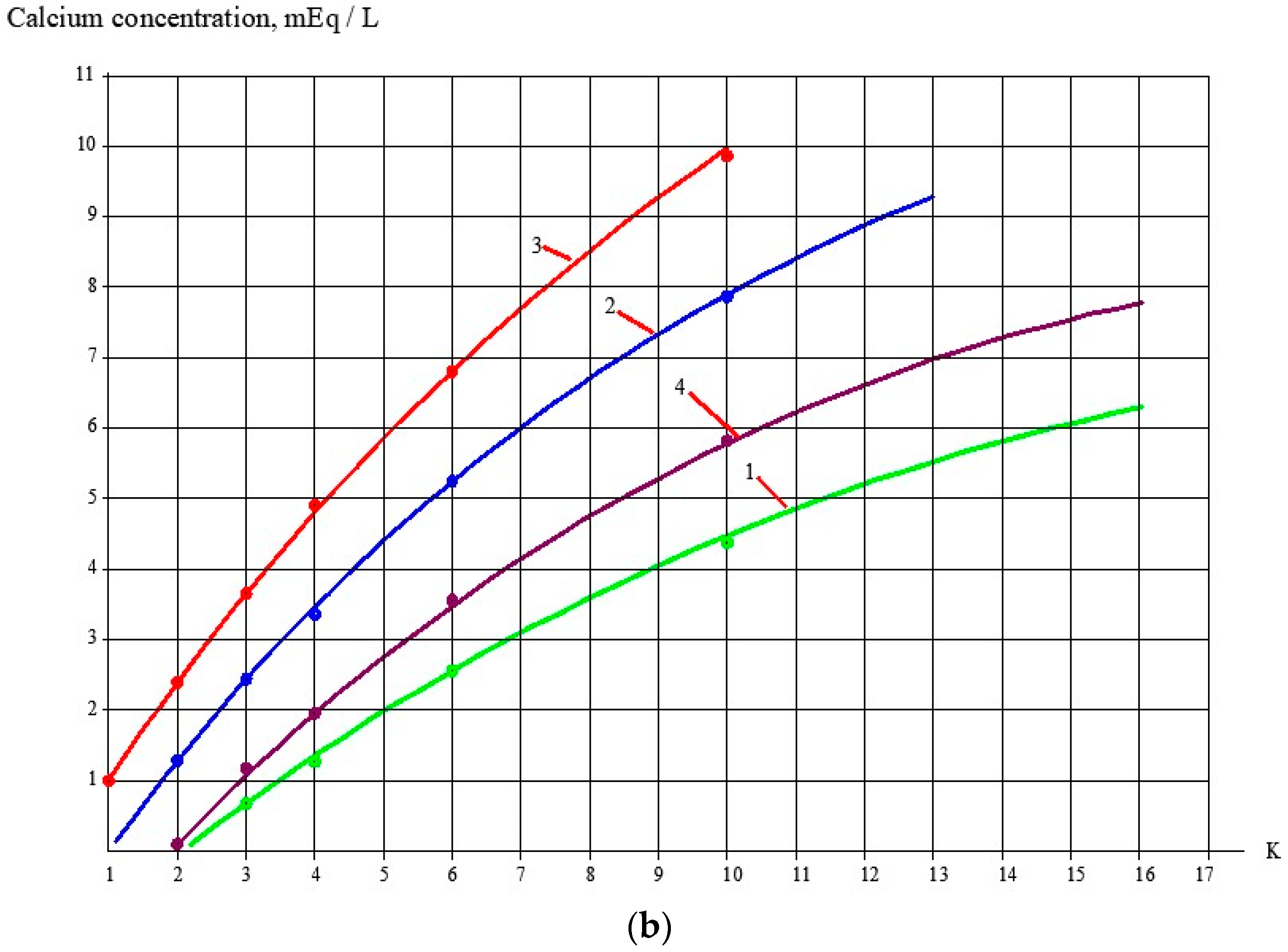
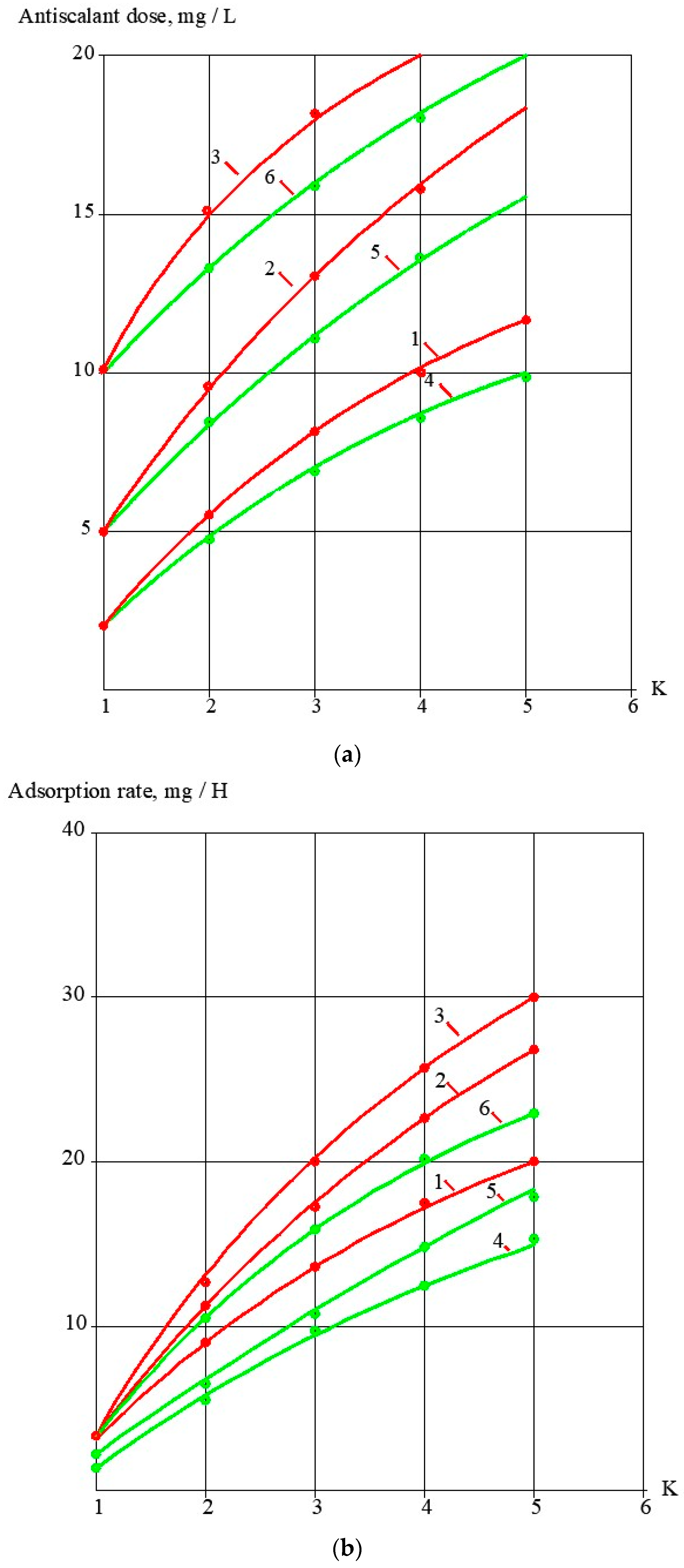
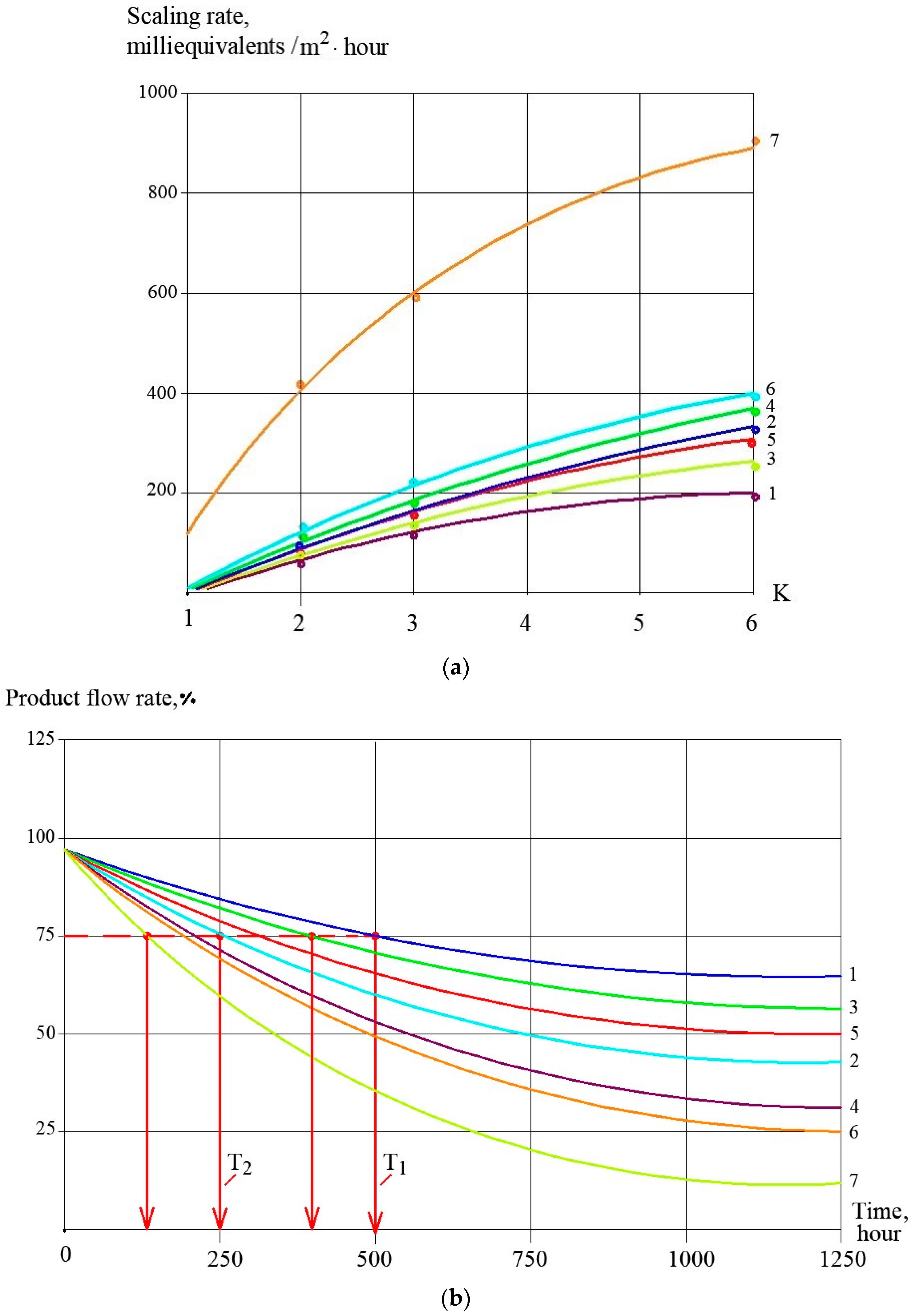
3.1. Effect of the Antiscalant Dose on Scaling Rates
3.2. Effect of Membrane Type on Scaling Rates
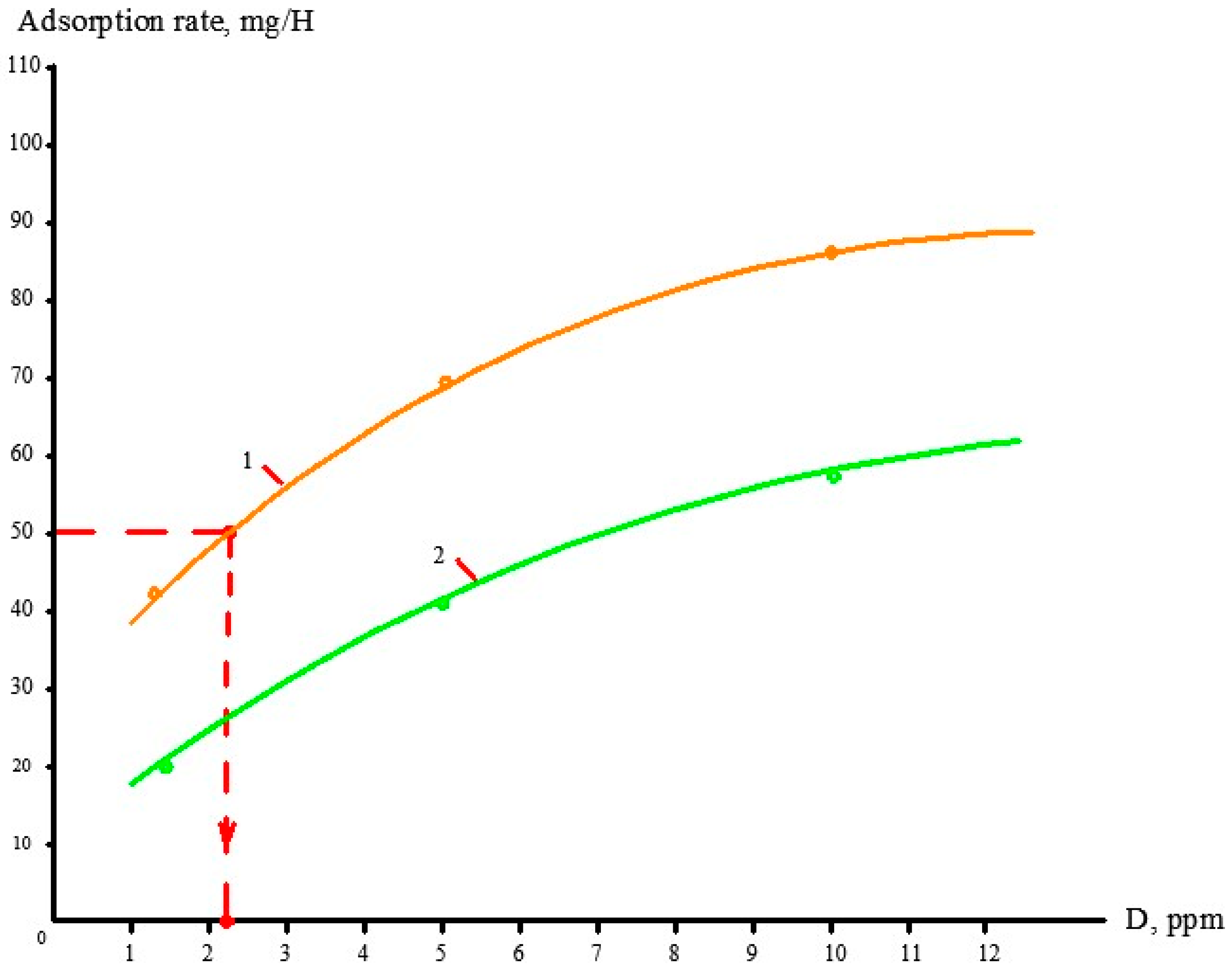
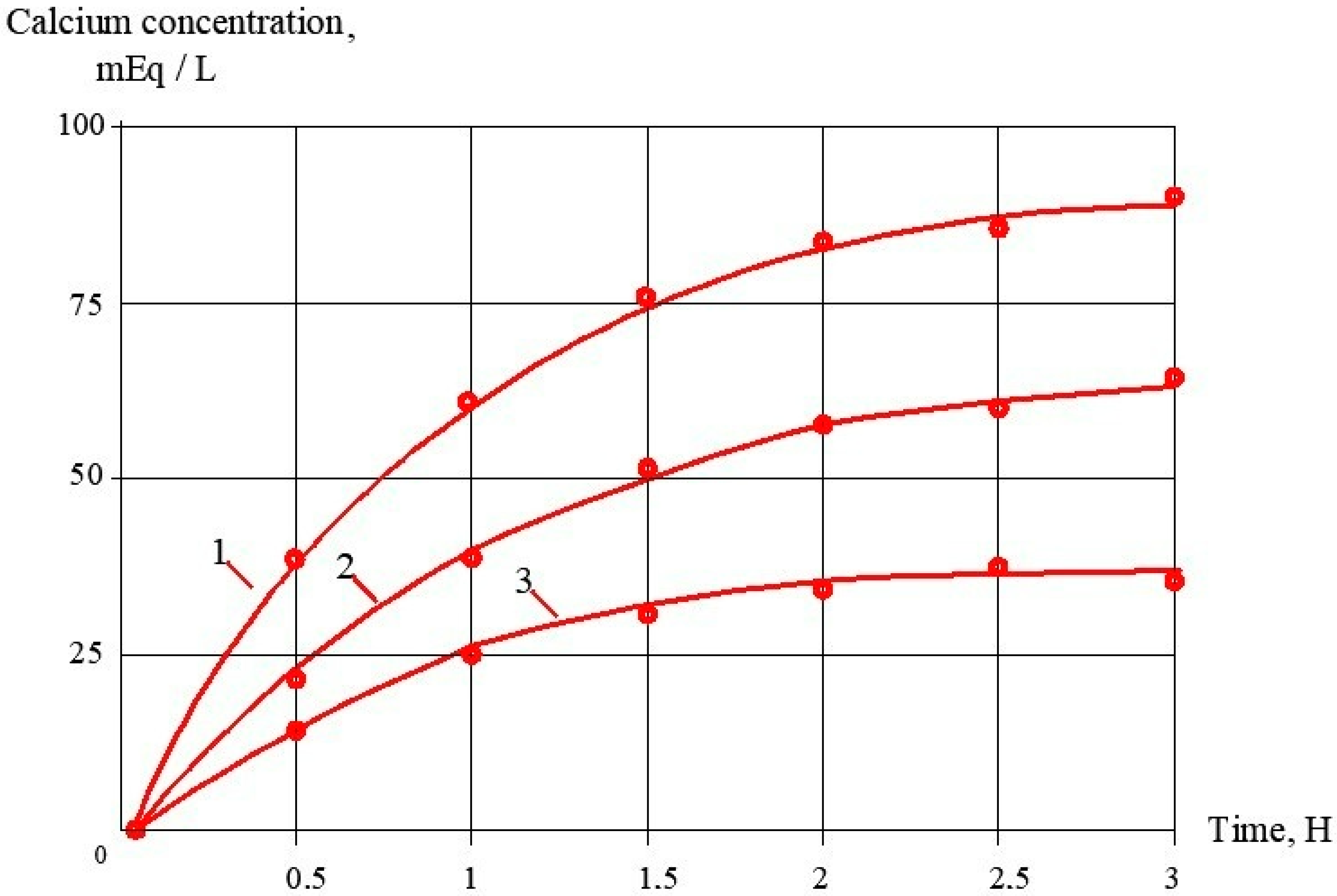
3.3. Evaluation of Antiscalant Adsorption Rates and Their Influence on Scaling Rates
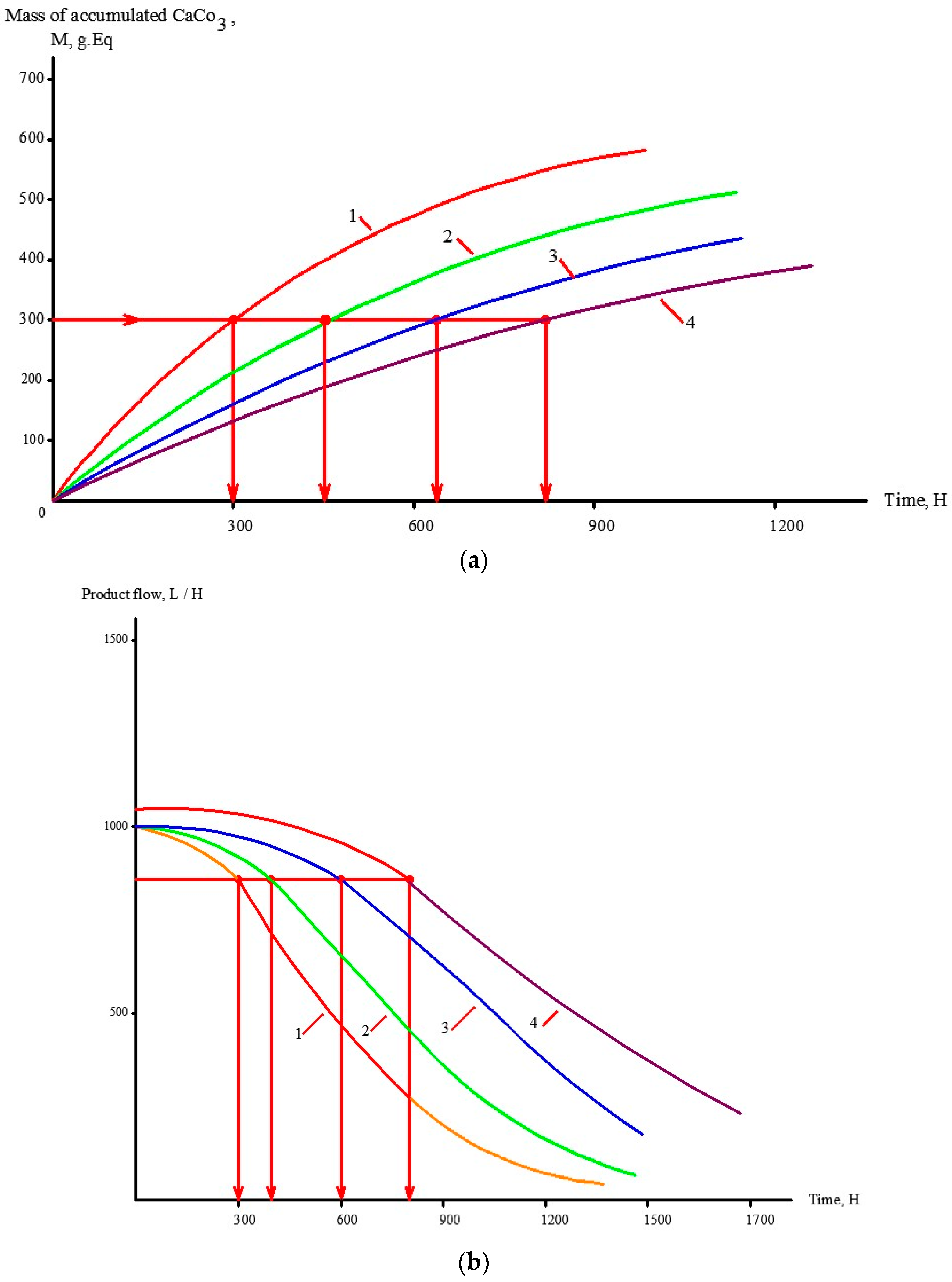
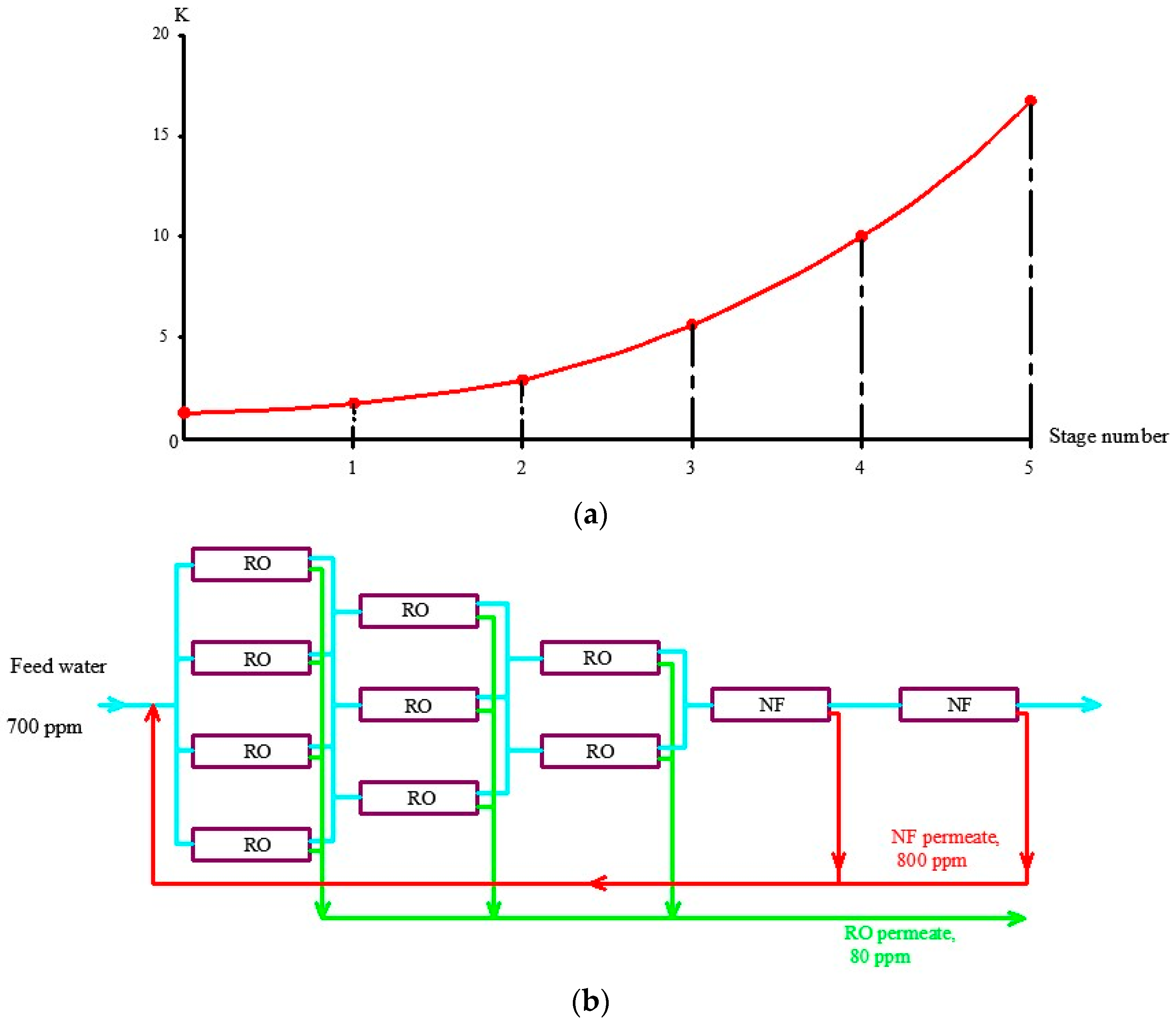
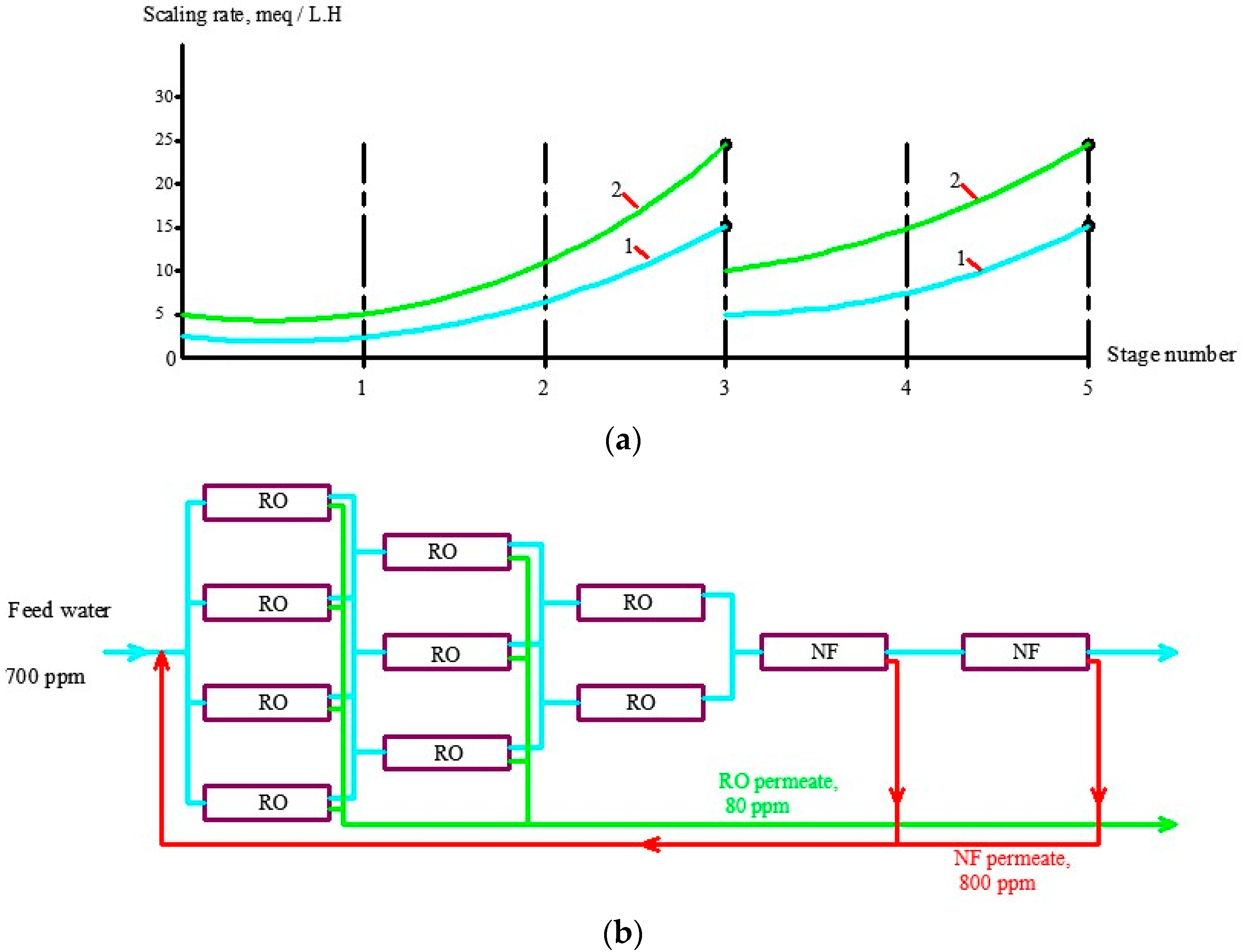
3.4. Selecting Antiscalant and Membrane Type to Reduce Operational Costs
4. Industrial Application of the Results
5. Conclusions
- Antiscaling efficiency of the inhibitor depends on its ability to adsorb on the surface of growing crystals. The higher adsorption rate, the better the antiscaling behavior of the inhibitor.
- To efficiently control scaling and to reduce operational costs of the RO unit it is recommended to tailor the membrane plant with nanofiltration membranes. The joint use of low rejection membranes and efficient antiscalant provides substantial decrease in scaling rates in membrane modules and reduces operational costs.
- High adsorption abilities of phosphonic-based antiscalant enables us to reduce the antiscalant dose in the feed water without compromising effectiveness of scale control and thus reduce reagent consumption and operational costs.
- High efficiency of phosphonic antiscalants is attributed to the content of aminotris (methylene=phosphonic acid) in the product. Application of Nuclear Magnetic Resonance method helps to identify the presence of ATMP and to avoid buying low-quality products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pervov, A.; Andrianov, A. Deposition of Calcium and Magnesium from RO concentrate by means of seed crystallization and production of softened water for technical purpose. Desalination Water Treat. 2018, 110, 10–18. [Google Scholar] [CrossRef]
- Pervov, A.G.; Nguen, X.Q. Application of reverse osmosis and nanofiltration techniques at municipal drinking water facilities. E3S Web Conf. 2019, 97, 06004. [Google Scholar] [CrossRef]
- Suratt, W.B.; Adrews, D.R.; Pujals, V.J.; Richards, S.A. Design considerations for major membrane treatment facility for groundwater. Desalination 2000, 131, 37–46. [Google Scholar] [CrossRef]
- Salman, M.A.; Al-Nuwaibit, G.; Safar, M.; Al-Mesri, A. Performance of physical treatment method and different commercial antiscalants to control scaling deposition in desalination plant. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Turek, M.; Mitko, K.; Dydo, P.; Laskovska, E.; Jakobic-Kolon, A. Prospects for high water recovery membrane desalination. Desalination 2017, 401, 180–189. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman MH, D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 95, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hasson, D.; Shemer, H. Flow conditions affecting the induction period of CaSO4 scaling on RO membranes. Desalination 2018, 431, 119–125. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Tesla, F.; Freeman, B.D. The effect of antiscalant addition on calcium carbonate precipitation for a simplified synthetic brackish water reverse osmosis concentrate. Water Res. 2010, 44, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.K.; Choi, Y.-G. The comparative study for scale inhibition on surface of RO membranes in wastewater different antiscalants. J. Membr. Sci. 2018, 546, 61–69. [Google Scholar] [CrossRef]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pecoul, N.; Perrot, H.; Ledion, J.; Cheap-Charpentier, H.; Homer, O. State of art of natural inhibitors of calcium carbonate scaling: A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Pervov, A.; Andrianov, A.; Rudakova, G.; Popov, K. A comparative study of some novel “green” and traditional antiscalants efficiency for the reverse osmotic Black Sea water desalination. Desalination Water Treat. 2017, 73, 11–20. [Google Scholar] [CrossRef]
- Zimmer, K.; Hater, W.; Icart, K.; Jaworski, J.; Kruse, N.; Braun, G. The performance of polycarboxylates as inhibitors for CaCO3 scaling in reverse osmosis plants. Desalination Water Treat. 2016, 57, 48–49. [Google Scholar] [CrossRef]
- Al-Qadami, E.; Ahsan, A.; Mustafa, Z.; Abdurrasheed, A.; Yusof, K.; Shah, S. Nanofiltration membrane technology and its applications in surface water treatment: A review. J. Desalination Water Purif. 2020, 18, 3–9. Available online: http://ababilpub.com/download/jdwp18-2 (accessed on 27 December 2022).
- Guo, H.; Li, X.; Yang, W.; Yao, Z.; Mei, Y.; Peng, L.E.; Yang, Z.; Shao, S.; Tang, C.Y. Nanofiltration for drinking water treatment: A review. Front. Chem. Sci. Eng. 2021, 16, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.W.; Hilal, N.; Darwish, N.; Al-Zoubi, H. Prediction of permeate fluxes and rejections of highly concentrated salts in nanofiltration membranes. J. Membr. Sci. 2007, 289, 40–50. [Google Scholar] [CrossRef]

| Component | Intake 1 | Intake 2 | Intake 3 | |
|---|---|---|---|---|
| 1 | Ca2+ | 4.4 | 3.9 | 3.5 |
| 2 | Mg2+ | 2.3 | 1.4 | 2.5 |
| 3 | SO42− | 0.63 | 0.2 | 0.7 |
| 4 | Cl− | 0.29 | 0.3 | 0.15 |
| 5 | HCO3− | 6.5 | 5.8 | 6 |
| 6 | PH | 7.2 | 7.3 | 7.2 |
| 7 | TDS | 590 | 510 | 480 |
| Characteristics | Present Technique (Antiscalant Dose 6 ppm) | Recommended Technique (Antiscalant Dose 4/2 ppm) | |
|---|---|---|---|
| 1 | Feed flow, (m3/H) | 130/910,000 | 130/910,000 |
| 2 | Product flow, Designed, (m3/H)/ Real, (m3/year) | 100 700,000/ 520,000 | 100 700,000/ 630,000 |
| 3 | Concentrate flow, | 30 | 30/1.5 (After recovery increase using NF) |
| 4 | Pressure, Bar | 14–16.5 | 14–15 |
| 5 | Annual power consumption, KW | 112,000 | 105,000 |
| 6 | Power costs, USD/year | 8000 | 7500 |
| 7 | Antiscalant type, and dose, ppm | Jurbysoft, 6 ppm | Aminat-K, 4/2 |
| 8 | Annual antiscalant consumption, kg | 5460 | 3640/1820 |
| 9 | Antiscalant costs, USD per ton | 2857 | 3571 |
| 10 | Annual antiscalant cost, USD | 15,600 | 13,000/6500 |
| 11 | Number of cleanings per year | 8 | 4 |
| 12 | Reagent consumption; Caustic cost, USD/liter, Acid cost, USD/liter | 6000/100 4800/120 | 3000/100 2400/120 |
| 13 | Annual cleaning costs; caustic, USD acid, USD | 8571 8229 | 4286 4114 |
| 14 | Membranes; model/number/cost, USD | Lewabrane RO B 370 HF/108 | 500 |
| 15 | Annual costs for membrane replacement, USD | 9000 | 9000 |
| 16 | Annual operational costs, USD | 49,400 | 31,400 |
| 17 | Water cost price, USD/ | 0.097 | 0.05 |
| 18 | Membrane elements to reduce concentrate flow: model/number/cost, USD | 8040 70 NE/24/750 | |
| 19 | Additional annual costs for membrane replacement, USD | - | 3000 |
| 20 | Concentrate discharge flow, /hour/tax, USD/ | - | 0.33 |
| 21 | Annual payment for concentrate discharge, USD | 70,000 | 3500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitsov, D.; Aung, H.Z.; Pervov, A. The Selection of Efficient Antiscalant for RO Facility, Control of Its Quality and Evaluation of the Economical Efficiency of Its Application. Membranes 2023, 13, 85. https://doi.org/10.3390/membranes13010085
Spitsov D, Aung HZ, Pervov A. The Selection of Efficient Antiscalant for RO Facility, Control of Its Quality and Evaluation of the Economical Efficiency of Its Application. Membranes. 2023; 13(1):85. https://doi.org/10.3390/membranes13010085
Chicago/Turabian StyleSpitsov, Dmitry, Htet Zaw Aung, and Alexei Pervov. 2023. "The Selection of Efficient Antiscalant for RO Facility, Control of Its Quality and Evaluation of the Economical Efficiency of Its Application" Membranes 13, no. 1: 85. https://doi.org/10.3390/membranes13010085
APA StyleSpitsov, D., Aung, H. Z., & Pervov, A. (2023). The Selection of Efficient Antiscalant for RO Facility, Control of Its Quality and Evaluation of the Economical Efficiency of Its Application. Membranes, 13(1), 85. https://doi.org/10.3390/membranes13010085








